Introduction
Duck meat is a preferred protein source for consumers because of its excellent nutritional value and flavor (Biswas et al., 2019; Cha et al., 2025; Ofori and Hsieh, 2014). With growth in the duck meat industry, the production of byproducts during the slaughter process has also increased (Shim et al., 2018). Maximizing the utilization of these duck by-products can reduce industrial waste, efficiently utilizing the available proteins to minimize environmental pollution (Jayathilakan et al., 2012; Wan et al., 2002). Blood is one of the first by-products produced during the slaughter of livestock, consisting of approximately 17%–18% protein and 75%–82% water, with hemoglobin accounting for approximately 70% of the total blood protein found in red blood cells (Leoci, 2014; Tarté, 2011). Duck blood contains protein and iron, making it an excellent source of protein for meeting the amino acid requirements of adults (Silva and Silvestre, 2003; World Health Organization [WHO] et al., 2007). Therefore, duck blood is a valuable source of protein and iron. Additionally, blood plays a functional role in sausage production by binding fats, pork, and various other ingredients, thereby maintaining the sausage shape (Choi et al., 2009; Gräsbeck et al., 1982). Currently, blood is widely utilized as a protein source in various countries across Europe and Asia, and is used commercially in products, such as blood sausages, black pudding, blood curd, and blood tofu (Lui, 2002). Blood sausages are traditionally favored as meat products and consumed in various countries. The ingredients used may vary depending on the production region (Diez et al., 2009; Stiebing, 1990).
Traditional Korean fermented foods are acidic foods that break large nutrient molecules into smaller ones, promoting protein digestibility and enhancing flavor and texture. Microorganisms involved in fermentation are beneficial and edible, producing hydrolytic enzymes and other enzymes that break-down carbohydrates, lipids, and proteins (Park, 2012). Fermentation is a biochemical reaction that converts complex organic substances into relatively simpler ones, thereby enhancing the nutritional value of food and increasing the absorption rate in the body. This process plays an important role in enhancing the functional and nutritional characteristics of food (Hwang et al., 2017). Proteins or peptides with different molecular weights exhibit different physicochemical properties (Sato et al., 1995). Consequently, factors that influence protein interactions can also alter gel characteristics. Traditional fermented foods contain proteolytic enzymes, which break down proteins into low-molecular-weight amino acids (Ann, 2011). The application of fermented foods to blood sausage could potentially interfere with the gelation of myofibrillar and blood proteins during heating, resulting in lower gel strength. Lower gel strength may cause the product to degradation more quickly during digestion, leading to higher protein digestibility. Therefore, this study aimed to evaluate the effects of replacing salt with traditional fermented foods on the protein digestibility and physicochemical properties of blood sausages, with the goal of enhancing the application of fermented foods in meat products.
Materials and Methods
Fresh pork ham, back fat, skin, and duck blood were purchased from a local market in Jeonju, South Korea. Blood sausages containing traditional fermented food materials were prepared by modifying the manufacturing methods described by Choi et al. (2009). The control blood sausage was prepared by mixing 55% pork ham, 20% blood, 15% back fat, 5% skin, 5% ice, and 1.5% salt. Five types of traditional fermented materials were used as salt substitutes: Doenjang (T1), Gochujang (T2), Cheonggukjang (T3), Magjang (T4), and Kimchi (T5). The salinity of each traditional fermented food material (T1: 7.7%, T2: 9.5%, T3: 3.3%, T4: 6.8%, T5: 1.9%) was measured, and materials corresponding to a salt concentration of 1.5% were added. Pork ham, back fat, and skin were chopped using a 6 mm plate. Salt and traditional fermented materials (1.5% salinity and 0.01% nitrite) were added to chopped pork ham, back fat, and skin. The mixture was then tumbled for 1 h and subsequently heated for 30 min at 80°C. The heated materials, along with blood, ice, and sub-materials (0.8% sugar, 4.0% onion powder, 3.0% garlic powder, 0.7% ginger powder, 0.3% black pepper, 9.7% monosodium glutamate, 0.7% carrageenan, and 2.0% isolated soy protein) were combined in a silent cutter, mixed for 5 min, and stuffed into casings. Subsequently, the sausages were heated for 30 min at 80°C and then cooled to 4°C.
The compositional properties of blood sausages containing traditional fermented food materials were analyzed using the methods outlined by the AOAC (2000). The moisture content was determined using a drying oven (AOAC 950.46B), protein content was measured using the Kjeldahl method (AOAC 981.10), and fat content was assessed using the Soxhlet method (AOAC 960.69). The ash content (AOAC 920.153) was quantified in a muffle furnace.
To estimate the amino acid composition of the heat-induced mixed protein gel, a cooling gel was prepared and analyzed using the method described by Fountoulakis and Lahm (1998). Briefly, the amino acid composition of the hydrolyzed protein, processed under nitrogen using 6 M HCl, was analyzed using an L-8800 amino acid analyzer (Hitachi, Tokyo, Japan). The hydrolysate was filtered using a 0.20 μm membrane filter and an ion-exchange resin column (4.6 mm inside diameter×60 mm). The nutritional value, particularly the essential amino acid index, of the gel was calculated according to the FAO et al. (1985) guidelines.
The pH was measured using a pH meter (Accumet Model AB15+, Thermo Fisher Scientific, Hampton, NH, USA). For pH measurement, 5 g of the sample was homogenized with 20 mL of distilled water at 8,000 rpm for 3 min.
The color of the blood sausages was measured using a colorimeter (CR-210, Minolta, Osaka, Japan). The CIE L*, CIE a*, and CIE b* values were measured thrice using illuminant C. The colorimeter was calibrated using a white plate (CIE L*=+97.83, CIE a*=–0.43, CIE b*=+1.98).
In vitro digestion of blood sausages was performed as described by Lee et al. (2020). Blood sausage sample (3 g) was mixed with distilled water (9 mL) and heated in a water bath at 80°C for 30 min. After cooling at room temperature (20°C), the mixture was then homogenized at 13,000 rpm for 1 min. The homogenate (4 mL) was treated with 10 mL of gastric digestive juice (pepsin=182 units/mg protein and gastric lipase=21 units/mg protein dissolved in 0.15 M NaCl, pH=1.8, adjusted with 0.1 M HCl) and digested at 37°C for 2 h in a shaking water bath. Duodenal (10 mL) and bile fluids (5 mL) were added to the material from the gastric phase, and digestion was performed under identical conditions. The compositions of the duodenal and bile fluids were as follows: duodenal fluid (trypsin=34.5 units/mg protein, chymotrypsin=0.4 units/mg protein, and pancreatic lipase=2,000 units/mg protein dissolved in distilled water, pH=7.5, adjusted with 1 M NaOH) and bile fluid (4 mM bile extract dissolved in distilled water, pH=7.5, adjusted with 1 M NaOH). For the control, the same amounts of distilled water and digestion solution were used instead of the sample during digestion. The digesta after digestion was stored at –70°C and stored until further analysis and the protein content was determined by the Kjeldahl method (AOAC, 2000). The digesta samples were fractionated based on size through filtration using centrifugal filters (Amicon Ultra-15, Millipore, Billerica, MA, USA) with molecular weight cut-offs of 3 kDa. The in vitro protein digestibility of the digesta after gastrointestinal digestion was calculated using the following Eq. (1):
The textural properties were analyzed using a texture analyzer (TA-XT2i, Stable Micro Systems, Surrey, UK) under the following conditions: maximum load=2 kg, pre-test speed=2.0 mm/s, post-test speed=5.0 mm/s, force=10×g, distance=8.0 mm, and head speed=2.0 mm/s.
The apparent viscosities of the samples were measured using a Brookfield viscometer (DV3T, Brookfield, MA, USA). The emulsion temperature was maintained at 20°C via distilled water circulation using a refrigerated circulator bath (VB-07, U1TECH, Suwon, Korea), and the viscosity change was measured at 10 rpm for 30 s using an SC4-29 standard spindle.
The dynamic viscosities of blood sausages containing traditional fermented food materials were measured using a Physica MCR 102 rheometer (Anton Paar, Graz, Austria). A flow curve test was conducted using a parallel plate with a diameter of 25 mm and a gap of 1 mm. The angular frequency ranged between 0.1–100 rad/s at a strain of 1%, with the temperature maintained at 25°C. Storage modulus (G’), loss modulus (G”), and loss factor (tan δ=G”/G’) were recorded using RheoCompass v.1.19 software.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed to determine the distribution of proteins based on their molecular weight. The samples were prepared at identical concentrations (1 mg/mL). The sample buffer was mixed with 20 μg of protein sample (Bio-Rad Laboratories, Hercules, CA, USA) at a ratio of 3:1 (protein to sample buffer). The mixture was heated at 100°C for 5 min, and subsequently cooled to 25°C for 5 min. Protein bands were separated using 12% Mini-PROTEIN® TGXTM Precast Protein Gels (Bio-Rad Laboratories), and Precision Plus ProteinTM Dual Color Standards (Bio-Rad Laboratories) were used as standard proteins. After electrophoresis, the gels were stained with Coomassie Brilliant Blue R-250 (Bio-Rad Laboratories).
Results and Discussion
The proximate compositions of blood sausages containing traditional fermented food materials are shown in Table 1. The moisture content was the highest in T3 (Cheonggukjang) at 61.50% (p<0.05), with no significant differences observed in other treatments. Arrese et al. (1991) reported soybean protein exhibit a high water-holding capacity. Meanwhile the protein content was higher in T3 compared to the other treatments. Therefore, it is thought that the high moisture content in T3 was due to its higher protein content and water-holding capacity. The protein content tended to be higher in sausages prepared with traditional fermented foods than in the control. Lee (1973) reported that soybean, soybean paste, and red pepper paste contained 13.31%–38.06% protein, and Kim et al. (1998) reported the presence of protein in Kimchi ingredients. Additionally, Lee and Lyu (2008) found that the protein content increased with the addition of Cheonggukjang powder to patties. The fat content was the lowest in T4 (Magjang) at 11.93%, with no significant differences observed in other treatments (12.67%–13.12%). Sausages with added blood have a fat content of approximately 20% (Pereira, 2000; Santos, 2007; Santos et al., 2003).
The amino acid compositions of blood sausages made from traditional Korean fermented foods are presented in Table 2. There was no significant difference in the total amino acid content of blood sausages containing traditional fermented foods, but there was a difference in the amino acid composition (p<0.05). Cheonggukjang has been reported to generate amino acids such as glutamic acid (Glu) and aspartic acid (Asp) through the breakdown of soybean protein by Bacillus spp. during the fermentation process (Rozan et al., 2000). Glu and Asp values were higher in T3 than in the control (p<0.05), indicating the influence of Cheonggukjang addition. Similarly, Doenjang and Gochujang have been reported to have increased levels of Glu and Asp during fermentation, which is considered to have influenced the blood sausage as well (Choi et al., 2000; Lee et al., 1980; Lee et al., 2012). Kimchi also has relatively high levels of Glu in its amino acid composition (Woo et al., 2006). Therefore, adding fermented foods could influence the amino acid composition of blood sausage due to the amino acids contained in the fermented foods.
The pH and color characteristics of the blood sausages containing traditional Korean fermented foods are shown in Table 3. The pH showed no significant difference between the control and the blood sausages with traditional fermented foods. Gašperlin et al. (2014) reported that the pH of blood sausages (Krvavica) in various regions ranged between 6.60–6.94, which is slightly higher than the values observed in the present study. CIE L* was the highest in T3 (Cheonggukjang) and lowest in the control (p<0.05). CIE a* was prominent in T2 (Gochujang) and T3, with no significant differences observed in other treatments. CIE b* was the highest in T4 (Magjang; p<0.05), with no significant differences observed in other treatments. The addition of blood to meat products results in a dark color because of the presence of hemoglobin in the blood. Heat treatment of blood can affect the color because of the destruction of heme, as reported by Oellingrath and Slinde (1985). The differences observed in the treatment groups in the present study can be attributed to the unique color of traditional fermented foods.
Table 3 shows the protein digestibility of blood sausages containing traditional Korean fermented foods. The proteolysis of free amino acids and small peptides indicates their absorption into the body, highlighting the importance of assessing the protein digestibility of meat products to determine their health profile (Hsu et al., 1977). The protein digestibility of sausages with traditional Korean fermented foods showed the highest in T3 (Cheonggukjang). Protein digestibility increases with smaller particle size or higher low-molecular-weight protein content (Sicard et al., 2018). SDS-PAGE suggests that T3 showed higher digestibility because it contained more low-molecular-weight proteins. Additionally, T3 exhibited lower gel hardness and elasticity. During protein gelation, a three-dimensional network of polypeptides that can enclose water is formed. This process involves the stages of protein denaturation and aggregation. Protein gelation is affected by various factors such as protein concentration, temperature, pH, salt, or other additives (Opazo-Navarrete et al., 2018). Changes in the form or structure of proteins affect the digestibility of meat proteins (Kaur et al., 2014). These results suggest that fermented foods interfere with the gelation of myofibrillar and blood proteins during the heating process, leading to rapid dissociation of the product during digestion, thus increasing protein digestibility. Meanwhile, Gan et al. (2009) reported that an improvement in gel strength leads to a decrease in digestibility, which is consistent with the results of this study.
Table 4 shows the texture analysis of blood sausages containing traditional fermented foods. The hardness of the control was the highest at 2.21 kg, whereas T3 exhibited the lowest among the blood sausages with traditional Korean fermented foods (p<0.05). Springiness and cohesiveness did not differ significantly between the control and any of the experimental samples, with values between 0.40–0.47 and 0.26–0.28, respectively. The gumminess and chewiness of the control group were the highest, whereas those of the T3 group were the lowest (p<0.05). Although the texture index of blood sausages containing traditional Korean fermented foods decreased, no significant difference was observed in cohesiveness, indicating that the structural tightness between the components of the sausage was not significantly affected. Koak et al. (2011) reported a reduction in breaking strength during a study on the physical properties of sausages with the addition of a tenderizer (over-matured fruits). Furthermore, several studies have shown tenderizing effects on physical properties, such as reduction in hardness and shear force, by applying proteolytic enzymes to meat, which aligns with the results of the present study (Choi et al., 1992; Han and Chin, 2004; Yang, 2006).
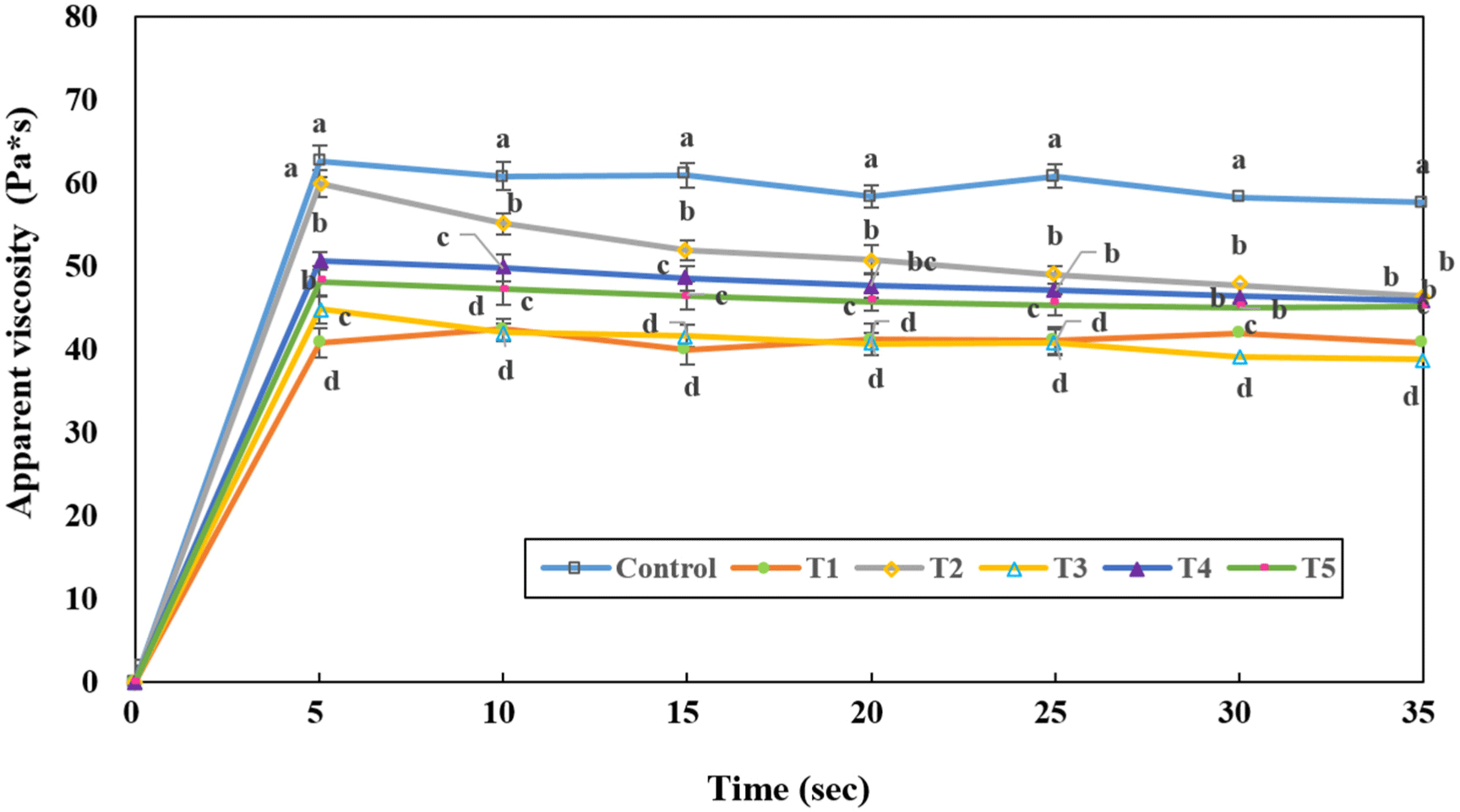
The apparent viscosities of the blood sausages prepared using traditional fermented foods are presented in Fig. 1. Blood sausages prepared with traditional fermented foods showed lower values than the control; T3 (Cheonggukjang) and T1 (Doenjang) tended to have the lowest values. Degradation of meat proteins increases the viscosity of meat emulsions because of an increase in the number of degraded protein molecules (Richardson, 1977). However, in the present study, the lower apparent viscosity of blood sausages prepared with traditional Korean fermented foods compared to the control was attributed to the suppression of emulsification caused by the application of these fermented foods.
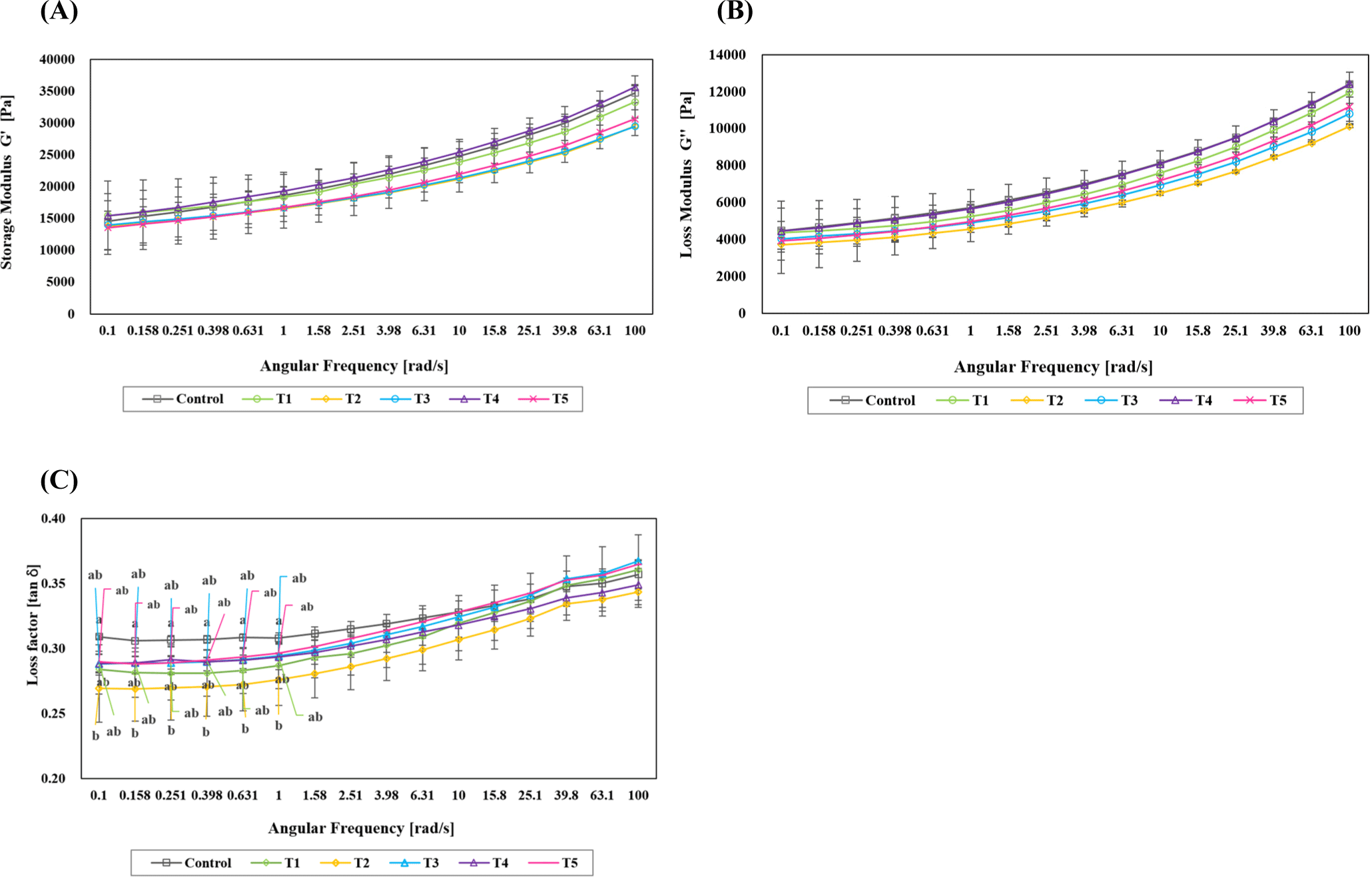
The structural characteristics of blood sausages prepared from traditional Korean fermented foods are shown in Fig. 2. Storage modulus (G′), loss modulus (G’’), and tan δ are indicators used to explain the physical properties of viscoelastic materials. The storage modulus is a value representing the elastic response of a material, indicating how elastically the material reacts to external deformation forces. The higher the storage modulus, the stronger the material’s resistance to external deformation forces. The loss modulus represents the viscous response of a material and indicates how viscously the material flows in response to external strain. The higher the loss modulus, the more flexibly the material flows under external strain, consuming more energy. Tan δ represents the relative relationship between the viscosity and elasticity of a material. It is defined as the ratio of the loss modulus to the storage modulus (Lee and Kim, 2017). Both storage modulus (G’) and loss modulus (G’’) exhibited an increasing trend with increasing angular frequencies, indicating a similar trend across all treatment groups. Over the entire frequency range, all the samples formed stable gel systems without any crossover points. Storage modulus (G’), loss modulus (G’’), and tan δ were the lowest in T2 (Gochujang), followed by T3, T5, and T1, with T4 and the control showing similar values. The G’ decreased for all treatments except T4 compared to the control, indicating that the elasticity of the blood sausage gels decreased in the treatments added with fermented products. These results suggest that the addition of fermented foods inhibited emulsification and gel formation. Tan δ remained below 1 for all treatments, indicating predominantly elastic behavior rather than viscous behavior (Liu et al., 2019).
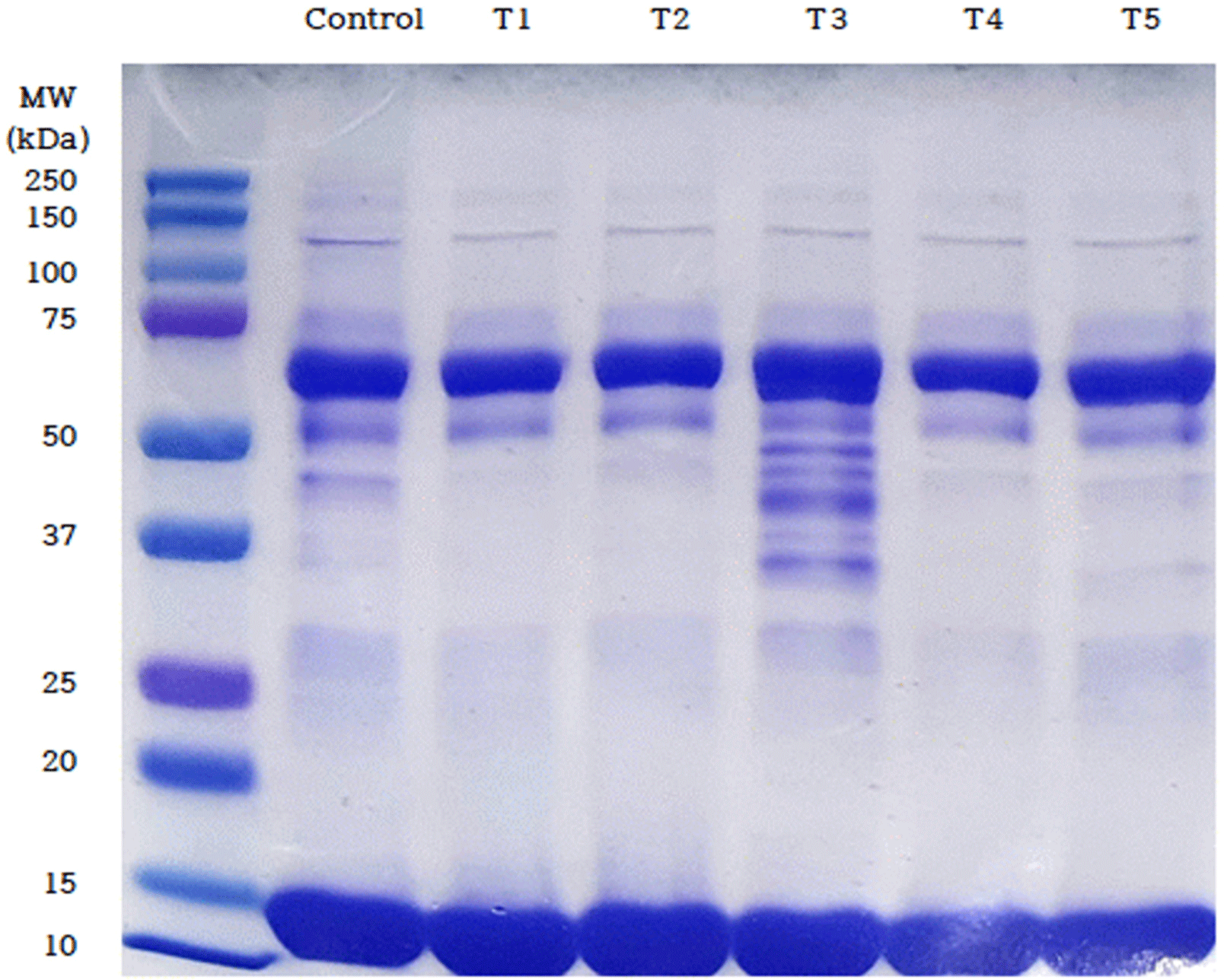
The SDS-PAGE results for blood sausages prepared with traditional Korean fermented foods are shown in Fig. 3. The protein degradation patterns of both the control and the groups treated with traditional fermented foods showed a similar trend. Low-molecular-weight bands at approximately 60 kDa and 10–15 kDa were observed for all samples. However, unlike the other treatment groups, T3 exhibited bands in the 30–60 kDa range. The protein content of Cheonggukjang is the highest among the added fermented foods (18.8%, Table 5). The Cheonggukjang has been reported to have high proteolytic enzyme activity (Kim et al., 2004). Furthermore, higher crude protein content has been associated with increased protease activity (Baek et al., 2014). These findings suggest that the interaction between the proteins in the blood sausage and the proteolytic enzymes in Cheonggukjang resulted in the presence of fractions in the 30–60 kDa range, which were not observed in other treatments. While protein-degrading enzymes derived from fruits exhibit high activity around 50°C, the enzymes from traditional fermentation agents have relatively high activity at higher temperatures, a finding corroborated by the results of the present study (Yoo et al., 2013).
Conclusion
In this study, we evaluated the effects of incorporating traditional Korean fermented foods to blood sausages on their protein digestibility and physicochemical properties. Blood sausages made with traditional fermented foods exhibited improved digestibility and decreased gel hardness and elasticity. Among them, the Cheonggukjang treatment exhibited more extensive degradation into small molecular peptides and the highest protein digestibility. This effect is likely due to the inhibition of emulsification and gel formation by the fermented foods. Therefore, the application of Cheonggukjang to blood sausage is expected to contribute to the utilization of blood by-products and to have a positive effect on digestion and absorption by degrading them into low molecular amino acids.













