Introduction
Fermentation involves inoculating food products with strains that are beneficial to humans to improve their quality, functionality, and shelf life (Kumar et al., 2017). During fermentation, high-molecular weight polypeptides in fermented meat and meat products are decomposed into low-molecular weight monopeptides or amino acids by endogenous or microbial proteolytic enzymes (Zhao et al., 2016). The high level of proteolysis results in high digestibility in the body, and small peptides have bioactivities such as antioxidant activity or anti-inflammatory properties (Ketnawa and Ogawa, 2019; Zhang et al., 2021).
Fermented sausage is a representative fermented meat product that is manufactured by inoculating a starter culture composed of lactic acid bacteria (LAB), yeast, and mold. The extent of proteolysis differs depending on the type of strain used, and a higher rate of proteolysis is associated with more diverse flavors and textures (Afraei et al., 2022; Ikonić et al., 2016). Therefore, one of the methods to increase consumer preference is to use strains with a high level of proteolysis during the manufacture of fermented sausages.
To assess consumer preference, the food industry regularly conducts sensory evaluations while developing food and fermented meat products. It primarily evaluates traits according to the proteolysis level of the starter culture (Ruiz-Capillas et al., 2021). The texture of food varies greatly depending on the molecular weight of proteins constituting the food (Isaschar-Ovdat and Fishman, 2018). Numerous low-molecular weight proteins can be produced while manufacturing fermented sausages using starter cultures with a high proteolysis rate (Jokanovic et al., 2017). Therefore, there exists a need to develop strains with a high proteolysis rate in the food industry to manufacture fermented sausages with high consumer preferences.
Several studies have been conducted worldwide on strains derived from fermented foods. For instance, Thakkar et al. (2018) reported that strains derived from Sauerkraut (German traditional fermented food) have antioxidative, proteolytic, and antihypertensive potential. Similarly, Dey et al. (2019) reported that Weissella confusa derived from Kimchi (a traditional Korean fermented food) possessed proteolytic activity and served as an effective strain for fermented meat products. Jeon et al. (2016) reported high proteolytic activity in strains derived from Doenjang (Korean traditional fermented food) with a NaCl content of 7% or less.
The above studies demonstrate that strains derived from traditional fermented foods in Korea undergo a high rate of proteolysis and are effective for manufacturing fermented sausages. Therefore, we analyzed the proteolysis and conducted a sensory evaluation of fermented sausages using strains derived from fermented foods in Korea.
Materials and Methods
The strains (LAB, yeast, and mold) derived from traditional fermented foods in Korea were used in this study. LAB were identified via 16S rRNA sequencing and yeast and mold were identified via 26s rRNA sequencing. LAB used Pediococcus pentosaceus SMFM2016-GK1 (GK1) and P. pentosaceus SMFM2016-NK3 (NK3). These strains were derived from Kimchi and incubated in de Man-Rogosa-Sharp broth at 37°C for 24 h. Yeast used Debaryomyces hansenii SMFM2021 D1 (D1), and mold used Penicillium nalgiovense SMFM2021 S6 (S6). Yeast was derived from Deonjang and incubated in yeast malt broth at 25°C for 48 h. Each broth was centrifuged, and the supernatant was removed. Then, LAB and yeast were stored at −90°C after freeze-dried. Mold was derived from spontaneous fermented sausages and incubated in potato dextrose agar (PDA) at 25°C for 72 h. Then PDA agar was mixed with 10 mL of sterile saline and used. The strains of control used LAB powder (21 mixed LAB, Biotopia, Chuncheon, Korea) and P. nalgiovense Sarterkulturen Edelschimmel. LAB are composed of 13 types of Lactobacillus spp., 5 types of Bifidobacterium spp., 2 types of Leuconostoc spp., and Streptococcus thermophilus, and the mold was supplied by the National Institute of Animal Science (Wanju, Korea) and mixed with sterile saline. All strains were inoculated at a level of 8 Log colony-forming units (CFUs)/g.
Fermented sausage was manufactured using the method described by Jeong et al. (2022). Meat was purchased from local market, which were lean pork from crossbred (Landrace×Yorkshire×Duroc) taken in 24 h after slaughter. Lean pork (85%) and fat (15%) were ground to 3 mm, and salt (2%), black pepper (0.3%), ascorbic acid (0.03%), glucose (0.8%), garlic (0.5%), pepper (0.1%), grape wine (2%), and strain (0.06%) were mixed for 6 min. The sausage emulsion was stuffed in a fibrous casing (Ø 40 mm) weighing approximately 250 to 300 g, and the casing was rinsed with cold water. A sausage pricker was used to create a hole inside the sausage to supply oxygen. After the suspension, the liquid mold was sprayed all over. Sausages were fermented for 3 days at 25°C and 70% RH, and finally dried at 14°C with 68% RH for 28 days with gradually decreasing temperature and RH.
The ratio of strains was conducted by selecting two strains predicted to exhibit optimal sensory characteristics in a previous study (Jeong et al., 2022). The control and treatments were classified as follows: (1) commercial starter culture (CST), (2) mixed with GK1, D1, and S6 (GKDS), and (3) mixed with NK3, D1, S6 (NKDS). Sausages were collected and used at the end of the fermentation (3 days) and drying periods (31 days). Two random sausages per batch were analyzed at each sampling. Texture profile analysis (TPA) and sensory evaluation were performed using only the final product (31 days).
Proximate composition was measured using the method described by Anderson (2007). Approximately 150 g of ground sausages was placed in a 140 mm sample dish, which was placed in a food scanner (DA6200, PerkinElmer, Waltham, MA, USA). The scanner identification was entered, and the software was set to meat product profile. Then, the moisture, protein, fat, and ash indicated on the scanner were recorded.
For the measurement of pH, the fermented sausages were finely ground under sterile conditions. Then, it was homogenized (6,451×g, 1 min) with deionized water (DIW) at a ratio of 1:4 using an Ultra turrax (HMZ-20DN, Poonglim Tech, Seongnam, Korea). The pH of homogenate was measured using a pH meter (Model S220, Mettler-Toledo, Greifensee, Switzerland), and it was calibrated with the standard buffer solutions.
A total of 2.5 g of fermented sausage and 10 mL of 3 mM phosphate buffer (pH 7.0) were homogenized (5,614×g, 2 min) using a homogenizer (AM-5, Nihonseiki Kaisha, Tokyo, Japan), and the supernatants were extracted by centrifugation (6,387×g, 4°C). Next, supernatants, 3 mM phosphate buffer, and 5× sample buffer were mixed to prepare the sample. The sample was heated for 5 min in a water bath (JSWB-30T, JSR, Gongju, Korea) at 100°C. Each sample (20 μL) was added to precast gels and processed for 2 h, following which the gel was incubated overnight in a fixing solution for 16 h and subsequently stained with Coomassie brilliant blue for 30 min. The gel dye was then removed using a destaining solution.
The protein solubility (total, myofibrillar, and sarcoplasmic proteins) of the fermented sausage was determined using the method described by Lee et al. (2020). For the total protein solubility, 2 g of fermented sausage and 20 mL of 1.1 M potassium iodide in 0.1 M potassium phosphate (pH 7.4) were homogenized (5,614×g) for 2 min using a homogenizer (AM-5, Nihonseiki Kaisha). For sarcoplasmic protein solubility, 2 g of fermented sausage and 20 mL of 0.025 M potassium phosphate buffer (pH 7.4) were homogenized (5,614×g) for 2 min using a homogenizer (AM-5, Nihonseiki Kaisha). The homogenate was incubated overnight for 16 h after blocking the light under refrigeration conditions (2°C), and the supernatants were extracted by centrifugation for 15 min (6,387×g, 4°C). The protein solubility of sausages was measured using the Biuret method. The absorbance of the sausage was measured at 540 nm using a multi-mode microplate reader (Spectra Max ID3, Molecular Devices, San Jose, CA, USA) to obtain the OD, and bovine serum albumin was used as the standard material. The following equation was used to calculate the protein content:
where a is supernatant diluent multiples; b is buffer dilution factor.
In vitro digestibility was determined using the method described by Wang et al. (2021). The fermented sausages and 10 mM PBS (pH 7.0) were mixed at a ratio of 1:4 and homogenized (6,451×g, 1 min) using an Ultra Turrax (HMZ-20DN, Poonglim Tech). The pH of homogenate was adjusted to pH 2.0 using 1M HCl to achieve the same conditions as the inside of the stomach. Then, the pepsin digestion was performed at 37°C for 2 h after adding 6 mg/mL of pepsin to the homogenate. After pepsin digestion was completed, the pH of homogenate was adjusted to pH 7.5 using 1 M NaOH to stop pepsin activity. The trypsin digestion was performed at 37°C for 2 h after adding 6 mg/mL of trypsin to the homogenate. After trypsin digestion completed, the homogenate was heated min in a water bath (JSWB-30T, JSR) at 90°C for 5 min to stop the trypsin activity. Digestibility was calculated to evaluate the total protein ratio of the homogenate after pepsin digestion and pepsin/ trypsin digestion. The protein content of the homogenate was measured using the Biuret solution, which consisted of 1.5 g of CuSO4·5H2O, 6 g of sodium potassium tartrate, 700 mL of DIW, and 10% of 300 mL NaOH. The absorbance of sausages was measured at 540 nm using a multi-mode microplate reader (Spectra Max ID3, Molecular Devices) and compared to a standard curve. Bovine serum albumin was used as the standard. The following equation was used to calculate the protein content:
where a is the protein content of fermented sausage before digestion and b is the protein content of fermented sausage after digestion.
The diameter (Ø) and height of fermented sausages were set at 2.5 and 2.0 cm, respectively, using a cylinder probe. TPA was used to measure the texture properties (hardness, springiness, gumminess, chewiness, and cohesiveness) of samples using a texture analyzer (TA 1, Lloyd, Largo, FL, USA) under the following conditions: pre-test speed, 5.0 mm/s; maximum load, 2 kg; head space, 2.0 mm/s; distance, 2.0 mm; force, 5 g; compression level, 80%.
Eighteen panelists (male: 9, female: 9, age: 20–34) were trained in commercial fermented sausages to ensure familiarity with their sensory properties. Fermented sausages were provided with a thickness of 5 mm, and the flavor, texture, and overall acceptability of samples were evaluated using a 10-point descriptive scale (flavor: 1=extremely inadequate, 10= extremely adequate; tenderness: 1=extremely hard, 10=extremely soft; texture: 1=extremely tough, 10=extremely tender; overall acceptability: 1=extremely unacceptable, 10=extremely acceptable). The sensory evaluation was approved by the Ethics Committee of the Kongju National University (Authority no: KNU_IRB_2021-54).
All experiments were repeated at least in duplicate and consisted of three batches. Proximate composition, pH, protein solubility, in vitro digestion, TPA, and sensory evaluation were performed using one-way analysis of variance (ANOVA) in the SAS program. The results are presented as the mean value and SEM. Significant differences between the data were analyzed using Duncan’s multiple range test (p<0.05).
Results and Discussion
Table 1 shows the proximate composition and pH of CST, GKDS, and NKDS. The protein contents of the NKDS and GKDS were significantly higher than that of the CST on days 3 and 31, respectively (p<0.05). The higher protein contents in those groups could be attributed to higher water loss (data not shown). This phenomenon is due to the rapid decrease in pH during fermentation, the faster the formation of gel, and syneresis occurs, resulting in increased water loss (Kenneally et al., 1998).
The pH of the CST and GKDS on day 3 was significantly lower than that of the NKDS (p<0.05). The pH of fermented sausages decreased rapidly during fermentation due to the growth of LAB (data not shown). Therefore, we recorded a higher growth of LAB in the GKDS during fermentation than in the NKDS. Fermented sausages must exhibit high levels of LAB that disintegrate the proteins into free amino acids and enhance the flavor and texture of the final products (Cao et al., 2019). The pH of the CST and GKDS on day 31 was significantly lower than that of the NKDS (p<0.05). During fermentation, as the microorganisms grow and decompose proteins, basic metabolites, such as NH3, and other produced metabolites accumulate in the fermented sausages and increase the pH (Wang et al., 2022a). However, because the pH on day 31 showed the same trend as the pH on day 3, the level of metabolites (NH3, etc.) produced did not show a significant difference between the CST and treatment. Therefore, both the CST and GKDS showed lower pH values than the NKDS on days 3 and 31, indicating a high level of LAB. However, excessive acidity can cause negative sensitivity in consumers, suggesting that additional studies, such as sensory evaluation, are warranted.
Fig. 1 shows the relevant protein levels in CST, GKDS, and NKDS. Although a 100 kDa protein band (α-actinin) was observed on day 0, none of the CST, GKDS, and NKDS were observed on day 3. Wang et al. (2017) reported that α-actinin is rapidly degraded during the early stages of fermentation to produce degradation products varying in size from 50 to 75 kDa. As the fermentation and drying period increased, a 53 kDa protein band (desmin) in all emerged due to the decomposition of α-actinin into desmin. The thickness of the 42 kDa protein band (actin) reduced as the fermentation and drying period increased. In the GKDS, the thickness of the actin band was less on day 3, with no significant change in the NKDS. In the GKDS, the thickness of the actin band was less on day 3, with a slight change in the NKDS. These results are attributed to the high proteolysis rate in inoculated GKDS compared to NKDS. The thickness of the 35 kDa protein band (tropomyosin) in all increased as the fermentation and drying periods increased, which is attributed to the decomposition of myosin heavy chain to tropomyosin by endogenous or microbial enzymes during fermentation and drying (Mora et al., 2019). The thickness of the 25 kDa protein band (myosin light chain) was greater in the GKDS than that in the NKDS on day 31. In both GKDS and NKDS, a protein band of 25 kDa or less was not observed, indicating that the GKDS contained more low-molecular weight proteins (25 kDa) than the NKDS. A higher content of low-molecular weight protein is associated with greater digestibility of food, indicating that the digestibility of the GKDS was higher than that of the NKDS (Ketnawa and Ogawa, 2019). Therefore, the use of GKDS in the manufacture of fermented sausages using strains derived from fermented foods in Korea will result in a high rate of proteolysis.
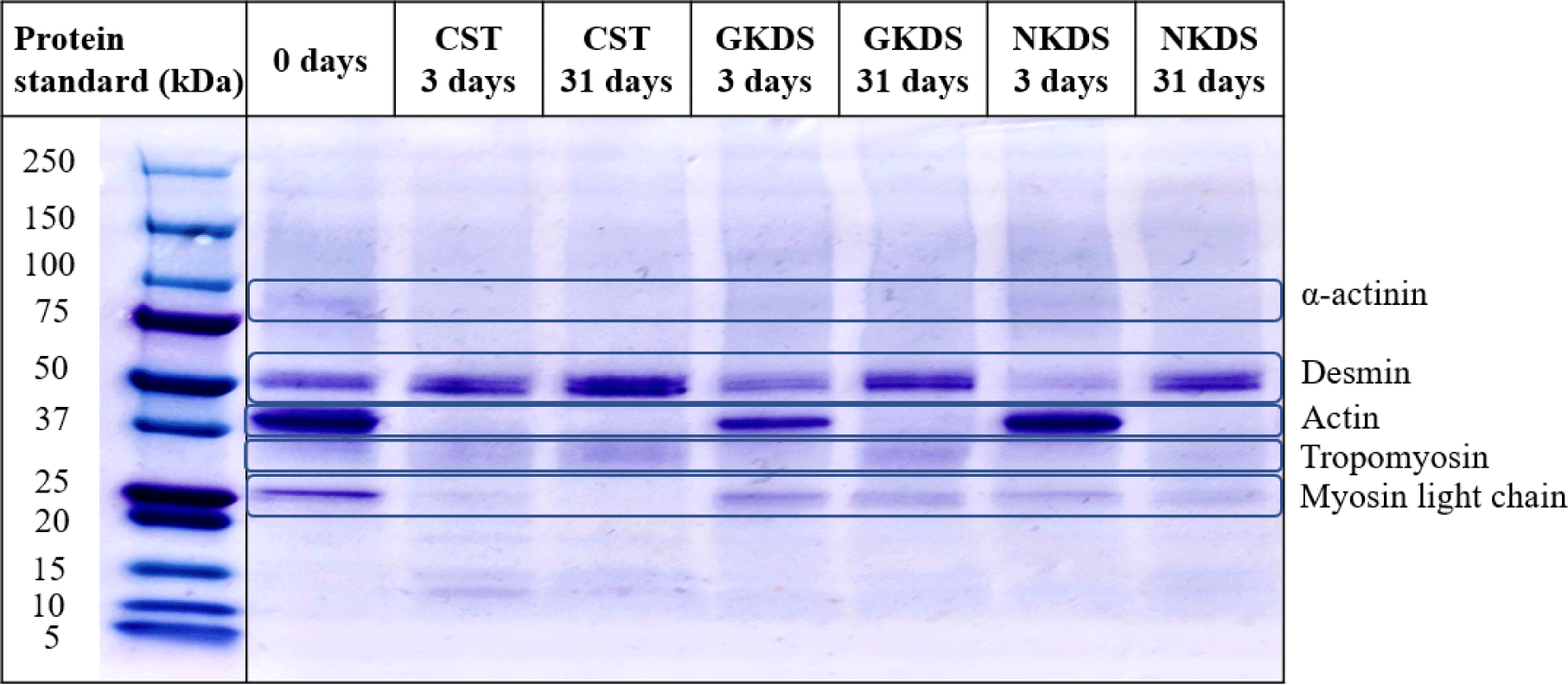
Table 2 shows the protein solubility of CST, GKDS, and NKDS. Protein solubility was determined by factors involved in protein–protein interactions or protein–water interactions, such as molecular weight, hydrophobicity, the charge of amino acids or pH, and ionic strength (Krunić et al., 2019). On day 3, no significant difference was observed in the total protein solubility between the CST and treatments; however, on day 31, the NKDS showed a higher level than the CST (p<0.05). Sarcoplasmic protein solubility was not significantly different between the CST and treatments. Gao et al. (2016) reported that sarcoplasmic protein was primarily related to cellular metabolism, indicating that the starter culture exerted no effect on cellular metabolism in this study. On days 3 and 31, myofibrillar protein solubility in the GKDS was significantly higher than that in the CST (p<0.05). Myofibrillar proteins are largely composed of myosin, actin, titin, tropomyosin, troponin, and nebulin (Mijailovich et al., 2019). Lee et al. (2020) demonstrated the significance of high levels of myofibrillar protein solubility because salt-soluble proteins contribute to the enhanced texture of meat products. Salt-soluble proteins are generated by combining oil, water, and protein, and their content increases with an increase in the number of surfactants (Han et al., 2021). In the case of fermented meat products, metabolites produced by proteolysis can act as surfactants (data not shown). We found higher production of metabolites in the GKDS and NKDS than in the CST, suggesting an increase in the content of myofibrillar proteins (Cropotova et al., 2019). Therefore, both GKDS and NKDS, which showed higher myofibrillar protein solubility than the CST, exerted a positive effect on the texture of fermented sausages.
Pepsin converts proteins to peptones in the presence of hydrochloric acid secreted from the stomach, following which peptones are decomposed into amino acids and dipeptides by trypsin secreted into the pancreatic juice (Wang et al., 2020). Table 3 shows the in vitro digestion of CST, GKDS, and NKDS. Although pepsin digestion did not show a significant difference between the CST and treatments on day 3, the final product, the CST, and GKDS showed significantly higher values on day 31 than the NKDS (p<0.05). Protein digestibility increases with smaller particle size or higher low-molecular weight protein content (Sicard et al., 2018). SDS-PAGE showed that the CST, and GKDS had more low-molecular weight proteins than the NKDS and therefore showed higher digestibility. Proteins that remain undigested by trypsin in the small intestine get excreted from the body (Hur et al., 2011). Trypsin digestion on days 3 and 31 was significantly higher in the GKDS than in the NKDS (p<0.05), which could be attributed to the degradation of numerous proteins into low-molecular weight peptides because the proteolysis in the GKDS was higher than in the NKDS. The results of in vitro digestion analysis showed higher pepsin- and trypsin-mediated digestion in GKDS than in NKDS, suggesting it to be a suitable starter culture for fermented sausages using strains derived from fermented foods in Korea.
TPA is an analytical method to mechanically measure the texture felt while ingesting food and subsequently express it numerically (Chandra and Shamasundar, 2015). Table 4 shows the textural properties of CST, GKDS, and NKDS. Hardness was significantly higher in the CS and GKDS than in the NKDS (p<0.05). The hardness of fermented meat products is affected by the drying period and stickiness (Ledesma et al., 2016; Wang et al., 2022b). The GKDS and CST had more metabolites (alanine, leucine, etc.) than the NKDS due to an increased rate of proteolysis, suggesting a high extent of stickiness. Springiness was significantly higher in GKDS and NKDS than in the CST (p<0.05). The springiness of meat or meat products is affected by muscle structure and biochemical components, such as moisture, liquid neutral lipids, and myofibrillar proteins (Xiong et al., 2015). Therefore, it is suggested that the strain derived from Korean fermented food decomposes proteins and affects the muscle structure or the content of myofibrillar proteins. The gumminess, chewiness, and cohesiveness in the GKDS were not significantly different from those recorded in the CST. The TPA analysis revealed that the GKDS exhibited similar mechanical levels to the CST, resulting in little difference in texture and commercial product when consumers select fermented meat products. Therefore, GKDS is considered a suitable starter culture for manufacturing fermented sausages using strains derived from fermented foods in Korea.
Table 5 shows the sensory evaluation of CST, GKDS, and NKDS. The flavor recorded in the GKDS was significantly higher than that in the CST (p<0.05). The flavor of fermented meat products was determined by free amino acids and free fatty acids produced by the decomposition of proteins and fats in the starter culture (Cai et al., 2020). Thus, the GKDS produced numerous free amino acids and free fatty acids compared to other and received a high score (Yoo et al., 2015). Tenderness, texture, and overall acceptability exhibited the highest value in the GKDS. The tenderness and texture of fermented meat products were determined by the level of proteolysis in the starter culture (Chen et al., 2022). The higher level of proteolysis in the GKDS than in the CST and NKDS resulted in an elevated content of low-molecular weight proteins and improved tenderness. Therefore, GKDS is a suitable candidate as a starter culture when manufacturing fermented sausages using strains derived from fermented foods in Korea.
| Traits1) | CST | GKDS | NKDS | SEM |
|---|---|---|---|---|
| Flavor | 7.88b | 9.10a | 9.00ab | 0.787 |
| Tenderness | 8.83b | 9.75a | 8.58b | 0.550 |
| Texture | 8.67b | 9.42a | 8.67ab | 0.664 |
| Overall acceptability | 8.67b | 9.67a | 8.50b | 0.483 |
Conclusion
This study analyzed the effects on proteolysis and conducted sensory evaluation when strains derived from fermented foods in Korea (Kimchi, Doenjang, and spontaneously fermented sausage) were inoculated into the fermented sausages. Moisture content decreased during fermentation and drying in the control and all treatments, but the final moisture content was lowest in the GKDS. GKDS showed lower pH than NKDS after fermentation (day 3), it was predicted to have rich organoleptic characteristics. Total protein and myofibrillar protein solubility were higher in GKDS and NKDS than in the CST. An analysis of relevant protein levels showed that the GKDS had a thicker low-molecular weight protein band (25 and 37 kDa) than the NKDS, whereas the GKDS displayed a higher digestibility of pepsin and trypsin than the NKDS. The texture of the GKDS was mechanically similar to that of the CST, it was predicted that there would be less resistance from consumers. In addition, panelists assigned higher scores to the GKDS for all traits than the NKDS and CST. In this study, sausages fermented using LAB (GK1), yeast (D1), and mold (S6) derived from fermented foods in Korea exhibited a high proteolysis rate and excellent sensory evaluation results.













