Introduction
Hypertension is a primary risk factor for cardiovascular diseases (CVDs), including stroke, heart attack, heart failure, and other complications related to structural damage to the cardiovascular system. In 2021, the World Health Organization (WHO) reported that CVDs are the leading cause of death globally. People who died from CVDs in 2019 were estimated at 17.9 million, representing 31% of all deaths worldwide. Heart attacks and strokes are the main causes of these deaths (85%) (WHO, 2021). Human blood pressure is regulated by a system called the “renin angiotensin aldosterone system”, in which angiotensin-I-converting enzyme inhibition (ACEI) plays an important role. ACE could catalyze the conversion of the decapeptide angiotensin I to the potent vasoconstrictor angiotensin II. Furthermore, this enzyme hydrolyzes bradykinin and stimulates the release of aldosterone which causes vasoconstriction and fluid retention which increases blood pressure (Rai et al., 2017). Therefore, the treatment of clinical hypertension could be done by controlling ACE activity. ACE inhibitors such as captopril, enalapril, alacepril, lisinopril and ramipril are widely used in the clinical treatment of hypertension. However, the use of these synthetic drugs in some cases causes side effects such as coughing, increased blood calcium levels, decreased kidney function, angioedema, and skin rashes (Zeng et al., 2013). Several researchers through in vivo studies on rats with spontaneous hypertension (SHR) and humans with hypertension showed that ACE inhibitors without side effects could be obtained from food protein (Bravo et al., 2019; Chen et al., 2014; Seppo et al., 2003).
Food protein from milk and dairy products such as fermented milk is a source of ACEI peptides (Begunova et al., 2021; Wu et al., 2019). Among them, there have been reported from fermented goat milk. The presence of ACE inhibitors in fermented milk is associated with the presence of lactic acid bacteria (LAB). LAB are the dominant group of bacteria involved in fermenting milk such as yogurt and kefir. Kefir is a fermented goat milk that has been consumed for hundreds of years and is believed not only as a source of antihypertensive peptides but also as a source of antioxidants and immunological agents (Ibrahim et al., 2017; Parmar et al., 2020).
During fermentation, milk protein could be hydrolyzed by LAB into peptides and amino acids. The abundance and characteristics of peptides released from milk proteins by LAB are strain-dependent (Wang et al., 2015). Among these peptides, the presence of bioactive peptides could be identified (Li et al., 2017). Bioactive peptides differ in size and sequence. Bioactive peptides that have functional properties as ACEI activity have the characteristics that their molecular weight (MW) is generally <3 kDa and the presence of the amino acids proline and phenylalanine in the sequence (Gonzalez-Gonzalez et al., 2013; Wu et al., 2006).
The ability of LAB to release bioactive peptides in fermented milk (Ayyash et al., 2020; Kim et al., 2017), and the status of LAB as “generally recognized as safe” (GRAS) for application in food, have increased the utilization of certain strains of LAB for production of fermented milk with certain functional properties. The purpose of this study was to investigate ACEI activity in goat milk fermented using LAB from fermented foods and breast milk. The potential of ACEI peptides was identified in the <3 kDa fraction of fermented goat milk with the highest ACEI activity. The proteolytic specificity of the LAB used was also evaluated. The ten strains used were selected because they effectively released ACEI peptides in fermented cow milk in our previous study (Rubak et al., 2020).
Materials and Methods
The ten LAB isolates from fermented foods and breast milk used in this study were obtained from culture collections of the Laboratory of Food Microbiology, Southeast Asian Food and Agricultural Science and Technology (SEAFAST) Center, IPB University (Bogor Agricultural University). The isolates were refreshed in de Man, Rogosa, and Sharpe (MRS) broth and incubated at 37°C for 24 h, then adapted in fresh skimmed milk for 2 rounds (24 h, 37°C) before being used as a starter culture in the experiment.
Goat skimmed milk 11% (w/v) was pasteurized at 95°C for 10 min. After cooling (45°C), LAB starter culture (2%) was inoculated followed by incubation at 37°C until pH 4.6 (700 Eutech) was reached. The fermentation process was stopped by heating (75°C for 1 min) followed by centrifugation (Hettich, Zentrifugen, Mikro 22R) at 6,000×g for 10 min, 4°C. The supernatant was collected for analysis of peptide content and ACEI activity (Cushman and Cheung, 1971). Viable cell counts of LAB and titratable acidity were also analyzed from unheated samples.
Hippuryl-L-Histidyl-L-Leucine (HHL, Sigma-Aldrich, St. Louis, MO, USA) was used as an enzyme-substrate. A total of 50 μL of the substrate (50 mM HHL in 0.1 M sodium borate buffer containing 0.3 M NaCl at pH 8.3) was added into a 50 μL sample and incubated at 37°C for 5 min. To initiate the reaction, 50 μL of 0.1 U/mL ACE (Rabbit lung, Sigma-Aldrich) solution was added, and the mixture was incubated at 37°C for 5 min. The reaction was stopped by adding 250 μL 1 M HCl. The resulted hippuric acid (HA) was extracted with 1.5 mL ethyl acetate and centrifuged at 2,000×g for 5 min. An aliquot (0.8 mL) of the ethyl acetate layer was transferred to a clean tube and evaporated at 85°C for 60 min. Distilled water (4 mL) was then added to dissolve the HA in the tube, and the amount of HA formed was measured by measuring the optical density at 228 nm (UV-2800, Hitachi, Tokyo, Japan). The extent of inhibition was calculated as 100% [(B – A) / B] where A is the optical density in the presence of ACE and ACEI components, and B is the optical density without the ACEI component.
The IC50 of the sample having the highest ACEI activity was calculated from the linear regression equation by plotting the ACE inhibition (%) versus the inhibitory concentration for each sample dilution. The percentage of ACEI activity was divided by the peptide concentration to obtain the IER value.
The supernatant of fermented goat milk (4 mL) was pipetted into ultrafiltration centrifuge tubes molecular weight (MW) cut-off of 3 kDa; Merck, 4 mL, IRL , then centrifuged at 4,000×g for 30 min, 4°C. The fractions (<3 kDa and >3 kDa) were collected and the volume was adjusted to 4 mL by addition of water. Fractions were analyzed for ACEI activity.
Peptides in <3 kDa fraction were analyzed by using LC Ultimate 3000 series system Tandem Q Exactive Plus Orbitrap HRMS (Thermo Scientific, Dreieich, Germany). The samples (5 μL) were injected into the nano liquid chromatography mass spectrometry (LC-MS/MS) system. The samples were trapped on a trap column (164649, 30 μm×5 mm; Thermo Scientific) and washed for 6 min with a gradient of 98% solvent A [water/acetonitrile (98:2, v/v), 0.1% formic acid] and 2% solvent B [water/acetonitrile (2:98, v/v), 0.1% formic acid] at a flow rate of 5 μL/min. The eluted peptides were loaded and separated on a capillary column (PepMap RSLC-C18, 75-μm×150 mm, 3.5 μm particle size, 100 pore size, Thermo Scientific, ES800) at a flow rate of 300 nL/min with a gradient at 2% to 35% solvent B over 30 min, then from 35% to 90% over ten min, followed by 90% solvent B for 5 min, and finally 5% solvent B for 15 min. Electrospray was performed at an ion spray voltage of 3500 eV. Automatically, the peptides were analyzed using Proteomic Discoverer 2.2 software. The range of m/z values was 200–2,000.
Identification of ACEI peptides was carried out through a literature search. The target of investigation is a peptide that provides 100% similarity to the ACEI peptide that has been previously reported by the researchers.
Result and Discussion
Milk has been known as a suitable growth medium for LAB. The population of the ten LAB in fermented goat milk reached 9 Log CFU/mL. Viable cell counts of the LAB ranged from 9.18±0.46 to 9.79±0.39 Log CFU/mL. When the pH reached 4.6, there was no difference in population (Table 1), which is in accordance with previous results obtained by Elkhtab et al. (2017) and from fermented cow milk using similar cultures (Rubak et al., 2020). However, the fermentation time to reach pH 4.6 was different between isolates that ranged from 18 to 48 h with titratable acidity ranging from 0.77±0.06 to 0.94±0.04%. A short fermentation time (18 h) was observed in the fermentation by Lactobacillus rhamnosus R2, while the longest fermentation time (48 h) occurred in the fermentation by Lactobacillus fermentum S206, Lactobacillus delbrueckii BD7, and Lactococcus lactis ssp. lactis BD17. In our research the fermentation was ended at pH 4.6 to obtain high production of peptides. The release of a bioactive peptide from the protein matrix by culture could decrease when the pH value falls below 4.5 (Gonzalez-Gonzalez et al., 2013). Increase in coagulation could inhibit bacterial cell diffusion to protein tissue, thus inhibiting access of Cell Envelope Proteinase (CEP) to milk protein for hydrolysis. Further acidification could be avoided by stopping fermentation when it reaches pH 4.6, or the pH must be controlled by adding alkaline solutions such as sodium hydroxide (Chen et al., 2015).
ACEI activity was detected in all supernatants of goat milk fermented in the range of 20.44±2.33 to 60.79±8.78% (Table 2). The highest percentage of ACEI activity (>50%) was obtained in goat milk fermented by Lb. delbrueckii BD7 and Lc. lactis ssp. lactis BD17, but it was not significantly different (>0.05) from that of Lb. kefiri YK4 and Lb. kefiri JK17. It has been reported that goat milk could be used as a potential precursor for the production of ACE inhibitors through the fermentation process (Izquierdo-González et al., 2019). Starter cultures of LAB, growth conditions, and substrate are factors that influence ACEI production in fermented milk (Li et al., 2017; Shu et al., 2015; Wang et al., 2015). Lactobacillus species are known to produce high ACEI activity (>50%) in fermented milk (Hati et al., 2018; Wu et al., 2019). The variation of ACEI activity between LAB in milk fermented is related to its proteolytic activity. The proteolytic activity of LAB is determined by the specificity of its proteolytic components (Chen et al., 2015).
The proteolytic activity of LAB in goat milk was measured by the OPA method, with results ranging from 3.55±0.26 to 5.69±0.21 mg/mL (Table 2). Goat milk fermented by P. pentasaceus 1 W2SR04 and Lb. kefiri YK4 (>5 mg/mL) showed high peptide content. In a study by Toe et al. (2019), P. pentasaceus species also showed high proteolytic activity. However, high peptide content was not always associated with high ACEI activity in samples. This is also seen in the results of our study. ACEI activity is more related to the abundance of ACEI peptides that could be released during fermentation.
The IER values evaluated in ten samples showed that the highest IER values were obtained in fermented goat milk of Lc. lactis ssp. lactis BD17. The IC50 value was also determined in this sample and the result was 0.297±0.10 mg/mL. The IC50 value reflects the peptide concentration required to inhibit 50% ACE. The IC50 value in fermented milk <1 mg/mL (Gútiez et al., 2013). Our results show that the obtained IC50 value is lower than that of fermented milk of other Lactobacillus species, as reported by Qian et al. (2011) in fermented milk by Lb. delbrueckii (IC50 67.71±7.62 mg/mL), by Moslehishad et al. (2013) in fermented milk by Lb. rhamnosus (IC50: 3.947±0.029 mg/mL) and by Chen et al. (2007) in fermented milk using several isolates (IC50: 0.65 mg/mL) and in koumiss (52.47±2.87 mg/mL) (Chen et al., 2010). Barla et al. (2016) have also reported from fermented milk by Lb. brevis, Lb. buchnery and W. hellenica (IC50: 0.28–0.83 mg/mL).
Filtration using a 3 kDa MW cut-off showed that the ACEI activity was concentrated in the MW fraction <3 kDa (Table 3). There was no significant difference (>0.05) in the ACEI activity of the <3 kDa fraction compared to the supernatant (without filtration). Bioactive peptides with ACEI activity have been reported as peptides with MW of <3 kDa (Gonzalez-Gonzalez et al., 2013).
| ACE inhibitory activity (%) | ||
|---|---|---|
| Supernatant (Without ultrafiltration) | >3 kDa | <3 kDa |
| 60.33±4.73a | 24.57±2.36b | 57.31±2.41a |
A total of 261 peptides were released in fermented goat milk by Lc. lactis ssp. lactis BD17 (Table 4 and Supplementary Data). Most of the peptides were hydrolyzed from casein (97%) and whey (3%). The main fraction of goat milk protein is casein, which is 80% of the total milk protein (Jandal, 1996). This explains the abundance of peptide hydrolyzed from casein in our results. Another thing that casein has a very flexible and open structure so it is very sensitive to proteolysis. While whey protein is more resistant which is explained by the presence of a globular structure (Swaisgood, 1993). According to the results, β-casein (54.02%) was the most accessible to the proteolytic system of Lc. lactis ssp. lactis BD17 to release a number of peptides. The cleavage site’s dominance on the β-casein was also shown by Lb. rhamnosus CGMCC11055 (Guo et al., 2016) and L. delbrueckii subsp. lactis ACA-DC 178 (Hebert et al., 2008).
∗ Protein access code at https://www.uniprot.org/.
The spectrum of MS analysis revealed a large number of peaks with retention times (RTs) of 8 to 65 min (Fig. 1), representing an abundance of released peptides with peptide mass/molecular charge (m/z) ranging from 310.1 to 1,146.0. The diversity of detected ions indicates that the nine peaks with RT of 11.29 to 30.97 corresponded to 13 peptides identified in goat milk fermented by Lc. lactis ssp. lactis BD17 (Table 5). ARHPHPHLSFM (ĸ-casein; RT 11.29, 11.89; m/z 665.33, 673.33) was a peptide that exhibited a prominent peak according to its abundance in the sample. This peptide was also identified as being present in goat milk kefir (Izquierdo-González et al., 2019) and goat milk (Ibrahim et al., 2017). Another signal was associated with the abundance of peptides in goat milk fermented by Lc. lactis ssp. lactis BD17 from the parent protein β-casein, namely MPFPKYPVEPF (RT 20.54; m/z 676.34), QEPVLGPVRGPFPI (RT 29.61; m/z 753.43) and RDMPIQAFLL (RT 23.59; m/z 602.33). This peptide has also been identified in goat milk kefir (Izquierdo-González et al., 2019) and bovine kefir (Ebner et al., 2015).
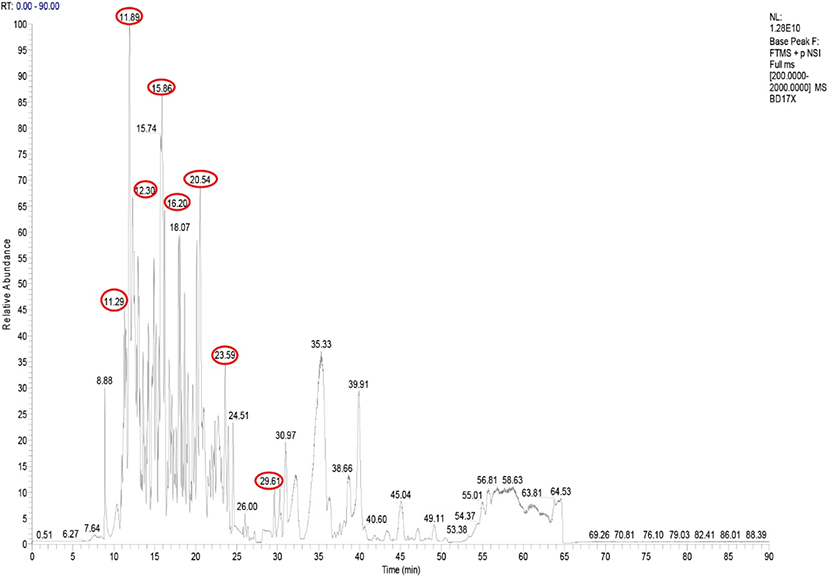
* Protein access code at https://www.uniprot.org/.
The release of peptides from the protein matrix is initiated by CEP, one of the essential enzymes in the LAB proteolytic system (Griffiths and Tellez, 2013) which cleaves the proteins resulting in peptides with 4 to 30 amino acids. The CEP of LAB is classified into three types based on the hydrolysis of casein (Kunji et al., 1996): (1) CEP type PI which specifically hydrolyzes β-casein, (2) CEP type PIII which hydrolyzes αS1-casein and κ-casein, (3) CEP intermediate type PI/PIII which hydrolyzes β-casein and αS1-casein. Based on this classification, our results indicate that the CEP of Lc. lactis ssp. lactis BD17 represents CEP types I and III. These types of CEP were also reported from Lb. casei PRA205 and Lb. rhamnosus PRA331 (Solieri et al., 2018), and Lb. paracasei ssp. paracasei (Nikolić et al., 2009).
Types and domains in the CEP region of each LAB provide variations in the specificity of the hydrolyzed substrate which have implications for the diversity of MWs and amino acid sequences of the released peptides (Raveschot et al., 2020). The MW of the peptide released by Lc. lactis ssp. lactis BD17 ranged from 659 to 2,201.1 dalton with amino acid residues ranging from 6 to 20. An investigation was carried out to determine the specificity of cleavage of CEP Lc. lactis ssp. lactis BD17 in goat milk parent protein during fermentation to release a number of peptides. The results are presented in Fig. 2. It appears that sites of Lc. lactis ssp. lactis BD17 dispersed throughout the parent protein, although certain amino acids are favorite for cleavage by CEP of Lc. lactis ssp. lactis BD17. In the αS1-casein region, the cleavage sites were frequently at serine, aspartate, and phenylalanine amino acid (f183-f184, f186-f187, and f184-f185), in αS2-casein region the sites were at amino acids tyrosine, leucine, and glutamine (f116-f117, f114-f115, f112-f113), and in the β-casein region the sites were at amino acids tyrosine, leucine, glutamic acid and proline (f208-f209, f207-f208, f123-f124, f125-f126). Moreover, in a κ-casein region the cleavage sites were at amino acids leucine, tyrosine, and alanine (f53-f54, f51-f52, f44-f45). These results indicate that the cleavage sites of casein by Lc. lactis ssp. lactis BD17 occur mostly in hydrophobic and aromatic amino acids. It seems that hydrophobic and aromatic amino acids are more easily accessed and released from parent protein. Similar results have been reported by Lozo et al. (2011) on Lb. paracasei subsp. paracasei BGHN14 (prtp), Lb. rhamnosus BGT10 (prtR), and Lb. helveticus BGRA43 (prtP), and Hebert et al. (2008) reported on Lb. delbrueckii subsp. lactis CRL 581.
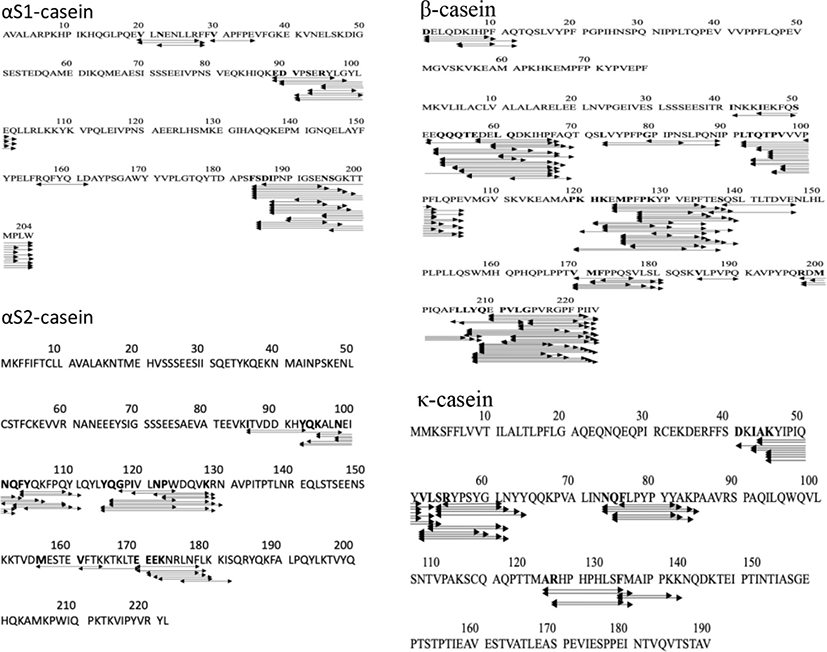
Twenty-one of the 261 peptides released by Lc. lactis ssp. lactis BD17 were identified as ACEI peptides (Table 6), most of which were released from β-casein. One of peptides namely ARHPHPHLSFM from parent protein κ-casein; 116-f117 was reported as an ACEI peptide (Ibrahim et al., 2017), and VLNENLR (αS1-casein; f39-f40) (Swaisgood, 1993). ACEI peptides could be identified based on their amino acid sequence (Lunow et al., 2015).
| Parent protein* | Sequence | m/z [Da] | MH+ [Da] | Charge | References |
|---|---|---|---|---|---|
| κ-Casein | ARHPHPHLSFM | 665.33 | 1,329.66 | 3 | Ibrahim et al. (2017) |
| β-Casein | DELQDKIHPF | 621.30 | 1,241.61 | 2 | Rodríguez-Figueroa et al. (2012) |
| β-Casein | DKIHPF | 378.71 | 756.41 | 2 | Fan et al. (2019) |
| β-Casein | DKIHPFAQ | 478.25 | 955.50 | 2 | Gobbetti et al. (2000) |
| β-Casein | EMPFPKYPVEPF | 740.86 | 1,480.71 | 2 | Papadimitriou et al. (2007) |
| β-Casein | ELQDKIHPF | 563.80 | 1,126.59 | 2 | Fan et al. (2019) |
| β-Casein | GPVRGPFPI | 470.27 | 939.54 | 2 | Amorim et al. (2019) |
| β-Casein | LGPVRGPFP | 470.27 | 939.54 | 2 | Hernández-Ledesma et al. (2004) |
| β-Casein | LTQTPVVVPPF | 599.34 | 1,197.68 | 2 | Villegas et al. (2014) |
| β-Casein | LVYPFPGPIHNSLPQN | 896.96 | 1,792.93 | 2 | Quirós et al. (2009) |
| β-Casein | LYQEPVLGPVRGPFPIIV | 997.58 | 1,994.14 | 2 | Pihlanto et al. (2010) |
| β-Casein | MPFPKYPVEP | 602.80 | 1,204.60 | 2 | Contreras et al. (2009) |
| β-Casein | MPFPKYPVEPF | 676.34 | 1,351.67 | 2 | Hayes et al. (2007) |
| β-Casein | QEPVLGPVRGPFP | 696.88 | 1,392.76 | 2 | Hernández-Ledesma et al. (2004) |
| β-Casein . | QEPVLGPVRGPFPIIV | 859.50 | 1,718.00 | 2 | Perpetuo et al. (2003) |
| αs1-Casein | VLNENLR | 485.78 | 970.56 | 2 | Zhao et al. (2019) |
| β-Casein | VLGPVRGPFP | 519.81 | 1,038.61 | 2 | Gútiez et al. (2013) |
| β-Casein | VVVPPF | 329.20 | 657.39 | 2 | Torres-Llanez et al. (2011) |
| β-Casein | YQEPVLGPVRGPFPI | 834.95 | 1,668.90 | 2 | Zhao et al. (2019) |
| β-Casein | YQEPVLGPVRGPFPIIV | 627.69 | 1,881.06 | 3 | Zhao et al. (2019) |
| β-Casein | YQEPVLGPVR | 579.31 | 1,157.63 | 2 | Kalyankar et al. (2013) |
* Protein access code at https://www.uniprot.org/.
The three amino acids located at the C-terminus could determine whether a peptide could act as an ACEI peptide (Wu et al., 2006). Amino acids from aliphatic groups (proline, isoleucine, valine) and aromatic amino acids (phenylalanine) are the dominant amino acids found in the ACEI peptide. Our investigation of other peptide released by Lc. lactis ssp. lactis BD17 which has not been identified as an ACEI peptide according to a literature search, demonstrated its potential as an ACEI peptide. A total of 36% of these peptides had a proline amino acid residue and 21% a phenylalanine amino acid residue at the C-terminus.
The characteristics of the ACEI peptide were not only observed in the presence of amino acids at the C-terminus. By other researchers, the presence of amino acids at the N-terminal was also evaluated. Aslam et al. (2019) showed that three identified ACEI peptides were released in goat milk fermented by Lb. helveticus cicc22171 has hydrophobic/aliphatic amino acids not only at the C-terminus but also at the N-terminus (valine and proline). Daliri et al. (2018) also presented their research results that four peptides identified as ACEI peptides were associated with the presence of negative amino acids (glutamate) and uncharged amino acids (glutamine) at their N-terminus. The presence of this amino acid in the Lc. lactis ssp. lactis BD17 peptide was also identified, namely in the peptide released from the parent protein β-casein and αS1-casein (Table 4).
In addition to the presence of certain amino acids in the ACEI peptide sequence, another characteristic of ACEI peptides is their MW. ACEI peptides are generally short peptides with MW <3 kDa, may consist of 6 to 16 amino acids (Ibrahim et al., 2017). However, ACEI peptides with 20 amino acid residues have also been reported (Elkhtab et al., 2017). The ACEI peptide identified in our study has a MW of <2 kDa (657–1,994 Da), consisting of 6 to 18 amino acids. Short peptides are known to easily bind to the active site of ACE (Aslam et al., 2019). ACEI peptide binding to the active of ACE is facilitated by hydrogen bonding, hydrophobic interactions, and disrupting the stability of the Zn2+ ion. The presence of ACEI peptide in fermented milk is highly dependent on the type of LAB used for the fermentation process. It is therefore very important to use isolates that have been shown to have the ability to release ACEI peptides. Lc. lactis ssp. lactis BD17 used in this study was an isolate isolated from kefir. Although the ability of this strain has only been explored in this study, as a comparison, the results of studies using kefir grain could be presented. Ebner et al. (2015) stated that kefir microbes were able to release 12 ACEI peptides in fermented milk. The same thing was also conveyed by Dallas et al. (2016) that kefir microbes release 29 bioactive peptides, including ACEI peptides in fermented cow milk. A recent study by Izquierdo-González et al. (2019) showed that in goat milk using kefir grains, five ACEI peptides were identified.
Conclusion
ACEI activity was detected in all fermented goat milk using isolates from fermented food and breast milk. Goat milk fermented using Lc. lactis ssp. lactis BD17 produced the highest ACEI activity (IC50: 0.297±0.10 mg/mL) after 48 h of incubation. A total of 261 peptides were hydrolyzed by Lc. lactis ssp. lactis BD17 during fermentation, most of which were released from casein (β-casein). The peptide has a MW of 659 to 2,201.1 dalton, consisting of 6–20 amino acid residues. The CEP specificity of Lc. lactis ssp. lactis BD17 towards goat milk parent protein, was dominated by cleavage on the amino acids tyrosine, leucine, glutamic acid, and proline. A total of 21 peptides were identified as ACEI peptides, having 100% homology to the reported ACEI peptides. Several characteristics of ACEI peptides are present in peptides hydrolyzed by Lc. lactis ssp. lactis BD17. These peptides mostly have hydrophobic and aromatic amino acids at the C-terminus. The results of this study add to the information that Lc. lactis ssp. lactis BD17 is a candidate that could be considered as a starter culture to obtain fermented milk that has functional properties as a source of ACEI peptides.













