Introduction
Lactobacilli have a long history of applications in fermented foods, beverages and milk production due to their safety property (Gaspar et al., 2013). These bacteria are frequently found in nature such as; animals, plants and considered as the commensal microbiota in humans (Corbo et al., 2017; Garcia-Hernandez et al., 2016). Up to present, some strains/species of lactic acid bacteria (LAB) have been proven as safe for applications in pharmacological, food industry and animal production areas. In the pharmaceutical application, the LAB strains have been used for probiotic products (live micro-organisms) production for human consumption aiming to improve the nutritional and microbiological balance of the gastrointestinal tract (Penner et al., 2005), or to prevent and treat cases of acute infectious diarrhoea, antibiotic-associated diarrhea and Clostridiumdifficile-associated diarrhea etc. (Domingo, 2017; Szajewska et al., 2011).
Among the meat products, fermented sausages are well known as the popular meat products which play important role in many diets and are highly appreciated by consumers. In the fermented sausages production, the presences of LAB strains play some central roles in intensifying rapid acidification through the production of organic acids, developing flavor and taste, and improving the biological safety of the products (Leroy and Vuyst, 2004; Leroy et al., 2006). Nowadays, thanks to such beneficial roles, some species of LAB have used for production of a wide ranges of fermented meat products as starter cultures (Ba et al., 2016; Leroy and Vuyst, 2004; Sun et al., 2017).
To date, a number of LAB species (e.g., L. plantarum, fermentum and curvatus etc.) have been isolated from some fermented foods (Corbo et al., 2017). Amongst, the L. plantarum has also been evaluated for its applicability as a starter culture in several food fermentations such as cheese (Songisepp et al., 2012) and fermented sausages (Sun et al., 2016). Although, there have been several studies focusing on evaluation of the effects of L. plantarum inoculation on the quality of fermented meat products (Sun et al., 2017; Yoo et al., 2014), however, how the fermenting conditions (e.g., temperature) or at which fermenting temperature condition the inoculated L. platarum could better improve the technological and eating qualities of the products were not studied.
On the other hand, apart from their advantages, some LAB species have also been reported to produce some toxic biogenic amines such as histamine (Bover-Cid and Holzapfel, 1999) which are harmful to human’s health (Stratton et al., 1991) when consuming foods containing high amounts of the amines. The roles of LAB in the formation of biogenic amines are known as their ability to decarboxylate amino acids to form biogenic amines (Fadda et al., 2010). Prior to the application, therefore, it is needed to determine whether the LAB strain (s) is the biogenic amines producer.
From such considerably beneficial effects of the LAB as mentioned above, therefore, the isolation, identification of new LAB species and then evaluating their applicability in the food industry as probiotic LAB strain/starter cultures are necessary. In the present work, the L. plantarum isolated from a naturally-fermented meat was used for producing fermented sausages as a starter culture. The objective of the work was to evaluate how the temperatures (20, 25 and 30°C) affecting the fermenting activity, quality traits, lipid oxidation and biogenic amines accumulation of fermented sausages inoculated with the tested L. plantarum. The final aim was to find out the optimum fermenting temperatures for the probiotic L. plantarum and using them for production of high quality fermented sausages as a starter culture.
Materials and Methods
The L. plantarum was isolated from a naturally-fermented meat (Seong et al., 2016). The culture, isolation, antimicrobial susceptibility tests of the bacteria were done following our protocol as shown in previous study (Ba et al., 2017a). Briefly, the colonies on the agar media were taken and applied for the 16S rDNA sequence analysis. Each colony was suspended in 60 μL of TE buffer (10 mM Tris-HCl and 1 mM EDTA, pH 8.0), boiled at 99°C for 10 min and DNA template was obtained following centrifugation at 8,200×g for 10 min. The universal primer: 27f (5'-AGT TTG ATC CTG GCT CAG-3') and 1490r (5'-GTT ACC TTG TTA CGA CTT C-3') was used to amplify the 16S rDNA fragment. PCR amplification was carried using the following conditions as described in our previous work (Ba et al., 2017a). After being purified, the DNA was sequenced using ABI-Prism Big Dye Determinator Cycle Sequencing Ready Reaction kit and ABI-Prism 377 Sequencer (Applied Biosystems Inc). Homology searches of the 16S rRNA sequences were performed at DDBJ (http://www.ddbj.nig.ac.jp) with Blast analysis program.
Following the first 16S rRNA sequencing, the whole DNA of the bacteria was isolated and then subjected to the genomic sequencing using a denovo Assembly Analysis method as described in our previous study (Ham et al., 2013). The procedures for the denovo Assembly Analysis method are summarized in Fig. 1A. Finally, the bacteria were identified as L. plantarum and then registered at the Korean Agriculture Culture Collection (KACC) as L. plantarum KACC 92189.
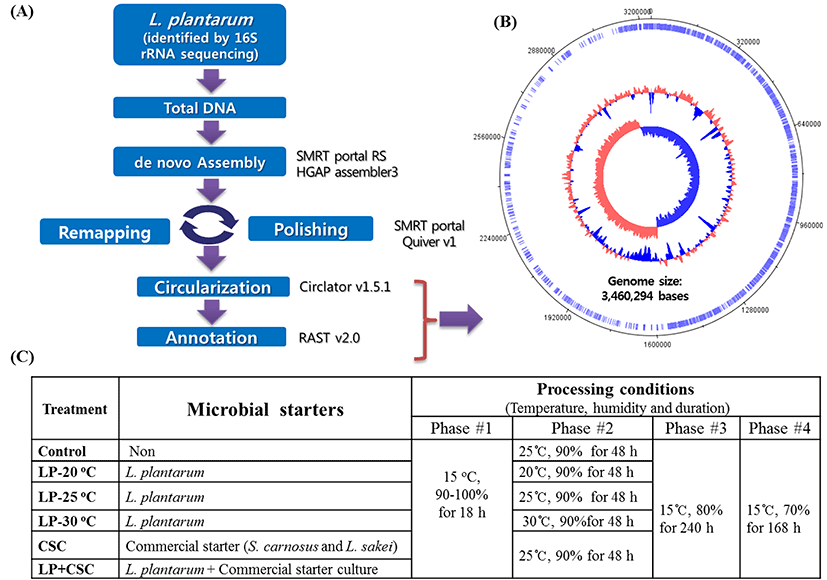
The L. plantarum was cultured in 500 mL de Man, Rogosa and Sharpe broth and allowed to grow at 30°C for 24 h. The bacteria were collected by centrifuging at 10,000 g for 10 min, and the pellets were washed with 0.85% (w/v) saline solution. The number of washed cells were estimated by using a spectrophotometer and then immediately used for production of fermented sausages.
In order to determine the effect of fermenting temperature on applicability of L. plantarum as starter culture, 6 different treatments of fermented sausage: 3 inoculated with L. plantarum at three different temperatures (20, 25 and 30°C), and other 3 treatments (1 with commercial starter culture, 1 with its mixture with L. plantarum and 1 non-inoculated control) under the same processing conditions were prepared. All treatment batches (about 10 kg each) were prepared with 80% pork ham and 20% pork fat, 2% NaCl, 1.5% sugar, 0.2% black pepper, 0.2% polyphosphate, 0.2% sodium ascorbate, 0.01% sodium nitrite and 0.005% sodium nitrate. To determine the effects of fermenting temperature on the quality of products added with L. plantarum, the test L. plantarum at number of 105 CFU/g meat batters was added to each treatment. The number of L. plantarum used was referred to those used in our previous study (Ba et al., 2017a) and previous examinations (data not shown). The batch added with 0.02% (w/w) commercial starter culture containing Staphylococcus carnosus and Lactobacillus sakei was served as the positive control. Another batch was also made with a mixture of L. plantarum (105 CFU/g meat batter) and 0.02% (w/w) of above commercial starter culture.
The processing procedures and conditions (humidity and temperatures) were the same for all treatments as described in our previous work (Ba et al., 2016), except the fermenting temperature used in the treatments with L. plantarum alone which were carried out at either 20 or 25 and 30°C as shown in Fig. 1B. The present study, the fermenting temperature of 25°C was considered as the standard fermenting temperature because it was standardized in our previous works. At the end of the ripening/drying (20th day), the samples were collected and used for analyses.
The pH values of samples during processing were measured in triplicates using a pH meter (Model 340, Mettler-Toledo GmbH). Water activity (aw) was determined using a water activity measuring instrument (Model AW SPRINT-TH 300, Novasina Co.) The procedures used for determinations of pH and (aw) were the same as described in our previous work (Ba et al., 2016).
*: Mean ± standard error.
Means with different letters (a-c) within each parameter in the same row are significantly different (p<0.05).
Means with different letters (A-E) within a column are significantly different (p<0.05).
C: Control (without microbial starter culture) fermented at 25°C.
LP: Inoculated with L. plantarum and fermented at 20, 25, and 30°C.
CSC: Inoculated with commercial starter culture fermented at 25°C.
LP + CSC: Inoculated with a mixture of L. plantarum and commercial starter culture fermented at 25°C.
The proximate compositions (protein, fat and moisture) were analyzed using a Food ScanTM Lab 78810 (Foss Tecator Co., Ltd, Denmark), as described in our previous work (Seong et al., 2016).
The instrumental color was determined at 3 defined areas on the freshly cut surface of each sample using a Minolta Chroma Meter CR-400 (Minolta Camera Co., Ltd, Japan). Color was expressed according to the Commission International de l’Eclairage (CIE) system and reported as CIE L*(lightness), CIE a*(redness), CIE b*(yellowness), chroma and hue angle (h°). In which the chroma and hue angle were calculated as (a*2+b*2)0.5 and tan-1 (b*/a*), respectively.
The TPA was done using a puncture probe (7 mm diameter) attached to a texture Analyzer (Model 4465, Instron Corp. USA). For texture analysis, the samples from each treatment was cut into 2.5-cm long pieces; the cube was axially compressed twice until reaching each time 80% of its initial height. The speed of load cell was set at 120 mm/min and the following parameters were calculated: hardness (kg), cohesiveness (kg*mm), gumminess (kg) and chewiness (kg*mm).
The total LAB and aerobic plate count (APC) were determined during processing (0, 4, 14 and 20th day). The LAB and APC cultured on Man Rogosa Sharpe (MRS) agar and 3M Petrifilm Aerobic Count Plate (3M Health Care, USA), respectively as described in our previous work (Ba et al., 2017a). Each sample was done in duplicates and total count was expressed as log numbers of colony forming units/gram (CFU/g).
The TBARS content was determined to evaluate the lipid oxidation level in samples using the protocol of Pikul et al. (1989) as described in our previous work (Ba et al., 2016). The TBARS values were expressed as mg malonaldehyde/kg (MAD/kg) of sample. Three repetitions were applied for each sample in each treatment.
Biogenic amines were determined using the protocol as described in detail in our previous study (Ba et al., 2016). The samples were derivatized by dansyl chloride (5-dimethylaminonaphthalene-1-sulfonyl chloride, DCl) and then separated onto an Exlipse XDB-C8 column (150 mm × 4.6 mm × 5 μm particle size, Supelco) connected to a high performance liquid chromatography (HPLC Agilent 1100, USA). The separated amines were identified by comparison of the retention times of known standards. The concentrations of the identified amines were expressed in mg/kg of sample.
Ten randomly-selected sausages from each production batch were used. The panelists used were the members of Animals Products Development Division, National Institute of Animal Science, and they were chosen on the basis of previous experiences in sensory evaluation of fermented meat products. The sensory samples were prepared as described in by Ba et al. (2017a). Briefly, six 0.3-cm thick pieces were taken from each sausage sample, then placed onto dishes and coded with random numbers. The sensory samples were randomly allotted into sessions; each session had 6 panelists and each panelist evaluated 6 samples. The panelists were laid to seat in private seats under fluorescent lighting and were served with the sensory samples in a random manner. The panelists evaluated 4 major sensory traits including color, odor, taste and overall acceptability for each samples and rated using a 7-point scale (1 point=extremely undesirable, 7 point=extremely desirable).
The obtained data was statistically analyzed using a Statistic Analysis System (SAS) package (SAS Institute, USA). The data were analyzed by using the General Linear Model procedure considering treatment as the main effect. The differences between means were compared by using Duncan's Multiple Range Test, and significance was defined at p<0.05.
Results and Discussion
In the present work, the L. plantarum isolated from a naturally-fermented meat was firstly identified using the 16S rDNA sequencing and then again identified using the denovo Assembly Analysis, so that the genome of the bacteria was sequenced and a total of 3,460,294 bases were found as shown in Fig. 1C. After the identifications were completed the strain was cultivated and prepared for fermented sausages production as a starter culture. The fermentation temperatures tested in the present work were based on those recommended for the commercial L. plantarum starter culture (Starterkulturen Almi Rohschinken from Almi GmbH & Co., Germany).
The changes in pH and number of LAB during fermenting/ripening process are presented in Table 2. Regarding the pH, our results showed that its values varied among the treatments and depending on the stages (fermenting/ripening) examined. In most treatment batches, the pH values significantly decreased from the initial values (6.04-6.08) at 0 day to reached 4.40-5.27 after 12 days fermentation, and these values tended to increase with increased ripening time. In general, the control presented the highest pH values in comparison with the other treatments on all the stages examined. Regarding the temperature effects on the acidification activity within the treatments inoculated with L. plantarum, there were statistical differences in which the pH declining rate was faster as increasing the temperature up to 30°C. For instance, after 4 days the samples fermented at 30°C reduced pH by 1.57 units, followed by the samples at 25°C (by 1.30 units) and the slowest was found in samples fermented at 20°C (0.99 unit). In general, under the same fermenting temperature (25°C) the pH declining rate in samples made with L. plantarum (at 25°C) was almost equal to the ones made with commercial starter culture. However, in comparison to the pH declining rate (by 1.25 units) in treatment with commercial starter culture (at 25°C) after 12 days, the samples inoculated with L. plantarum fermented at 30°C showed higher rate (by 1.45 units). At the end of ripening (day 20th), among the three treatments with L. plantarum the samples fermented at 25 and 30°C significantly increased whereas the ones fermented at 20°C continuously decreased in their pH values. These results signify that the fermentation temperature largely affected the acidification activity of the L. plantarum in the products.
*: Mean ± standard error.
Means with different letters (a-c) within each parameter in the same column are significantly different (p<0.05).
Means with different letters (A-D) within a row are significantly different (p<0.05).
C: Control (without microbial starter culture) fermented at 25°C.
LP: Inoculated with L. plantarum and fermented at 20, 25, and 30°C.
CSC: Inoculated with commercial starter culture fermented at 25°C.
LP + CSC: Inoculated with a mixture of L. plantarum and commercial starter culture fermented at 25°C.
The pH decline could be attributed to the accumulation of organic acids, such as lactic acid etc. in the products as a result of the fermentation activity by the LAB (Zaho et al., 2011). Whereas, the increase in pH after 12 days of ripening could be due to the decrease in number of LAB or the increase in microorganism’s proteolytic activity resulting in production of free peptides and amino acids which have buffering effects on organic acids (Essid and Hassouna, 2013). Our findings are in agreement with those reported in previous studies which showed lower pH values in fermented sausages inoculated with starter cultures (Ba et al., 2016; Dominguez et al., 2016; Lorenzo et al., 2016). These results also support the previous findings of Tabanelli et al. (2012) and Essid and Hassouna (2013), who reported that the pH values of fermented sausages increased as increasing the ripening time.
Regarding the total LAB count, a similar trend as those of pH was observed in the samples during the fermenting/ripening process, in which the total LAB continuously increased from 0 day to 12th day of fermentation/ripening and decreased thereafter for all the treatments. At the 12th day, the total LAB counts were in the following order: L. plantarum (30°C) > L. plantarum + commercial starter culture (25°C) > L. plantarum (20°C) > Commercial starter culture (25°C) > Control (25°C) > L. plantarum (25°C), being their mean values of 10.41, 10.16, 9.68, 8.77, 8.65 and 8.60 log CFU/g, respectively. This order indicates that the samples added with the L. plantarum (at 30°C) presented the significantly higher LAB count in comparison with the control or commercial starter culture treatment. The rapid growth of LAB during the first days of fermentation could be responsible for the quick decline in the pH values in the products as described above. Also, among the treatments made with L. plantarum the samples fermented at 30°C showed their higher LAB count than those fermented at lower temperatures (20 or 25°C), suggesting that the fermenting temperature affected the growth rate of LAB. Furthermore, comparing to the number of LAB (8.13 log CFU/g after 14 days) reported for the sausages inoculated with 7 log CFU/g of L. plantarum (Essid et al., 2013), all the samples made with L. plantarum at all the temperatures in the present study presented much higher LAB count after 12 days of ripening. Our LAB results might suggest that the added L. plantarum strain could adapt and grow well in the meat mixtures during fermenting and ripening process.
Table 2 presents weight loss, water activity (aw), TBARS and TVBN contents in the samples. After 20 days of ripening, the weight loss levels ranged among the treatments from 48.31% to 49.397% and the fermenting temperature did not affect the loss levels in samples added with L. plantarum. The aw-values ranged among the treatments from 0.82 to 0.85, and the values in all treatments were not significantly different from the control, except the treatment with L. plantarum (fermented at 20°C). The higher aw-values in the control samples could be related to their significantly higher moisture content (Table 3). It is well recognized that the shelf-life stability of a food product is usually depended on the water activity; low aw can increase the shelf-life stability. Ba et al. (2017a) and Ba et al. (2017b) reported higher aw-values (0.85-0.88) for the same product type; this could be attributed to higher moisture contents in samples in these studies.
*: Mean ± standard error.
Means with different letters (a-c) within a row are significantly different (p<0.05).
C: Control (without microbial starter culture) fermented at 25°C.
LP: Added with L. plantarum and fermented at 20, 25, and 30°C.
CSC: Added with commercial starter culture fermented at 25°C.
LP + CSC: Added with a mixture of L. plantarum and commercial starter culture fermented at 25°C.
The lipid oxidation is associated with quality deterioration and health risks (Grun et al., 2006). At the end of ripening (20th day), the TBARS content significantly differed among the treatments, in the following order: L. plantarum + commercial starter culture (25°C) > Commercial starter culture (25°C) > L. plantarum (20°C) > L. plantarum (25°C) > L. plantarum (30°C) > Control (25°C), being their mean values 2.39, 1.85, 1.62, 1.00, 0.53 and 0.28 mg MDA/kg sample, respectively. This means that the treatment with commercial starter culture presented the highest lipid oxidation level, followed by those made with L. plantarum whereas the control had the lowest level. In fact, previous studies have also found that the inoculation with starter cultures (e.g. LAB or Enterococci) resulted in increased lipid oxidation in fermented sausages (Ba et al., 2016; Ba et al., 2017a). This phenomenon could be attributed to the increased lipolysis activity by the microbial lipase in these inoculated batches, resulting in generation of free fatty acids which are easy to be oxidized under the processing condition. Our TBARS results were in contrast with those reported by Sun et al. (2017), who found that the samples inoculated with starter cultures had lower TBARS values than the non-inoculated control. Interestingly, we observed that the fermenting temperature significantly affected the lipid oxidation levels, as increasing the fermenting temperature from 20 to 30°C, the lipid oxidation levels tended to decrease in the samples made with L. plantarum. The present study, for the first time the effect of fermenting temperatures on lipid oxidation in fermented sausages was investigated. The reason why increasing the fermenting temperature resulted in the decreased lipid oxidation in the products is remained unknown and further study may be needed to elucidate the phenomenon.
TVBN content is composed of ammonia, hydrogen sulfide and ethyl mercaptan etc. which are formed from the decomposition/degradation of proteins by spoiled bacteria or endogenous enzymes (Huang et al., 2014). The TVBN therefore is usually used as an important indicator indicating the freshness of raw meats or shelf-life and microbial quality of meat processed products. Our results depict that the TVBN contents varied among the treatments, ranging from 0.49 mg% to 0.71 mg%. The TVBN content in the present study was much lower compared to levels (20-25 mg%) reported for same product type at 25th day of ripening (Rai et al., 2010). Although the products made with L. plantarum increased in the TBNV contents as increasing fermenting temperature, however, all of these treatments at all fermenting temperatures presented significantly lower levels in comparison with the other remaining treatments or control. The results could be due to the capacity of TVBN content neutralization by organic acids (e.g. lactic acid) or bacteriocin produced from the inoculated bacteria (Yin et al., 2002).
APC is generally used as an indicator reflecting the microbiological quality and self-life stability of meat products during processing and storage. Our results showed that the APC showed significant differences among the treatments on all days examined, and the increasing or decreasing rate of the APC also differed depending on the treatments. For instance; the control presented the highest APC on all days examined, and it also showed the highest increasing rate (by 4.37 log CFU/g) followed by the treatments with L. plantarum (by 0.78-1.74 log CFU/g) or commercial starter culture (0.26 log CFU/g) after 4 days of fermentation. Our results are in agreement with findings of Sun et al. (2016), who also reported that APC in fermented sausages made with L. plantarum significantly increased with increased fermenting time after 9 days of fermentation. These authors, however, reported higher APC (7-8 log CFU/g) in comparison to the level (6.15-6.87 log CFU/g) in the 12 days-fermented samples in our work, and this may be related to the higher initial microbial load in samples in their study. Additionally, the control rapidly reached a maximum APC (8.26 after log CFU/g) after 4 days and tended to decrease after 12 days of fermentation, however, the APC in these samples still remained at the highest levels in comparison with the inoculated treatments.
At the end of ripening (20th day), the APC were in the following order: Control (25°C) > Commercial starter culture (25°C) > L. plantarum + commercial starter culture (25°C) > L. plantarum (25°C) > L. plantarum (20°C) > L. plantarum (30°C), being their mean values 7.91, 7.75, 7.06, 5.94, 5.86 and 5.15 log CFU/g sample, respectively. This order signifies that all the treatments with L. plantarum had lower APC than those made with commercial starter culture as well as control. The APC in all samples made with L. plantarum at the end of ripening was similar to those (6.54 log CFU/g) reported for the same product type made with mushroom extract in literature (Ba et al., 2017b), but much lower than the level (above 8 log CFU/g) in samples ripened for 28 days (Chaves-Lopez et al., 2015). It is also noted that among the batches made with L. plantarum the increase of fermenting temperature up to 30°C resulted in lower APC (5.15 log CFU/g) as compared with those fermented at lower temperature (20-25°C), this could be attributed to the synergic effects of acidic environmental effects, low water activity and bacteriocins produced by this starter culture. Regarding this, previous study has indicated that some LAB strains have been found to produce bacteriocins (approximately 400-800 activity units/mL) which possess antimicrobial activity (Woraprayote et al., 2015).
The color, textural traits and chemical composition of fermented sausages measured at 20th day of ripening are presented in Table 3. The treatments added with L. plantarum presented L* (lightness), a* (redness) and b* (yellowness)-values of 40.75-43.67, 112.46-13.56 and 8.01-9.00, respectively. Among the treatments added with L. plantarum, samples fermented at lower temperature (20°C) had slightly lower L*-values, however, no statistical differences in a* and b*-values occurred between the temperature groups. The samples made with L. plantarum at all fermenting temperatures had a*-values similar to those in samples made with commercial starter culture but higher the value (11.69) in the control. Furthermore, all the values for the color traits obtained on the samples added with L. plantarum at all temperatures were comparable to those reported for the same product type inoculated with other commercial starter cultures (Ba et al., 2016). From the obtained results it might be said that the inoculation with L. plantarum resulted in products which had reddish degree similar to the ones made with commercial starter cultures, suggesting the nitrate to nitrite reduction capacity by the inoculated L. plantarum.
1)Mean ± standard error.
Means with different letters (a-c) within a row are significantly different (p<0.05).
C: Control (without microbial starter culture) fermented at 25°C.
LP: Added with L. plantarum and fermented at 20, 25, and 30°C.
CSC: Added with commercial starter culture fermented at 25°C.
LP + CSC: Added with a mixture of L. plantarum and commercial starter culture fermented at 25°C.
Regarding the textural profiles, the fermenting temperature did not affect all the textural traits in the products added with L. plantarum except hardness that was higher in the 20°C-fermented samples. Comparing to the inoculation with commercial starter culture, the addition of L. plantarum resulted in products which had similar cohesiveness, springiness, gumminess and chewiness values but softer. Whereas, the control presented lower hardness, gumminess and chewiness values in comparison with the other remaining treatments. These results could be related to the moisture differences among the treatments (Table 1) or could be due to the differences in biochemical changing levels which affected the water evaporation in the products during drying process.
According the chemical compositions, the total fat content ranged among the treatments from 25.98% to 29.14%, while the moisture and protein levels ranged from 20.40% -25.05% and 48.79-52.23%, respectively. The control had the lowest fat and protein, and had the highest moisture content while the samples made with the mixture of L. plantarum and commercial starter culture presented higher fat and protein contents, this could be due to the differences in moisture content among them. Also, comparing the levels of proximate compositions within the treatments made with L. plantarum at different temperatures (20, 25 and 30°C) shows no statistical differences (p<0.05), except for the protein content that showed lower in samples fermented at 25°C, probably due to its slightly higher moisture content.
It is well recognized that the ingestion of foods containing high amounts of biogenic amines has been reported to cause hazard to consumer’s health (Ruiz-Capillas and Jimenez-Colmenero, 2004). In the present study, cadaverine, an aliphatic diamine is the unique biogenic amine detected in the fermented sausage samples at the end of ripening, and its concentrations are shown in Fig. 2. The reason why the other common amines (e.g., histamine or tyramine) not found in the samples in the present study could be due to their absence or produced levels were too low under the detection limit.
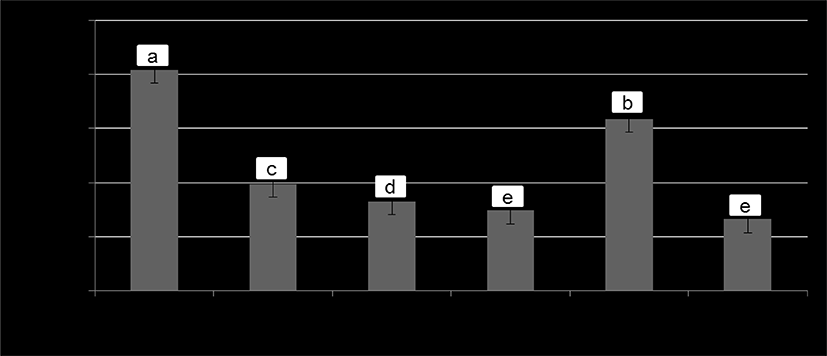
Previous researchers have demonstrated that the addition of starter cultures is an effective way to reduce the biogenic amines production in fermented sausages due to their preventative capacity of the outgrowth of the potential amines-producing bacteria by acidification (Latorre-Moratalla et al., 2007). However, it has also been found that some groups of microorganisms (e.g. Enterococci and LAB strains etc.) are among the biogenic amines producers (Bover-Cida and Holzapfel, 1999). For instance, Tabanelli et al. (2012) have reported a relatively high level (100-250 mg/kg) of cadaverine in Felino-type sausages inoculated with Staphylococcuscarnosus or S. xylosus. Similarly, Ba et al. (2017a) also found a higher level (9.78-30.99 mg/kg) of cadaverine in the same product types inoculated with Enterococcus faecalis. From our obtained results, therefore, it may be said that the L. plantarum is not an amines-producing LAB strain whereas its use could reduce the amines accumulation. Furthermore, among the three treatments with L. plantarum the increase in fermenting temperature resulted in a decreased level of cadaverine, this could be related to the lower number of APC in the samples fermented at higher temperature (Table 2).
Among all the obtained results, those on sensory evaluation might be the most noticeable since a positive perception was received from the panelists for all the samples inoculated with the L. plantarum regardless of fermenting temperature effect. For the treatments with L. plantarum, the fermenting temperature produced an important impact on most sensory traits examined, as the scores for odor, taste and overall acceptability were increased as increasing the fermenting temperature from 20 to 30°C. Particularly, the statistical analysis showed that the samples inoculated with L. plantarum at 30°C presented significantly (p<0.05) higher odor, taste and acceptability scores in comparison with those fermented at lower temperature (e.g., 20°C). These results could be related to the intensified level of proteolysis as increasing fermentation temperature, resulting in elevated production of flavor and taste-active components (e.g., amino acids and peptides etc.) in these samples. Also, comparing the sensory scores among the treatments fermented under the same temperature (25°C) shows statistical differences in which the samples inoculated with L. plantarum had much higher scores for all sensory traits than those with commercial starter culture. These obtained results are in agreement with finding of Sun et al. (2016), who also reported higher odor and taste scores for samples inoculated with the same strain in comparison with samples inoculated with Staphylococcus xylosus. Additionally, when compared to the odor, taste and acceptability scores in the control under the same fermenting temperature (25°C) those made with L. plantarum presented significantly higher values. These results again suggest that the inoculation with L. plantarum produced a better improvement in sensory quality of the product in comparison with the commercial starter culture.
Furthermore, it should be noted that the addition of commercial starter cultures resulted in the fermented sausage products with the lowest acceptance rate by the panelists in comparison with all the other remaining treatments or control. These results may be related to the higher level of lipid oxidation occurred in these samples (as indicated by TBARS values in Table 2). Regarding this issue, it has been reported that the TBARS values above 0.5 mg MDA/kg indicates a level of lipid oxidation products which impart a rancid flavor and undesirable odor that can be detected by consumers (Wood et al., 2008).
In conclusion, the fermenting temperature considerably affected the quality of the L. plantarum - added products. The inoculation with L. plantarum produced importantly beneficial effects on the quality improvement such as; increased acidifying activity and reduced spoilage bacteria count in the products. Also, the addition of Lactobacillus plantarum significantly improved the eating quality especially the taste and odor of the product when comparing to the commercial starter culture. Our results also showed that the Lactobacillus plantarum was negative with biogenic amines production. Based on the results obtained from our investigation it is suggested that the isolated Lactobacillus plantarum could be considered as a probiotic strain to be used as a starter culture, and the fermenting temperature at which the strain showed the greatest quality improvement of fermented sausages was 30°C. Further study is needed to elucidate whether the strain has capacity of bacteriocins production, and determine the levels of flavor-enhancers (e.g. free amino acids and peptides) produced by the strain in the product.













