Introduction
The 2015 report by the World Health Organization (WHO) and the International Agency for Research on Cancer (IARC) classified red meat as ‘probably carcinogenic’ and processed meat as ‘carcinogenic,’ causing major repercussions worldwide (IARC, 2018). Since this report, many people have become more aware that the consumption of processed products has been linked to colorectal cancer (CRC). However, some studies have found no significant relationship between the intake of meat and processed meat products and CRC (Carr et al., 2015; Hur et al., 2019). Nevertheless, several studies have reported various mechanisms, including the consumption of processed products with natural materials, to reduce the risk of CRC (Kang et al., 2022; Lee et al., 2020a; Lee et al., 2020b). Fermented foods are generally considered good for human health and Korean fermented foods, such as kimchi, soybean paste, red pepper paste, soy sauce, jeotgal, and makgeolli, reportedly have many health benefits, such as antioxidant, anti-obesity, anti-inflammatory, neuroprotective, antibacterial, and anticancer effects (Han et al., 2022; Islam and Choi, 2009; Kim et al., 2020; Ko et al., 2019; Nile, 2015; Perumal et al., 2019). Several studies have reported that the beneficial effects of dietary fermented foods are related to the improvement of the gut microbiota balance and the gut barrier function related to digestive health, which, in turn, protects against CRC (Bell et al., 2018; Gagnière et al., 2016). Nevertheless, the main mechanisms and effects of the dietary intake of processed meat products with fermented foods on the risk of CRC and the changes in gut microbiota remain largely unknown. Therefore, the purpose of this study was to analyze the effects of the dietary intake of processed meat with fermented foods on the risk of CRC and the changes in gut microbiota.
Materials and Methods
All materials were purchased from a local market (Anseong, Korea). This study used ham, sausage, and bacon as processed meat products, and kimchi, soybean paste, and red pepper paste as fermented foods. The composition of the processed meats used is described with a focus on the meat composition: 1) ham was made from pork picnic (69.99%), purified water, and additives (including corn syrup, purified salt, hydroxypropyl starch, potassium lactate, sodium diacetate, sodium triphosphate, potassium chloride, sodium erythorbate, and sodium nitrite) and purchased from Bar-S Foods (Scottsdale, AZ, USA); 2) sausage was made from pork (94.59%), purified water, and additives (including corn syrup, sugar, L-sodium glutamate, sodium erythorbate, and sodium nitrite) and purchased from Johnsonville sausage. LLC (Sheboygan Falls, WI, USA); and 3) bacon consisted of pork belly (91.32%), purified water, and additives (including sodium, sodium acid pyrophosphate, purified salt, sodium erythorbate, and sodium nitrite) and purchased from Swift Pork company (Greeley, CO, USA). Kimchi was prepared using cabbage, radish, purified salt, salted shrimp, and L. mesenteroides, and purchased from Bibigo (CJ Cheiljadang, Seoul, Korea). Soybean paste was prepared using soybeans, wheat flour, purified salt, soybean paste, fermented soybean (meju) powder, ethyl alcohol, koji obtained from Aspergillus oryzae, defatted soybean powder, flavor enhancer, and Bacillus spp., and purchased from Haechandle (CJ Cheiljadang). Red pepper paste (pepper paste) was prepared using red pepper powder, purified salt, garlic, onion, starch syrup, wheat flour, brown rice powder, meju, glutinous brown rice powder, yeast powder, and Bacillus subtilis, and purchased from Cungjungone (DAESANG, Seoul, Korea).
All processed meat samples were cooked using an electric grill (55×31×31 cm; KitchenArt, Incheon, Korea) at 180°C 200°C. Before cooking, the cooking temperature was adjusted using an infrared thermometer (TM-969, Lutron, Taipei, Taiwan). The ham was cut into 0.8 cm-thick slices and cooked back and forth for 3.5 min until it was completely cooked. The bacon was cut into 4 cm-wide slices and cooked on each side for 2.5 min. The sausage was cooked for 3 min (until it was completely cooked). The cooked meat products were cooled, vacuum-packed, and then frozen (20°C) until use.
All procedures involving mice were approved by the Institutional Animal Care and Use Committee of Chung-Ang University (approval number: 202000050). For animal experiments, Institute of Cancer Research (ICR) female mice were purchased from Orient Bio (Seongnam, Korea). Thirty-nine of 24 wk old (adult) and 39 of 80 wk old (elderly) mice were housed, and acculimatized for a wk before the animal experiments. During the acclimatization period, the mice were fed a normal diet (Pico 5030; Orient Bio). Mice were housed under standard laboratory conditions of 22.0±0.6°C temperature, 65±5% humidity, and a 12 h light/dark cycle. After the acclimatization period, the mice were divided into 26 treatments [13 treatments×2 ages (adult and elderly)] and fed the ground diet and processed meats mixed with fermented foods for 33 d, as presented in Table 1. Body weight, feed intake, and water intake were monitored every 3 d (data are not shown). Furthermore, mice feces were collected a day before the mice were sacrificed to analyze the composition of gut microbiota.
The composition of the gut microbiota of mice was characterized through next-generation sequencing (NGS)-based analysis of fecal samples following the method of Lee et al. (2021a), with slight modifications. Microbial DNA was isolated from fecal samples using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany). Briefly, 1 g of the collected feces was suspended in 5 mL of stool lysis buffer and then homogenized in TissueLyser II at 20 Hz for 5 min. DNA was extracted and analyzed for quality using agarose gel electrophoresis and a Qubit 3.0 fluorimeter (Thermo Fisher Scientific, Waltham, MA, USA). The extracted DNA samples were diluted to 5 ng/μL. The gut microbial community was characterized based on an approximate 450-bp-long sequence of the 16S rRNA gene (V3 V4 region), directly amplified using primers 341F (5’-CCTACGGGNGGCWGCAG-3’) and 805R (5’-GACTACHVGGGTATCTAATCC-3’). The Illumina Nextera XT DNA Library Prep Kit and Nextera XT Index Kit (Illumina, San Diego, CA, USA) were used for library preparation according to the manufacturer’s protocols. Paired-end sequencing of the libraries was performed on an Illumina MiSeq sequencer for 300 cycles, and the raw data were denoised using the DADA2 plugin (data2 denoise-paired option) in the QIIME2 software version 2019.7 (Bolyen et al., 2019). High-quality sequences were collected by eliminating chimeric sequences and taxonomically classified using machine learning techniques and the SILVA 16S rRNA gene database as a reference.
The large intestines of sacrificed mice were cut into small pieces and washed in ice-cold PBS (0.01 M, pH 7.4) to eliminate feces or contaminants. The pieces of the large intestine were weighed and then homogenized in PBS at a ratio of 1:9. Afterward, the homogenates were centrifuged for 5 min at 5,000×g, and the supernatant was used to analyze the carcinoembryonic antigen (CEA) concentration as the CRC-related parameter using a CEA kit (Elabscience, Houston, TX, USA). For the analysis, 0.1 mL of each sample or standard (0 4,000 pg/mL) prepared was added in a 96-well plate and incubated for 1.5 h at 37°C. Subsequently, 0.1 mL of biotinylated detection antibody working solution was added to each well and the plate was incubated for 1 h at 37°C. Following incuation, the plate was washed by adding a washing buffer to each well and then removing it; this step was repeated five times. Next, 0.1 mL of horse radish peroxidase conjugate working solution was added to each well and the plate was incubated for 30 min at 37°C. The washing step was repeated five times after incubation. Following this, 0.09 mL of substrate reagent was added to each well and the plate was incubated for 15 min at 37°C in a darkroom. Finally, 0.05 mL of stop solution was added to each well and the CEA concentration was determined at 450 nm using a Sunrise microplate reader (Tecan, Männedorf, Switzerland). All the steps were carried out according to the manufacturer’s manual.
Hematology parameters were assessed using a Beckman AU840 analyzer (AU840, Beckman Coulter, Brea, CA, USA). Whole blood samples were collected into CBC bottles containing K2EDTA (BD Microtainer 369?574, Becton Dickinson, Franklin Lakes, NJ, USA). Hematological parameters measured included neutrophils, monocytes, eosinophils, and basophils. One milliliter of blood was obtained from each animal to determine all study parameters. For the hematological analysis, 250 μL of whole blood samples were required.
All experiments were performed in triplicate, and the data were reported as the mean±SD. IBM SPSS Statistics for Windows (version 26, IBM, Armonk, NY, USA) was used for the statistical analysis. Significant differences were evaluated using Student’s t-test and one-way analysis of variance (ANOVA), and post-hoc analysis was performed using Tukey’s multiple comparison tests at the level of p<0.05.
Results and Discussion
The CEA concentrations in the large intestines of mice (adult and elderly) fed with a normal diet of processed meats and fermented foods were determined (Fig. 1). The results revealed that the CEA concentrations in treatment groups with processed meats and fermented foods were significantly lower (p<0.05) than those in control groups with a normal diet regardless of mouse age (adult and elderly). CEA concentration is a classic tumor marker for CRC (Jelski and Mroczko, 2020). Previous studies have identified that the CEA upper limit of normal levels differed depending on the institution and ranged from 3.0 to 5.0 ng/mL (Auclin et al., 2019). Moreover, a retrospective study showed that the optimal cutoff for preoperative CEA was 3.0 ng/mL (Kim et al., 2017). The CEA concentrations observed in all dietary groups in this study were overall safe levels (>620 pg/mL) and were not associated with a risk of CRC.

In adult mice fed with ham and fermented foods, the CEA concentration in groups fed with kimchi (AH2) and soybean paste (AH3) increased slightly, whereas that in the group fed with red pepper paste (AH4) was not significantly different from the group fed only ham (AH1; Fig. 1A). In adult mice fed with bacon, the group that was fed red pepper paste (AB4) exhibited a lower CEA concentration compared to the other groups (Fig. 1C). In elderly mice fed with ham and bacon, the CEA concentrations did not differ with the fermented foods fed (Figs. 1D and F). The CEA concentrations in groups fed with kimchi (ES2) and pepper paste (ES4) decreased significantly compared to those in other groups (Fig. 1E). Among the fermented foods, red pepper paste and kimchi decreased the CEA concentrations in elderly mice fed with sausage. A previous study reported that CEA concentration increased significantly in animals injected with 50 mg/kg of a carcinogenic agent, whereas CEA concentration decreased significantly in the animals fed with capsaicin and injected with a carcinogenic agent (El-kott and Bin-Meferij, 2018). Capsaicin, an essential component of red pepper, forms hydrophobic aggregates, resulting in a nonpolar phenolic structure that promotes its absorption in the gastrointestinal tract (Popescu et al., 2020). Capsaicin inhibited the development of CRC by activating the p53 gene (the suppressor gene located on chromosome 17) and promoted cell cycle control and apoptosis in tumors (McBride et al., 1986). The transient receptor potential cation channel subfamily V member 1 (TRPV1) is a deeply nonselective Ca2+ channel that can strongly inhibit CRC cell proliferation by creating an imbalance of calcium influx (Gueguinou et al., 2017). TRPV1 expression was significantly inhibited in CRC tissues. Furthermore, capsaicin, as a TRPV1 agonist, decreased the development of CRC and induced apoptosis by promoting the expression of the p53 gene (Hou et al., 2019). However, this study did not observe the CRC risk-reducing effects of dietary fermented foods such as kimchi and red pepper paste, which contain capsaicin.
Kimchi, the representative of traditional fermented foods in Korea, is fermented using probiotic lactic acid bacteria (Park et al., 2014). Kimchi promoted anticancer effects in human colon cancer cells by increasing apoptosis factors such as Bax, caspase-9, and caspase-3 and reducing pro-inflammatory factors (Kim et al., 2015). Lactic acid bacteria are important components of kimchi and are known to inhibit the activation of carcinogen-activating enzymes such as azoreductase, β-glucosidase, β-glucuronidase, 7-α-dehydrogenase, and nitroreductase and other related cancer-causing factors (Kwak et al., 2014; Lee et al., 2021b; Lee et al., 2022). One study reported that the CEA concentration is positively correlated with the levels of inflammatory cytokines such as interleukin (IL)-6, IL-8, and tumor necrosis factor-α (TNF-α; Li et al., 2018). CEA might affect the growth of CRC cells by binding to hNRNP M4, a receptor of CEA and a novel biomarker for CRC, and releasing inflammatory cytokines such as IL-6, TNF-α, IL-1 α, and IL-1β (Edmiston et al., 1997; Kammerer and von Kleist, 1996). A previous study found that kimchi and L. mesenteroides alleviate colitis by reducing the levels of inflammatory cytokines such as TNF-α, IL-6, and IL-1β and harmful intestinal bacteria (Moon et al., 2023). Thus, L. mesenteroides in kimchi and capcisine in red pepper paste that are representative components can help decrease CEA concentrations related to the development of CRC. This study found that the consumption of processed meats did not significantly affect the CEA concentration, a marker for the risk of CRC, and the CEA- lowering effects from consuming fermented foods such as kimchi, soybean paste, and red pepper paste differed depending on the dietary group regardless of mouse age. It is unclear whether the simultaneous consumption of processed meat products and fermented foods affects the risk of CRC; therefore, further research is necessary.
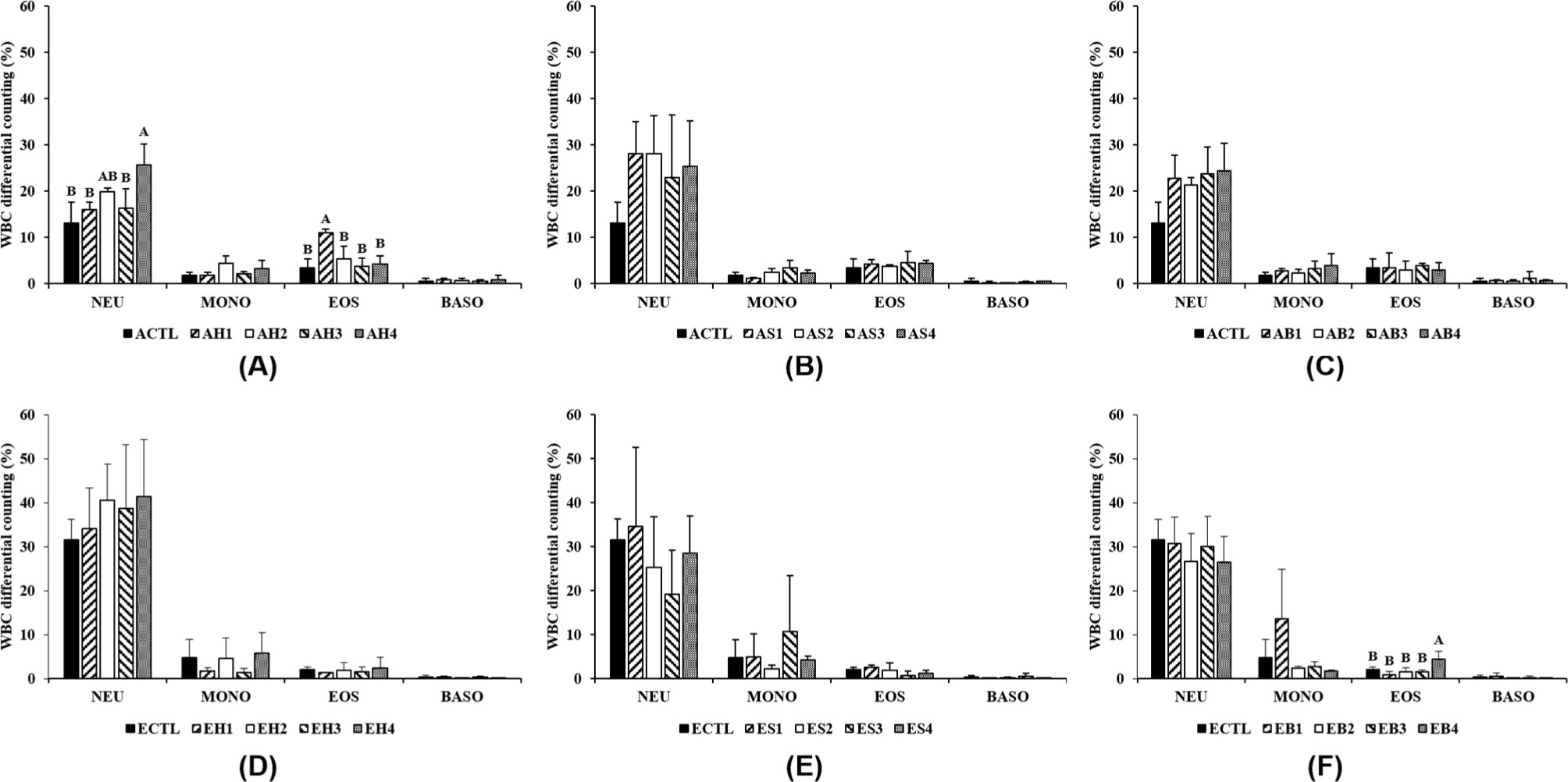
Hematological analysis was performed to determine the white blood cell (WBC) differential counting of whole blood and toxicology profiles of serum in mice fed with processed meat and fermented foods (Figs. 2 and 3). In adult mice fed with ham and fermented foods, the neutrophil content was significantly higher in the group fed with red pepper paste (AH4) compared to the control group, and the eosinophil content of the group fed with only ham (AH1) was higher than that of the other groups (Fig. 2A). Besides, the WBC differential counting content of the elderly groups fed with bacon and red pepper paste (EB4) was higher than that of the other groups (Fig. 2F). The neutrophil content of elderly mice tended to be higher than that of adult mice but were almost at a similar level. Furthermore, WBC differential counting was significantly different with no consistent trends among the groups regardless of age. Neutrophils, which are the richest leukocytes, are associated with defense in the innate immune system and they modulate inflammation and immune response (Rosales, 2018). The high neutrophil content of the group fed with ham and red pepper paste was similar to that reported in a previous study, which showed a neutrophil level of 34.70±0.14 in the control group of mice fed with processed meat (Jung et al., 2020). Therefore, the WBC differential counting was a limiting factor in determining the effectiveness of fermented foods because the intake of processed meats and fermented foods did not affect the overall WBC differential counting.
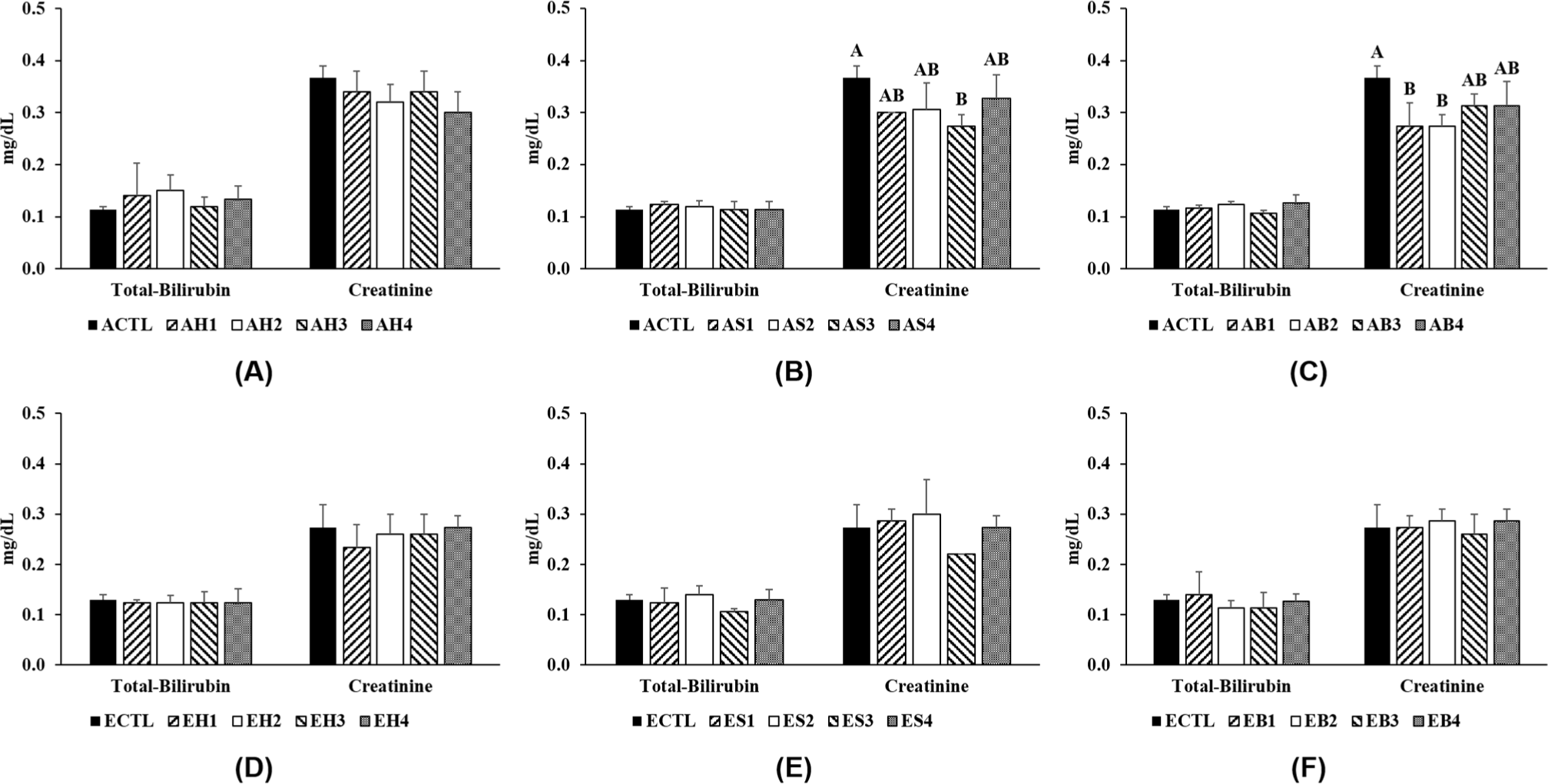
This study analyzed the total bilirubin and creatinine contents, that is, the serum toxicology profile of mice fed with processed meats and fermented foods (Fig. 3). Total bilirubin concentrations are used to determine liver injury, and high levels indicate the collapse of red blood cells in the liver (Ruiz et al., 2021). In this study, the total bilirubin concentration showed no significant difference between all treatments and was similar in adult and elderly mice. In adult mice, creatinine concentrations in the groups fed with sausage, bacon, and fermented foods were significantly lower than those in the control group. However, the creatinine content in elderly mice did not show a significant difference regardless of processed meat and fermented food consumption. Creatinine levels are often associated with CRC; therefore, a previous study suggested that creatinine levels can be used for CRC screening (Yang et al., 2021). Creatinine promotes cancer metastasis through MPS-1 by activating Smad2/3 (Zhang et al., 2021), and 1.2 mg/dL of serum creatinine in patients with primary epithelial ovarian cancer was associated with poor survival rates (Lafleur et al., 2018). The present study detected creatinine levels <0.4 mg/dL in mice serum, which is similar to those detected in a previous study (<0.47 mg/mL) and is considered normal (Jung et al., 2020). The present study also observed the impact of processed meat and fermented food intake on the organ morphology of mice (Fig. 4). No abnormalities were found overall and no significant difference in the visual observation of small intestine was found between treatments and between mice of different ages. Thus, the intake of processed meat is not toxic and does not alter the content of elements in blood regardless of mouse age.
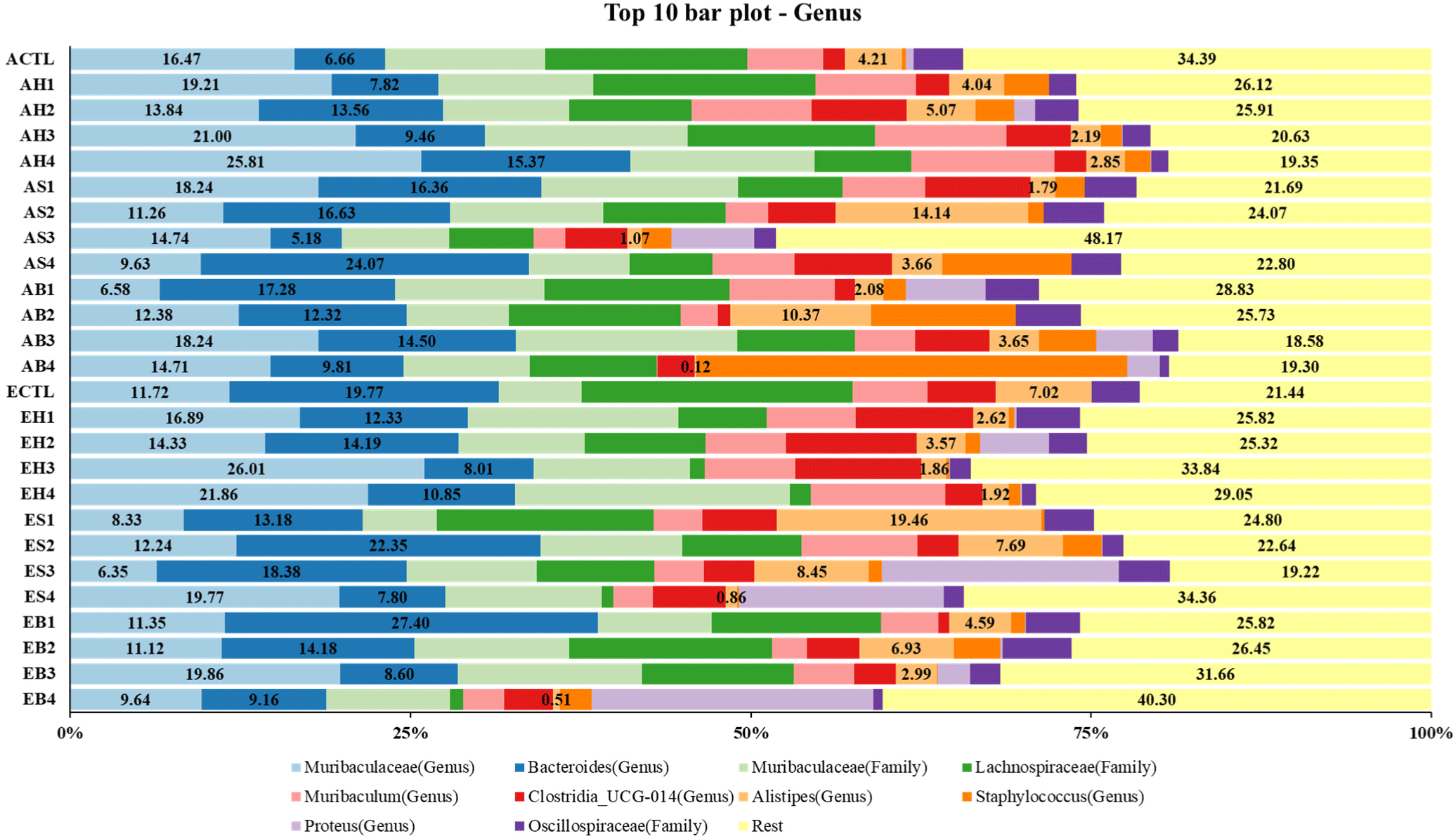
NGS was used to analyze the composition of gut microbiota of mice (adult and elderly) fed with processed meats and fermented foods (Fig. 5). The most prominent taxa at the genus level were Muribaculaceam Bacteroides, Muribaculaceae, Lachnospiraceae, Muribaculum, Clostridia UCG-014, and Alistipes.
Among the gut microbiota at the genus level, three bacteria related to disease and metabolism were selected. The proportions of the selected gut microbiota in feces of mice fed with ham and fermented foods are shown in Table 2. In adult mice, the proportions of Bacteroides in the groups fed with ham and fermented foods were higher than those in the control. The proportions of Alistipes in the groups fed with soybean paste (AH3) and red pepper paste (AH4) were lower than those in the control group and the group fed with only ham. Muribaculaceae was abundant in the groups fed with only ham (AH1), soybean paste (AH3), and red pepper paste (AH4). In elderly mice, the proportions of Bacteroides and Alistipes in the groups fed with ham and fermented foods were lower than those in the control group, while the proportion of Muribaculaceae was higher than that in the control group. The proportions of the gut microbiota in the feces of mice fed with sausage and fermented foods are shown in Table 3. In adult mice fed with fermented foods, the proportions of Bacteroides and Alistipes were low and the proportion of Muribaculaceae was high in the group fed with soybean paste (AS3), compared to those in the control group and the group fed with only sausage (AS1). Contrastingly, the group fed with red pepper paste (AS4) was rich in Bacteroids and low in Muribaculaceae, compared to the control group and the group fed with only sausage (AS1). In elderly mice, the proportions of Bacteroides and Alistipes in the groups fed with ham and fermented foods were lower than those in the control group, while the proportion of Muribaculaceae was higher than that in the control group. The difference in the proportions of gut microbiota between mice of different ages could not be confirmed because a consistent trend was not observed. The proportions of the gut microbiota in the feces of mice fed with bacon and fermented foods are shown in Table 4. In adult mice, the proportions of Bacteroides in the groups fed with bacon and fermented foods were higher than those in the control group. Moreover, the proportions of Alistipes were lower and those of Muribaculaceae were higher in the groups fed with soybean paste (AB3) and red pepper paste (AB4), compared to those in the control group and the group fed with only bacon. In elderly mice, the proportions of Bacteroides in the groups fed with fermented foods were lower than those in the control group and the group fed with only bacon. Moreover, the group fed with soybean paste (EB3) was low in Alistipes and rich in Muribaculaceae compared with the other groups. The age-wise difference in the proportions of gut microbiota in the feces of mice fed with ham and fermentation foods could not be confirmed because the results were not consistent.
ACTL: 100% ground normal diet; AH1: 50% ground normal diet+50% cooked ham; AH2: 50% ground normal diet+50% cooked ham with 15% kimchi per slice of ham; AH3: 50% ground normal diet+50% cooked ham with 1.5% soybean paste per slice of ham; AH4: 50% ground normal diet+50% cooked ham with 1.5% red pepper paste per slice of ham; ECTL: 100% ground normal diet; EH1: 50% ground normal diet+50% cooked ham; EH2: 50% ground normal diet+50% cooked ham with 15% kimchi per slice of ham; EH3: 50% ground normal diet+50% cooked ham with 1.5% soybean paste per slice of ham; EH4: 50% ground normal diet+50% cooked ham with 1.5% red pepper paste per slice of ham.
ACTL: 100% ground normal diet; AS1: 50% ground normal diet+50% cooked sausage; AS2: 50% ground normal diet+50% cooked sausage with 15% kimchi per sausage; AS3: 50% ground normal diet+50% cooked sausage with 1.5% soybean paste per sausage; AS4: 50% ground normal diet+50% cooked sausage with 1.5% red pepper paste per sausage; ECTL: 100% ground normal diet; ES1: 50% ground normal diet+50% cooked sausage; ES2: 50% ground normal diet+50% cooked sausage with 15% kimchi per sausage; ES3: 50% ground normal diet+50% cooked sausage with 1.5% soybean paste per sausage; ES4: 50% ground normal diet+50% cooked sausage with 1.5% red pepper paste per sausage.
ACTL: 100% ground normal diet; AB1: 50% ground normal diet+50% cooked bacon; AB2: 50% ground normal diet+50% cooked bacon with 15% kimchi per slice of bacon; AB3: 50% ground normal diet+50% cooked bacon with 1.5% soybean paste per slice of bacon; AB4: 50% ground normal diet+50% cooked bacon with 1.5% red pepper paste per slice of bacon; ECTL: 100% ground normal diet; EB1: 50% ground normal diet+50% cooked bacon; EB2: 50% ground normal diet+50% cooked bacon with 15% kimchi per slice of bacon; EB3: 50% ground normal diet+50% cooked bacon with 1.5% soybean paste per slice of bacon; EB4: 50% ground normal diet+50% cooked bacon with 1.5% red pepper paste per slice of bacon.
Alistipes is positively correlated with colonic tumor burden and is closely related to dysbiosis and disease (Baxter et al., 2014; Parker et al., 2020). A study by Yang et al. (2022) revealed that Alistipes was significantly higher in conventional CRC mouse models fed with a high-fat diet than in those fed with a control diet. Additionally, Alistipes finegoldii stimulated CRC growth via the IL-6/STAT 3 pathway (Moschen et al., 2016). Bacteroides spp. promote colitis in a host-genotype-specific fashion in inflammatory bowel disease mice (Bloom et al., 2011). Moreover, toxins from Bacteroides fragilis can cause chronic intestinal inflammation and epithelial injury, ultimately resulting in CRC (Cheng et al., 2020). Bacteroides fragilis toxin is implicated in the production of cyclooxygenase-2 and STAT 3, which induce inflammation and CRC. Muribaculaceae was significantly and negatively correlated with inflammation-associated parameters (Shang et al., 2021), and the abundance of Muribaculaceae was deeply related to the concentrations of propionate (Smith et al., 2019), which ameliorates dextran sodium sulfate-induced colitis via the STAT 3 signaling pathway (Tong et al., 2016). The present study found that the intake of processed meats did not affect the growth of gut microbiota such as Bacteroides and Alistipes, which affect the occurrence of colitis and CRC regardless of mouse age. In addition, as mentioned above, the main components in fermented foods inhibit the STAT 3 pathway and inflammatory cytokines that affect the growth of CRC cells. Therefore, the main components in fermented foods affect the proportions of gut microbiota both negatively or positively. Even if the difference in proportions of the gut microbiota between mice of different ages could not be confirmed because a consistent pattern was not observed, the intake of ham and fermented foods influences gut health without an overall negative effect. Based on the results of gut microbiota analyses related to colitis or gut health, the consumption of processed meats is generally less related to colitis, and the consumption of soybean paste along with meat products reduces the risk of colitis and improves gut health. However, further studies are needed to confirm the consistency of these results.
Several previous studies have reported that the dietary intake of large amounts of processed meat increases the risk of CRC, while the intake of fermented foods reduces the risk of CRC. However, in this study using ICR mice, the consumption of processed meats did not promote the risk of CRC. Moreover, no clear evidence related to the reduction of CRC risk due to the consumption of fermented foods was found. In addition, no age-related differences in experimental results were found. Over the past five years, the results obtained in all eight experiments measuring the changes in gut microbiota and the risk of CRC from feeding excessive amounts of processed meats and high-temperature cooked pork to mice were inconsistent. These inconsistencies probably occurred because CRC from consumption of processed meats may be caused by eating much more processed meat than we know or for a much longer period (although our experiments also fed 50% of total dietary intake as processed meat for more than three months). Another possible reason may be the differences in experimental methods and physiological characteristics between animals and humans. Moreover, many more factors (living habits, stress, drinking, smoking, obesity, or genetics) than we know may act synergistically to promote the development of CRC. Although previous studies have shown that the intake of fermented foods reduces the risk of CRC, it is not clear why such a reduction in CRC risk was not observed in the present study. The effects of CRC prevention probably vary depending on the type of fermented foods consumed. In addition, in this study, the CRC risk due to processed meat consumption was not significant, which may be why the CRC risk-reducing effects from fermented food consumption were not significant. Moreover, the intake of processed meat may not always be closely related to the development of CRC.
Conclusion
In this study, processed meat intake was not associated with the risk of CRC, and some fermented foods, such as kimchi, soybean paste, and red pepper paste tended to decrease CEA in mice regardless of their age. The hematological (WBC differential counting and total bilirubin and creatinine contents) and organ morphological analyses showed that the effects of fermented food with intake of dietary processed meat consumption were inconsistent. Thus, these factors are not sufficient to confirm whether fermented foods directly lower the risk of CRC. However, the hematological and organ morphological analyses revealed that processed meat products are non-toxic to human health. In addition, the intake of processed meats with fermented foods increased the abundance of Alistipes, Bacteroides, and Muribaculaceae. The fermented foods influenced gut health by decreasing the abundance of Alistipes and Bacteroides and increasing the abundance of Muribaculaceae. Furthermore, these results did not differ significantly between adult and elderly mice. In this study, the intake of processed meat products was not directly related to CRC risk; for this reason, the effect of fermented foods on reducing the CRC risk associated with the consumption of processed meat products was clearly not found. In addition, since the experimental results did not show a consistent trend, further studies should be conducted to clarify the results.













