Introduction
Fermented sausages are meat products manufactured using microorganisms generated during fermentation that degrade proteins and lipids to create the substances such as glycine, glutamic acid, oleic acid, which show the unique “fermented” taste and flavor, with subsequent dehydration to reduce the water activity to suppress the growth of pathogenic microorganisms (Bungenstock et al., 2020; Liang et al., 2020; Xiao et al., 2020). The certain amino acids, including valine, leucine, and isoleucine, are converted to such compounds as methyl aldehydes, acids, and alcohols during fermentation to create the “fermented” aroma of fermented sausages (Ikonic et al., 2019).
Manufacturing fermented sausage using starter cultures influence not only sensory properties but also microbiological stability (Chen et al., 2021b). The most used starter cultures are developed in western countries, and the manufacture of fermented sausages in Korea relies on imported starter cultures (National Institute of Animal Science, 2018). According to Hu et al. (2022), the taste and flavor may vary in fermented sausages depending on the type of microbial strain used in the starter culture, which implies the need to investigate starter cultures isolated from Korean fermented foods to enhance acceptability by Korean consumers.
Recently, various studies have been conducted using strains isolated from Korean fermented foods. Zhu et al. (2019) showed that the use of Lactobacillus pentosus isolated from Kimchi as the starter culture in manufacturing fermented sausages produced antimicrobial activities, thus, the authors claimed that sodium nitrite may partially be substituted. Jeong et al. (2016) verified the safety and potential use of Staphylococcus spp. isolated from Doenjang and Meju as the starter culture with respect to antibiotic susceptibility, hemolytic activity, and biogenic amine productivity. So far, studies on fermented meat products using a starter culture developed in Korea have mainly focused on physicochemical properties, with a general lack of studies on sensory characteristics.
Sensory evaluation is a process in the manufacture of food products to ensure preference by consumers through measuring the items that can be determined by the human sensory organs, such as the eyes, nose, and mouth (Singh-Ackbarali and Maharaj, 2014). While sensory evaluation is generally conducted based on trained sensory detection by the panelist, it is currently a challenge to discriminate the compounds produced during fermentation and maturation. Thus, the food industry has introduced such technologies as the electronic nose and electronic tongue to analyze the aroma and flavor of food products more objectively. Wen et al. (2022) distinguished the flavor attributes according to the fermentation period of beef jerky inoculated with three starter culture types using an electronic nose. Chen et al. (2021a) analyzed the taste profile of dry fermented sausages with the addition of varying proportions of NaCl and KCl according to the fermentation period using an electronic tongue. The use of an electronic nose and tongue has been determined to contribute to the development of unique starter cultures in Korea by enabling the early analysis of sensory characteristics of fermented sausages with long periods of fermentation and drying.
Therefore, starter cultures more suitable for Korean consumers should be developed through the use of microbial strains isolated from Korean fermented foods, followed by tests and analyses of microbial growth in fermented meat products and the aroma and flavor created as a result. Thus, this study applied lactic acid bacteria, yeasts, and mold isolated from Korean fermented foods as the starter culture for fermented sausages and analyzed microbial composition, pH, and electronic nose and tongue measurements.
Materials and Methods
The lactic acid bacteria Pediococcus pentosaceus SMFM2016-GK1 (GK1) and P. pentosaceus SMFM2016-NK3 (NK3) were isolated from Kimchi. The yeast and mold used were Debaryomyces hansenii SMFM2021-D1 (D1) isolated from Doenjang and D. hansenii SMFM2021-S8 (S8) and Penicillium nalgiovense SMFM2021-S6 (S6) isolated from spontaneously fermented sausage. The strains isolated from Korean fermented foods were supplied by Yoon Biotech (Seoul, Korea) in freeze-dried powder form (lactic acid bacteria and yeast) and in a liquid state (mold). As a control, Lactobacillus powder (21 mixed lactic acid bacteria, Biotopia, Chuncheon, Korea) mixed with 13 types of Lactobacillus spp., five types of Bifidobacterium spp., two types of Leuconostoc spp., and one type of Streptococcus spp. was used for the lactic acid bacteria, and P. nalgiovense Sarterkulturen Edelschimmel (Animal Products Utilization Division, National Institute of Animal Science, Wanju, Korea) was used for the mold.
The pork hind legs (Landrace×Yorkshire×Duroc, each weight approximately 110 kg) 24 h after slaughter were used to manufacture the sausages. The hind legs and pork back fat were ground to a 3 mm size using a grinder (PA-82, Manica, Barcelona, Spain). The pork hind legs (85%) and back fat (15%) were mixed with salt (2%), glucose (0.8%), black pepper (0.3%), garlic (0.5%), bird’s eye chili (0.1%), ascorbic acid (0.03%), red wine (2%), and starter culture (0.06%) for 6 min based on total weight using a mixer (RM-20, Manica). The starter cultures were adjusted to a level of 8 Log colony-forming units (CFU)/g. After the mixture was filled in a fibrous casing with a diameter of 40 mm, the sausage was divided into 250–300 g using a clip machine (Tischeinzelclipper, Lip Technik, Meißen, Germany). After the sausages were rinsed, a hole was punched into the casing surface using a sausage pricker. The sausages with hanging were sprayed back and forth twice using a sprayer containing liquid mold (approximately 4 mL). The sausages were classified into 10 types according to whether or not the starter cultures were inoculated and named using the initials of the inoculated lactic acid bacteria and yeast (Table 1). The sausages were fermented for 3 days at 20°C and 70% relative humanity.
The sample and sterile saline solution were placed into a sterile bag at a ratio of 1:2 and homogenized for 1 min with a stomacher (WH4000-2751, 3M Korea, Seoul, Korea). Then, 1 mL of the homogenate was diluted in a sterile saline solution, and this was repeated as many times as many multiples as needed. The aerobic bacteria plate count (AC) was measured using 3MTM petrifilm (3M, Saint Paul, MN, USA). Lactobacillus spp., Staphylococcus spp., and yeast and mold were measured using De Man, Rogosa and Sharpe (MRS) agar, mannitol salt agar (MSA), and potato dextrose agar (PDA), respectively. AC, MRS, and MSA were incubated at 37°C for 24 h, and PDA was incubated at 25°C for 48 h. The number of colonies were measured and expressed as Log CFU/g.
Deionized water was added to the sample with the casing removed and mixed at a ratio of 1:4 and homogenized for 1 min at a speed of 6,451×g using ultra turrax HMZ-20DN (Pooglim Tech, Seongnam, Korea). The pH of the homogenate was measured using a pH meter (Model S220, Mettler-Toledo, Greifensee, Switzerland) calibrated with buffer solutions (pH: 4.01, 7.0, and 10.0).
The electronic nose described by Kang et al. (2021) was used with some modifications. The aroma profile was measured using an electronic nose system (Heracles-II-e-nose, Alpha MOS, Toulouse, France) equipped with MXT-5 and MXT-1701 by placing 5 g of the sample with the casing removed in a vial. The electronic nose analysis conditions were as follows: Injected volume, 5 mL; injection speed, 200 μL/s; injection temperature, 200°C; detector temperature, 260°C. The measured aroma profile was expressed in the form of principal component analysis (PCA) and volatile compound using the Alpha software program (Alpha MOS). The classified aroma pattern was given as the primary component value (PC1) and the secondary component value (PC2).
The electronic tongue described by Lee and Kim (2021) was used with some modifications. After mixing 4 g of the sample with the casing removed and 16 mL of distilled water (DW), ultra turrax (HMZ-20DN, Pooglim Tech) was used for homogenization at a speed of 6,451×g for 1 min. The homogenate was extracted using filter paper (Whatman paper No. 1, Whatman, Maidstone, UK). The filtrate was diluted 1,000-fold with DW, and sourness, saltiness, and umami were analyzed using an electronic tongue system (Astree 5, Alpha MOS). The measured taste profile was expressed as AHS (sourness), CTS (saltness), NMS (umami), PKS, CPS, ANS, and SCS using the Alpha software program (Alpha MOS). In addition, 0.1 M HCl, 0.1 M NaCl, and 0.1 M monosodium glutamate (MSG) were used as reference materials for sourness, saltiness, and umami taste, respectively.
All data in this study (10 groups×4 fermentation periods×3 batches) were used for statistical analysis and presented as the mean values and SEM. Microbiology properties and pH were analyzed by one-way analysis of variance using the General Linear Models procedure in the SAS program (version 9.4 for window, SAS Institute, Cary, NC, USA). The significant difference was determined among the 5% level using Duncan’s multiple range test.
Results and Discussion
The starter culture used in this study consisted of S8 isolated from a fermented meat product and GK1, NK3, and D1 isolated from plant-based products, such as Kimchi and Doenjang. Chen et al. (2016) used a starter culture isolated from Nanx Wudl, a Chinese-style fermented meat product, which was shown to exhibit outstanding growth in fermented meat products. Lee et al. (2006) reported that lactic acid bacteria isolated from Kimchi exhibited high adaptability in fermented sausages. Thus, GK1, NK3, and D1 isolated from plant-based fermented products and S8 from an animal-based fermented product (sausage) were comparatively analyzed with respect to their effects on fermented sausages.
Table 2 presents the microbial population in fermented sausages produced with the addition of starter cultures isolated from Korean fermented foods. The AC and LABC of GD, GDS, ND, NS, NDS, GNS, and GNDS were significantly increased with increasing the fermentation period (p<0.05). The lag phase of lactic acid bacteria is around 5 h, after which the exponential phase commences (Halim et al., 2020). The LABC of control and treatments groups showed an early level of 3.96–4.99 Log CFU/g, which increased up to 8.72–9.50 Log CFU/g through rapid growth during the 3 days of fermentation. As LABC displayed no subsequent change (data not shown), it was determined that the bacteria entered the stationary phase. An increasing trend was shown by all treatment groups with an increase in the fermentation period for STPC, which reached 3.94–5.44 Log CFU/g on the third day of fermentation. Fermented sausages have been shown to require careful attention regarding the emergence of Staphylococcusaureus in low-temperature (20°C) fermentation (Stavropoulou et al., 2018). Although not explicitly shown, S. aureus was not detected in any of the control or treatment groups in this study after the 3 days of fermentation (data not shown). Thus, for STPC in this study, the experiment proceeded based on the presumption that the strains of Staphylococcus were of lactic acid bacteria in the control and treatment groups not displaying S. aureus. For YMC, an increasing trend was shown by the control and all treatment groups with an increase in the fermentation period, reaching 5.41–6.32 Log CFU/g on the third day of fermentation. The use of P. nalgiovense in the manufacture of fermented sausages is known to contribute to creating the flavor of fermented sausages as well as in preventing the toxic mold that produce mycotoxin (Moavro et al., 2020). Across the control and all treatment groups, no mycotoxin was detected (data not shown), which is presumed to be because S6 in the treatment group provided a protective culture as with P. nalgiovense Sarterkulturen Edelschimmel in the control group.
AC, aerobic bacteria plate count; LABC, Lactobacillus spp. plate count; STPC, Staphylococcus spp. plate count; YMC, yeast and mold plate count; CS, commercial starter culture; GD, Pediococcus pentosaceus SMFM2016-GK1+Debaryomyces hansenii SMFM2021-D1; GS, P. pentosaceus SMFM2016-GK1+D. hansenii SMFM2021-S8; GDS, P.pentosaceus SMFM2016-GK1+D. hansenii SMFM2021-D1+D. hansenii SMFM2021-S8; ND, P. pentosaceus SMFM2016-NK3+D. hansenii SMFM2021-D1; NS, P. pentosaceus SMFM2016-NK3+D. hansenii SMFM2021-S8; NDS, P. pentosaceus SMFM2016-NK3+D. hansenii SMFM2021-D1+D. hansenii SMFM2021-S8; GND, P. pentosaceus SMFM2016-GK1+P. pentosaceus SMFM2016-NK3+D. hansenii SMFM2021-D1; GNS, P. pentosaceus SMFM2016-GK1+P. pentosaceus SMFM2016-NK3+D. hansenii SMFM2021-S8; GNDS, P. pentosaceus SMFM2016-GK1+P. pentosaceus SMFM2016-NK3+D. hansenii SMFM2021-D1+D. hansenii SMFM2021-S8.
On day 0 of fermentation, AC and LABC in the control group showed significantly lower values than in the treatment groups (p<0.05). On day 3 of fermentation, however, AC showed a similar result to the GD and GS treatment groups, and LABC showed a similar result to the GD, GS, GDS, NS, NDS, GND, GNS, and GNDS treatment groups. This is presumed to be due to the differences in the growth rate according to the varying types of starters applied to the control and other groups. In addition, the GD, GS, and GDS treatment groups with GK1 alone, and the GND, GNS, and GNDS treatment groups with the mixture of lactic acid bacteria did not vary significantly from the control group (p>0.05). Nonetheless, the significantly lower value of the ND treatment group with NK3 alone compared to the control group indicated high adaptability of GK1 compared to NK3 in fermented sausages. The STPC of GD, GS, GDS, ND, GND showed no significant difference from the control on day 3 of fermentation. Staphylococcus strains, such as Staphylococcus xylosus and Staphylococcus carnosus, are the known microbial strains that contribute to the flavor formation in fermented meat products through the degradation of proteins and lipids (Schlegel et al., 2021; Wang et al., 2022). It is, thus, predicted that the GD, GDS, ND, and GND treatment groups exhibiting a high level of STPC would produce strong flavors in the final product. The YMC of GDS, GND, GNS showed significantly higher than the control in 3rd day (p<0.05). The treatment group with D1 only showed an overall higher level of YMC than the treatment group with S8 only. This is presumed to be due to the high capacity of growth of D1 compared to S8 in fermented sausages upon the inoculation of an identical lactic acid bacteria strain. The results, thus, indicated that S6 effectively inhibited the pathogenic microorganisms, and the GD, GDS, ND, and GND treatment groups are likely to be suitable for the manufacture of fermented sausages in terms of microbial activities as they are predicted to produce strong flavors in fermented sausages.
Table 3 presents the measured pH of fermented sausages with the addition of starters isolated from Korean fermented foods. On day 0 of fermentation, the pH of the control and treatment groups fell in the range of 5.89–6.04 with no significant variation from day 1 of fermentation. On day 2 of fermentation, a significant decrease was found in GD, GS, GDS, NS, NDS, GNS, and GNDS treatment groups compared to day 1 of fermentation, and a significant decrease was found in ND and GND groups on day 3 of fermentation (p<0.05). This is due to the acidification induced by the growth of lactic acid bacteria with a relatively low pH (Laranjo et al., 2019). On day 3 of fermentation, GS, NS, NDS, and GNDS treatment groups showed a low pH at 4.97, 4.98, 4.93, and 5.00, respectively. At a pH of 5.0 or below, the high acidity is known to cause the death of pathogenic microorganisms (Canon et al., 2020). Bis-Souza et al. (2019), on the other hand, reported that a rapid increase in acidity could influence the sensory characteristics of fermented meat products, and Ruiz et al. (2014) reported that consumer acceptability decreased as a result of greater moisture loss as meat proteins became closer to the isoelectric point at a pH of 5.2 or below.
CS, commercial starter culture; GD, Pediococcus pentosaceus SMFM2016-GK1+Debaryomyces hansenii SMFM2021-D1; GS, P. pentosaceus SMFM2016-GK1+D. hansenii SMFM2021-S8; GDS, P.pentosaceus SMFM2016-GK1+D. hansenii SMFM2021-D1+D. hansenii SMFM2021-S8; ND, P. pentosaceus SMFM2016-NK3+D. hansenii SMFM2021-D1; NS, P. pentosaceus SMFM2016-NK3+D. hansenii SMFM2021-S8; NDS, P. pentosaceus SMFM2016-NK3+D. hansenii SMFM2021-D1+D. hansenii SMFM2021-S8; GND, P. pentosaceus SMFM2016-GK1+P. pentosaceus SMFM2016-NK3+D. hansenii SMFM2021-D1; GNS, P. pentosaceus SMFM2016-GK1+P. pentosaceus SMFM2016-NK3+D. hansenii SMFM2021-S8; GNDS, P. pentosaceus SMFM2016-GK1+P. pentosaceus SMFM2016-NK3+D. hansenii SMFM2021-D1+D. hansenii SMFM2021-S8.
On day 0 of fermentation, the pH of the control group did not vary significantly from the pH of GDS, ND, NS, NDS, GNS, and GNDS treatment groups. In contrast, on day 3 of fermentation, the pH of the control group was significantly lower than that of the GD, ND, and GND treatment groups, and significantly higher than that of the GS, GDS, NS, NDS, GNS, and GNDS treatment groups (p<0.05). The GD, ND, and GND treatment groups with a higher pH than the control group on day 3 of fermentation were all inoculated with D1, while the GS, GDS, NS, NDS, GNS, and GNDS treatment groups with a lower pH were all inoculated with S8. The pH of D1 used in this study was approximately 6.76 with a relatively slow rate of fall of the pH, presumably due to the growth of yeast despite the growth of lactic acid bacteria. In general, the manufacture of fermented sausages involves at least 3 weeks of maturation after fermentation for the degradation of proteins and lipids by microorganisms. However, a steep fall in pH could lead to a low pH after drying maturation, with a potential negative impact on sensory characteristics. It is, thus, predicted that the treatment of GD, ND, and GND with a higher pH than the control would be suitable in the manufacture of sausages, considering the pH reduction that may occur in the post-fermentation drying maturation.
Electronic nose is a technology to analyze the aroma pattern as the sensor detects volatile compounds produced as the sample is heated (Aouadi et al., 2020). The results of electronic nose analysis of fermented sausages with the addition of starters isolated from Korean fermented foods are presented in Fig. 1A for PCA and in Fig. 1B for volatile compound. Based on PC1, the GD, GS, GDS, GND, GNS, and GNDS treatment groups inoculated with GK1 are found on the left side of the x-axis, while the ND, NS, and NDS treatment groups inoculated with NK3 are found at the right side of the x-axis (Fig. 1A). In contrast, the aroma profiles of treatment groups inoculated with D1 and those inoculated with S8 showed no correlation, implying that the flavor creation in fermented sausages during the fermentation period is mainly mediated by lactic acid bacteria rather than yeast.
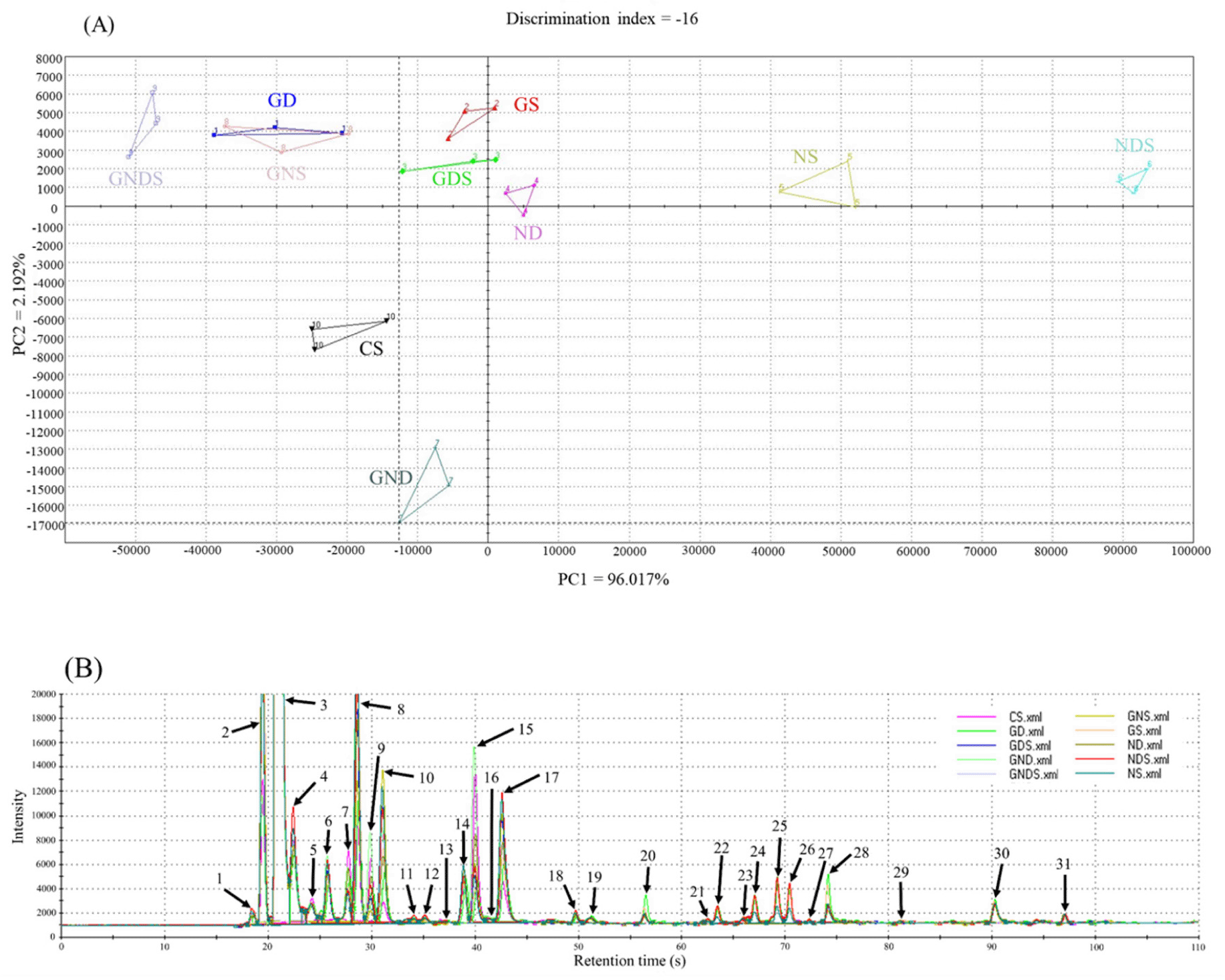
The volatile compounds produced according to the type of starter isolated from Korean fermented foods are shown in Fig. 1B. Among the compounds, ethanol at the 2nd peak had the highest level, followed by 2-propanol at the 3rd peak. The two compounds are responsible for the “alcoholic” flavor created in the process of conversion of glucose into ethanol, carbon dioxide, and other metabolic byproducts during fermentation mediated by yeast (Vamvakas and Kapolos, 2020). Hu et al. (2019) reported that in fermented sausages, lactic acid bacteria are engaged in flavor creation by producing organic acid and volatile compounds through carbohydrate fermentation. In addition, 16 volatile compounds (number of peaks: 4, 5, 6, 8, 10, 12, 13, 14, 15, 17, 18, 19, 21, 22, 26, and 28) and 7 volatile compounds (number of peaks 1, 6, 7, 10, 13, 17, and 27) responsible for the unique “fruity” and “cheesy” flavors of fermentation were detected. Among such volatile compounds, ethyl acetate and ethyl butyrate show a low sensory threshold value and have significant effects on the aroma of fermented sausages (Sun et al., 2010). Ethyl ester, which could have a strong effect on the flavor of fermented sausages, is formed by the esterase activity of lactic acid bacteria (Sionek et al., 2021). It is presumed that GK1 and NK3 isolated from Kimchi exhibit esterase activity and have a considerable positive effect on flavor creation. Butane-2,3-dione at the 6th peak is a compound responsible for flavor creation via lipid oxidation (Flores, 2018). The level of the 6th peak was higher in the control group than in the treatment groups, which is presumed to be due to the outstanding ability of the strains isolated from Korean fermented foods to inhibit lipid oxidation compared to the strains in the control group. For the 19th, 20th, and 30th peaks, the levels were higher in the GD treatment group than in any other treatment group and the control group, and the three respective compounds shared the ability to create the “fermented” flavor. This implied that the microorganisms in the GD treatment group had rapidly degraded the proteins and lipids so that the level of flavor caused by lipid oxidation was low, while a rich amount of compounds for the “fermented” flavor was detected. Based on this, treatment with GD is predicted to be suitable in the manufacture of fermented sausages as it would lead to adequate creation of the unique aroma and flavor of fermented meat products after drying maturation.
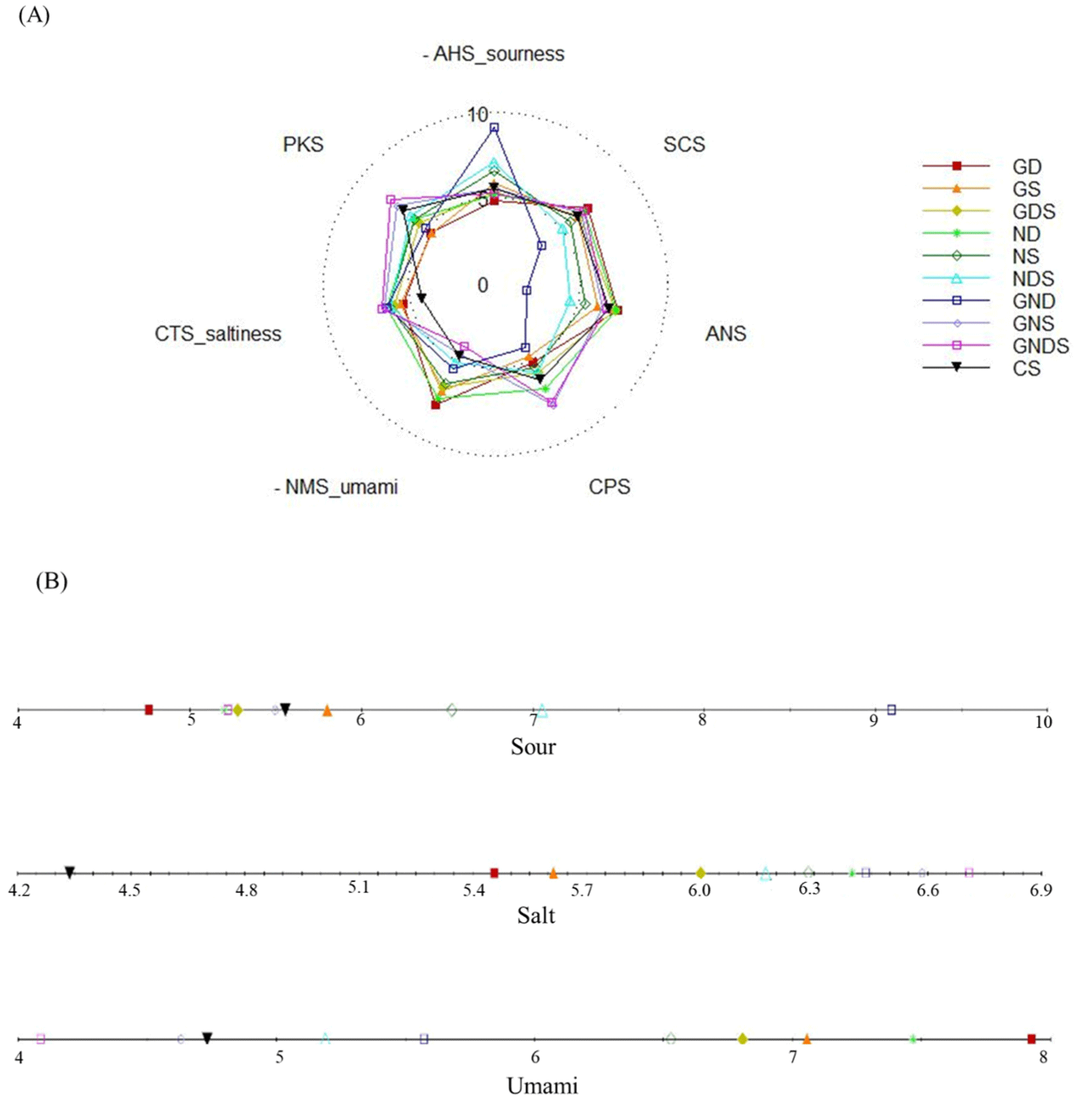
The results of the electronic tongue analysis of fermented sausages with the addition of starters isolated from Korean fermented foods are presented in Fig. 2A as a radar chart, while Fig. 2B shows the intensity of sourness, saltiness, and umami flavors. The sourness discriminated based on the difference in hydrogen ion potential with 0.1 M HCl as the reference material was lower in the GD and ND treatment groups than in the control group (Choi et al., 2014). This is likely to be due to the influence of the high pH in the GD and ND treatment groups compared to that in other treatment groups and the control group. Fermented sausages acquire intense sourness as a result of a fall in the pH due to the growth of lactic acid bacteria through additional post-fermentation drying maturation (Afifah et al., 2022). Thus, compared to the GS, NS, NDS, GND, and GNDS treatment groups, the GD, GDS, ND, and GNDS treatment groups with a lower level of sourness than the control group are predicted to be suitable for the manufacture of fermented sausages after drying fermentation. The saltiness was more intense across the treatment groups than in the control group. The microorganisms in fermented sausages grow rapidly when temperature, nutrient, and moisture conditions are adequate (Chen et al., 2018). Here, a large quantity of water was required for the rapid increase in microorganism levels, and as the count of aerobic bacteria was higher across the treatment groups than in the control group in this study, a greater amount of water is presumed to have been consumed in the process of fermentation. According to Zhang et al. (2017), the lowest moisture content is shown by fermented sausages with the highest total bacterial counts, which is in agreement with the results of the present study. The decrease in moisture content is one of the factors that can decrease the salinity of fermented sausages (Tian et al., 2020). When NaCl dissolves in water to dissociate into Na+ and Cl−, the migration of Na+ through the ion channels elicits depolarization, causing saltiness (Wang et al., 2021). The intense saltiness across the treatment groups is, thus, presumed to have arisen from the lower content of water than that in the control group. The umami flavor was more intense in the GD and ND treatment groups than in other treatment groups and the control group. Umami intensity is measured based on the contents of nucleotides: MSG, inosine monophosphate, guanosine monophosphate, adenosine monophosphate, and xanthosine monophosphate (Phat et al., 2016). A high level of umami has been reported to increase consumer acceptability as well as improve the flavor of meat products (Dermiki et al., 2013). Thus, the GD, ND, GS, GDS, NS, GND, and NDS treatment groups showing a higher value for umami flavor than the control group are likely to be suitable for the manufacture of fermented sausages.
Conclusion
This study was conducted to determine the microbial composition and sensory characteristics of the fermented sausages produced with the addition of microbial strains isolated from Korean fermented foods. The GK1 and D1 exhibited high adaptability compared to NK3 and S8, respectively. The result of electronic nose analysis showed that the contribution of GK1 was greater than that of NK3 with respect to flavor creation in fermented sausages. An abundance of compounds responsible for the “fermented” flavor; 4-methylpentanol, 2-furanmethanol, and propyl nonanoate, were detected in the GD treatment group. The results of electronic tongue analysis showed that the value of umami flavor was higher in the GD and ND treatment groups than in the control group. Therefore, for the addition of microbial strains isolated from fermented foods in the manufacture of fermented sausages, mixing GK1 and D1 is predicted to allow the substitution of imported starter cultures.













