Introduction
As consumers’ interest in functional food and safety has increased, products that promote health and wellness have developed (Tyopponen et al., 2003). Dairy products fermented by probiotic bacteria are well-known nutritional foods (Saxelin, 2000). Therefore, use of probiotics to fermented meat products could increase health benefits (De Vuyst et al., 2008; Lucke, 2000).
Biogenic amines are of worry in fermented meat products because of their toxicological effects on the blood pressure, intestinal system, and nervous system. Usual symptoms of biogenic amine poisoning, especially tyramine include headache, migraine, and increased blood pressure (Ladero et al., 2010). In addition, some studies were performed to evaluate the effects of the cytotoxicity of individual compounds on the in vitro mutagenicity and toxicity of biogenic amines (Badolo et al., 1998). Thus, excessive intake of these biogenic amines may cause health problems (Ten Brink et al., 1990). In addition to their toxicity, some biogenic amines can react with nitrite in meat products to form potentially harmful nitrosamines (Santos, 1996). These problems can be more serious for consumers with reduced monoamine and diamine oxidase activities; these enzymes are responsible for biogenic amine detoxification (Bardocz, 1995).
Vitamins C and E are representative natural antioxidants. These antioxidants can decrease the risk of various diseases, including cancer (Decker et al., 2000). Mitsumoto et al. (1991) reported that ascorbic acid scavenges oxygen and inhibits radical formation at double bonds. α-Tocopherol (vitamin E) is also an crucial antioxidant that can retard color changes and lipid oxidation in animal fats and meat products (Dziezak, 1986; Morrissey et al., 1994).
Anti-mutagenicity of L-ascorbic acid and α-tocopherol in sausage samples has been reported by Pourazrang et al. (2002). They reported that addition of L-ascorbic acid (>500 ppm) and α-tocopherol (>250 ppm) to pork sausages decreased mutagenicity to 61% and 54%, respectively. However, to the best of our knowledge, there are no studies on the effects of different starter cultures on mutagenicity in fermented sausages treated with vitamins C and E. Therefore, the objective of this study was to evaluate the effects of six different starter cultures on mutagenicity and biogenic amine contents in fermented sausages treated with vitamins C and E.
Materials and Methods
Six different types of fermented sausages were prepared (Kim and Hur, 2017). Fresh pork back-fat and ham were purchased from whole-sale meat market (Jeonju, Korea). The sausage was manufactured with pork ham (84.145%), pork back fat (9.4%), salt (1.0%), sodium erythorbate (0.045%), sodium nitrite (0.01%), pepper mix (2.3%), red pepper sauce (3%), and sugar (0.1%). Trimming, chopping, and additive mixing (CE93, RUHLE GMBH, Grafenhausen, Germany) were performed. After that, starter cultures were inoculated. According to our previous study (Kim and Hur, 2017), the following starter cultures were selected: T1, Pediococcus acidilactici; T2, Staphylococcus carnosus and P. pentosaceus; T3, P. pentosaceus, Lactobacillus curvatus, Debaryomyces hansenii, S. xylosus, and S. carnosus; T4, L. sakei and S. carnosus; T5, L. plantarum and S. xylosus; and T6, Penicillium nalgiovensis. These starter cultures were purchased from Almi Ges. M.g.H & Co GK (Oftering, Austria). Each starter culture was prepared as instructor’s guideline and inoculated at 104 CFU/g into the sausage batter (the bacteria were suspended in 50 mL of deionized water before inoculation). Subsequently, the sausage batter was stuffed into a pork small intestine casing (Naturin Viscofan Co, Tajonar-Navarra, Spain) and placed in a fermentation chamber (FT 50, Frigomeccania, Parma, Italia). The first fermentation step involved 8 days of fermentation at 4°C. The second fermentation step involved 28 days of fermentation at 10°C. The relative humidity (RH) values of the first and second fermentation steps were 70%–80% and 60%–70%, respectively. After fermentation, the fermented sausages were immersed in the same volume of deionized water, 0.1% vitamin C solution, 0.1% vitamin E solution, and 0.05% vitamin C and E solution (v/v) and then shaken at 4×g and 20°C for 4 h. The fermented sausage samples were stored at 4°C overnight before use.
Before measuring the mutagenicity of the fermented sausages, extraction step was performed (Weisburger et al., 2002). Briefly, 10 mL of methanol was added to 1 g of each fermented sausage sample and homogenized (HG-15D, Labtech, Ltd., Jeonju, Korea) for 1 min at 11,200×g and then centrifuged (Combi 514R, Hanil Scientific Inc., Gimpo, Korea) for 5 min at 10,000×g. Then, a rotary evaporator was used to remove the methanol. Dimethyl sulfoxide (5 mL) was added to residue. Mutagenicity was measured using the Ames test with Salmonella Typhimurium TA98, which is a frameshift mutation strain (Maron and Ames, 1983). S. Typhimurium TA98 strain was purchased from Moltox (Molecular Toxicology Inc., Boone, USA). S. Typhimurium culture (0.1 mL, 1−2×108 cells), each sample (0.1 mL), and maintained overnight, and Aroclor 1254-induced Sprague-Dawley rat liver S9 mix (0.5 mL) were added to 2 mL of top agar containing biotin and histidine (0.05 mM each), and then the mixture was vortexed and poured onto a minimal glucose agar plate (25 mL). After the solidification, the agar plate was incubated for 36–48 h (37°C). The mutagenicity was expressed as the mean number of revertants per agar plate (n=3). 4-Nitro-o-phenylenediamine (2.5 μg/plate) was used as positive control.
A total of 8 biogenic amines—tryptamine, phenylethylamine, putrescine, cadaverine, histamine, tyramine, spermidine, and spermine—were determined using high-performance liquid chromatography (HPLC; HP Agilent 1100; Hewlett Packard Co., Palo Alto, CA, USA) with an Eclipse XDB-C8 column (150 mm×4.6 mm; 5 μm) using a method of Eerola et al. (1993). Internal standard (1,7-Diaminoheptane, 1 mg mL−1, 125 μL) was added to sausage samples (1 g) and homogenized with 5 mL of 0.4 M perchloric acid with homogenizer (HG-15D, Labtech, Ltd., Jeonju, Korea) at 11,200×g. Then the mixture was centrifuged for 5 min at 1,008×g and filtered through Whatman No. 2 filter paper. Extraction procedure was repeated twice, and combined supernatants was adjusted to 20 mL with 0.4 M perchloric acid. Extracted sample (1 mL) was alkalized by adding 200 μL of 2 N NaOH, and buffered by adding 300 μL of saturated sodium bicarbonate. Dansyl chloride solution (2 mL, 10 mg mL−1) was added and incubated at 40°C for 45 min. An 100 μL of ammonia solution (10%) was added to remove residual dansyl chloride. After 30 min, 5 mL of acetonitrile was added to the mixture and centrifuged 5 min at 700×g, and the supernatant was filtered through 0.25 μm PTFE membrane filter, before HPLC injection. The mobile phase consisted of acetonitrile (solvent A) and water (solvent B). The gradient began at 50% and ended at 90% of A in 20 min. The volume of the injected sample and the flow rate were 20 μL and 1.0 mL/min, respectively. The detection wavelength was set at 254 nm. The retention times of tryptamine, phenylethylamine, putrescine, cadaverine, histamine, tyramine, spermidine, and spermine were 8.07, 9.42, 9.96, 10.63, 10.90, 14.19, 15.08, and 18.80 min, respectively.
The statistical analysis was performed for changes in the mutagenicity and biogenic amine concentrations in the fermented sausages using two-way analysis of variance (ANOVA) with IBM SPSS 22.0 software (Chicago, USA) as factors for six combinations of starter cultures (T1, T2, T3, T4, T5, and T6) and vitamin treatments (vitamin C, vitamin E, and vitamins C and E). All experiments were performed in triplicate. The Student–Newman–Keuls multiple range test was used to compare the mean values. Significant differences (p<0.05) between the mean values of the mutagenicity and biogenic amine concentrations were calculated.
Results
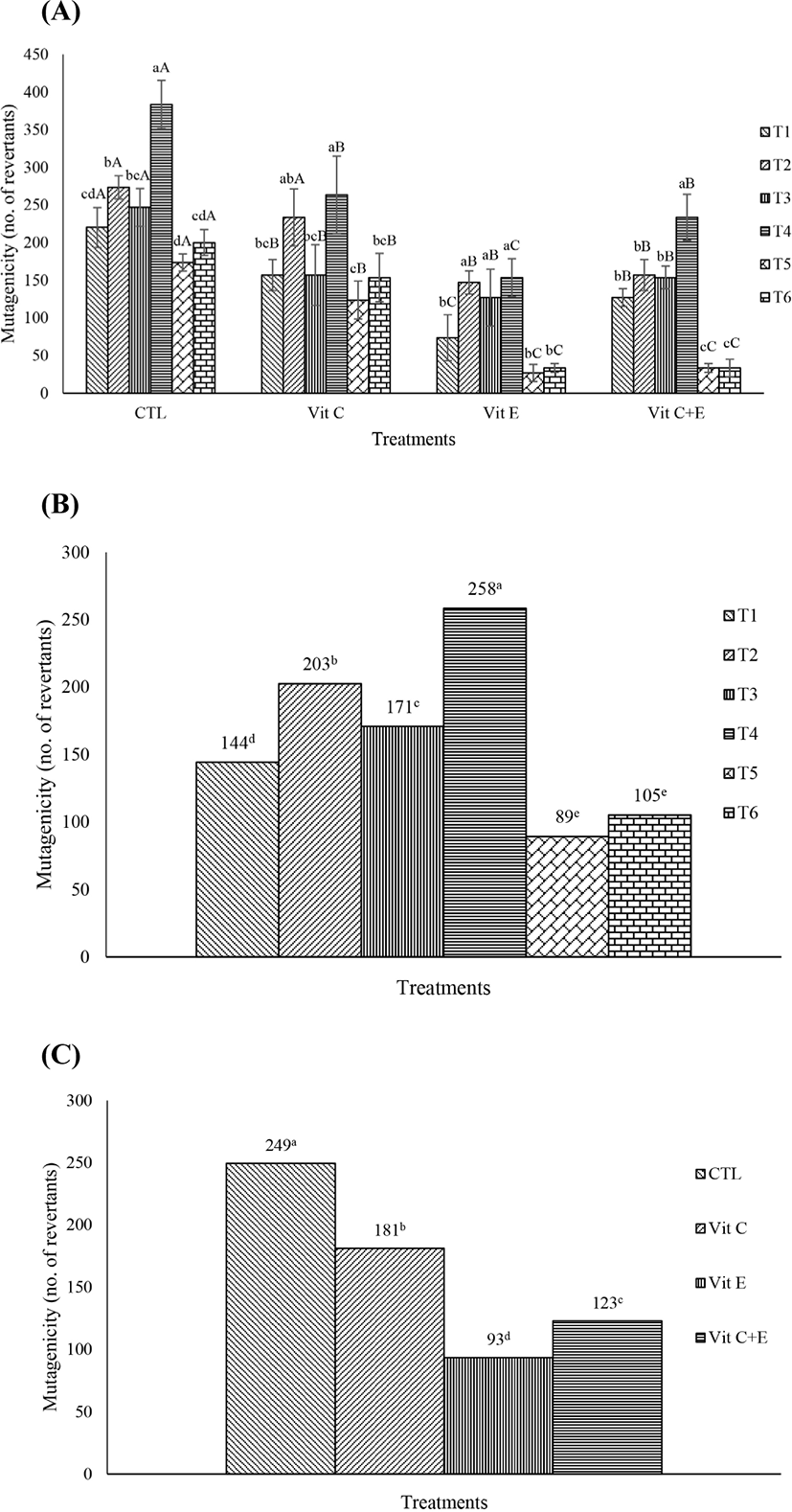
The mutagenicity of the 6 different types of fermented sausages is shown in Fig. 1A–C. The mean values of mutagenicity for the 6 fermented sausages in the control group were 220, 273, 247, 383, 173, and 200. Among the treatments, T4, sausages fermented with S. carnosus and L. sakei starter cultures, showed the highest mutagenicity, and T5, sausages fermented with S. xylosus and L. plantarum starter cultures, showed the lowest mutagenicity (Fig. 1B). However, their mutagenicity values were decreased by the addition of vitamin C and/or E (p<0.05), regardless of the starter cultures. In particular, vitamin E reduced mutagenicity more effectively than vitamin C (Fig. 1C; p<0.05).
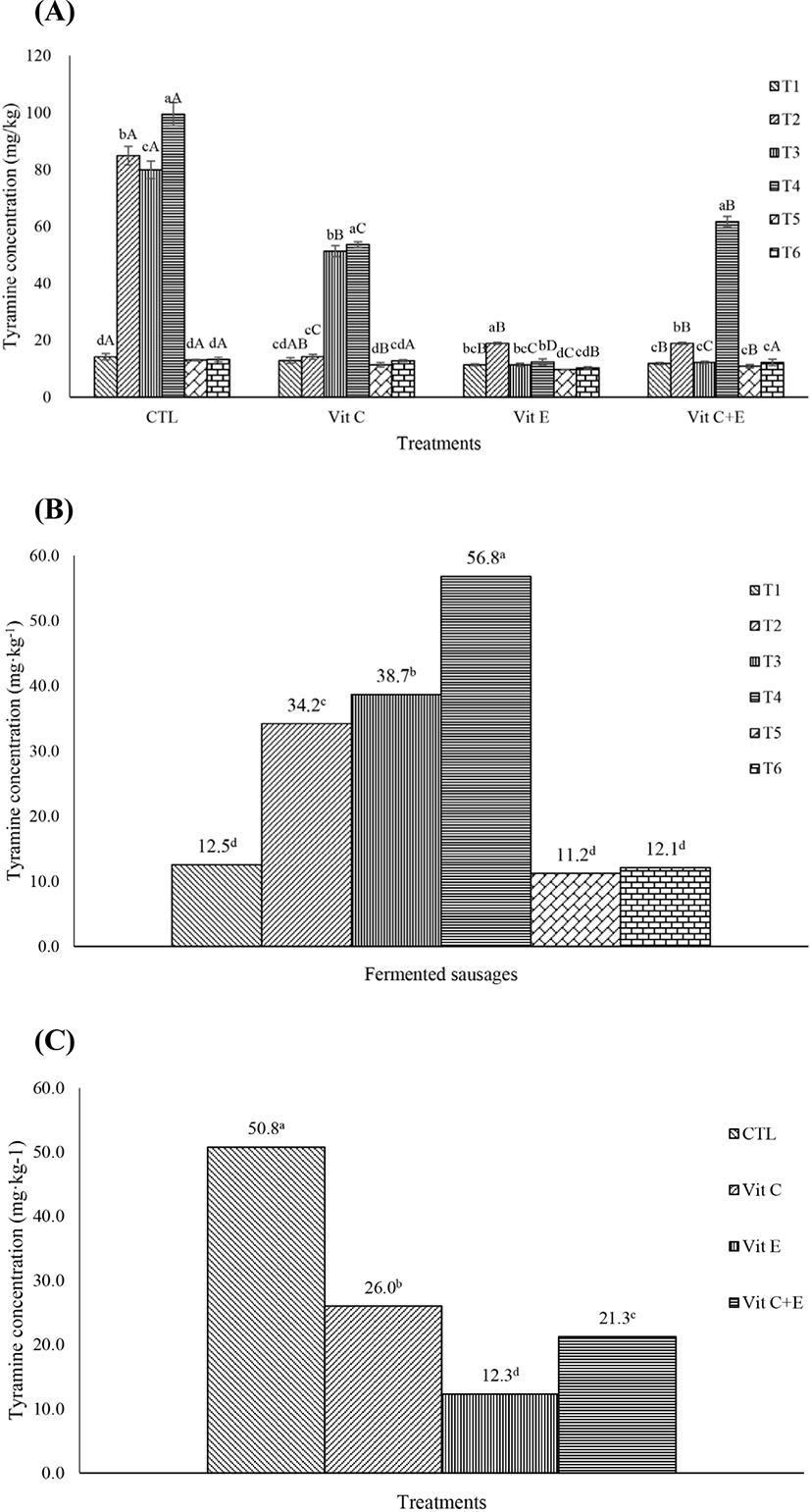
The levels of biogenic amines are shown in Fig. 2A–C. In this study, tyramine was the only biogenic amine found in the fermented sausages. The mean levels of tyramine in the 6 different fermented sausages were 14.1, 84.9, 79.9, 99.5, 13.0, and 13.2 mg kg−1. T4, sausages fermented with L. sakei and S. carnosus starter cultures, showed the highest biogenic amine concentration, and T1, T5, and T6, sausages fermented with P. acidilactici, S. xylosus and L. plantarum, and P. nalgiovensis starter cultures, respectively, showed the lowest biogenic amine concentration (Fig. 1B; p<0.05). The concentrations of biogenic amines in the six different fermented sausages were decreased by the addition of vitamin C and/or E (p<0.05). Similar to the mutagenicity results, addition of vitamin E effectively reduced the concentration of biogenic amines (Fig. 2C; p<0.05).
The correlations with factors that affect mutagenicity are listed in Table 1. Among the factors, starter cultures showed the highest correlation (r=0.81) with mutagenicity. In contrast, biogenic amine (r=0.54), vitamin C (r=0.22), and vitamin E (r=0.60) showed relatively low correlations. In addition, biogenic amines showed high correlations with starter cultures (r=0.85) and mutagenicity (r=0.54), whereas the effects of vitamin C (r=0.15) and vitamin E (r=0.34) on the concentration of biogenic amines were relatively low.
| Mutagenicity | BA | Starter cultures | Vitamin C | Vitamin E | |
|---|---|---|---|---|---|
| Mutagenicity | 1 | ||||
| BA | 0.54 | 1 | |||
| Starter cultures | 0.81 | 0.85 | 1 | ||
| Vitamin C | 0.22 | 0.15 | - | 1 | |
| Vitamin E | 0.60 | 0.34 | - | - | 1 |
Among the treatments, T4, which had the highest mutagenicity, showed the highest biogenic amine concentration, however the other treatments did not showed similar patterns after the vitamin C and/or E treatments. Therefore, the correlation between mutagenicity and biogenic amine content was not high (r=0.54).
Discussion
The use of appropriate starter cultures for the production of fermented sausages is necessary to control mutagenicity and biogenic amine concentrations. Some starter cultures has antimutagenic effect (Lankaputhra and Shar, 1998) by desmutagen factor (Hosono et al., 1987). Gram-positive and -negative bacteria have antimutagenicity by binding mutagens to their peptidoglycan layer and outer layer, respectively (Zhang and Ohta, 1991). In the case of biogenic amines, it is naturally produced compounds in the process of decarboxylation of the amino acid components in fermented foods (Ten Brink et al., 1990). Factors that control the formation of biogenic amines in fermented sausage include the use of amine-oxidizing or non-amine-forming starter cultures (Nieto-Arribas et al., 2009), control of pH (Hu et al., 2007) and temperature (Shalaby, 1996), however inhibiting decarboxylase activity is the most crucial factor (Wendakoon and Sakaguchi, 1995). Therefore, the use of amine-negative starter cultures can reduce the accumulation of biogenic amines (Bover-Cid et al., 2000). In this study, the six different combination of starter cultures may be affected the antimutagenicity and formation of biogenic amine by various factors, such as rates of Gram-positive and –negative bacteria, amine-oxidizing starter culture, pH, temperature, and inhibition of decarboxylase activity.
In previous studies, several vitamins, including vitamins C and E, have been shown to possess antimutagenic activities (Ramel et al., 1986). Vitamins C and E are known to be radical scavengers and strong antioxidants (Ramel et al., 1986). Inhibition of the mutation caused by oxygen radicals is possible with vitamins C and E. Vitamin C reduced the mutagenicity of activated aflatoxin B1 in S. Typhimurium TA100 more than 50% (Bhattacharya et al., 1987) by prevention of its metabolic activation (Firozi et al., 1986). Raina and Gurtoo (1985) reported that vitamin C reduced mutagenicity. Vitamin C showed antimutagenicity of sodium nitrite and spermidine mixture in the Ames test (Kokatnur et al., 1978). Experiments performed using bacterial cells showed vitamin E activity opposition to aflatoxin B1 mutagenicity in the TA100 and TA98 strains (Raina and Gurtoo, 1985), and antimutagenic activity was discovered with high levels of vitamin E. Shamberger et al. (1979) reported that vitamin E inhibited mutagenicity by the direct-acting mutagen malondialdehyde, which is produced during lipid oxidation, in a number of S. Typhimurium strains. Possibly, vitamin E prevents the conversion of promutagens into mutagens by inactivation of the free oxy radicals (Colin et al., 1991). Thus, antioxidant activity, including radical scavenging activity of vitamins C and E, may be the major mechanism for decreasing mutagenicity in fermented sausages. In particular, the use of vitamin E, a lipophilic antioxidant, was more effective in lowering mutagenicity than that of vitamin C, a hydrophilic antioxidant.
Formation of biogenic amine by the microorganisms is dependent on the availability of the amino acid substrate, level of decarboxylase activity, and specific bacterial strain(s) present (De Las Rivas et al., 2008; Suzzi and Gardini, 2003). Tyramine is the most abundant biogenic amine in fermented sausages (Coisson et al., 2004; Komprda et al., 2004). Santos (1996) reported that the toxic level of tyramine is 100–800 mg kg−1. Considering the already known harmful effects of biogenic amines (Ladero et al., 2010), it is crucial for the meat industry to produce safe fermented meat products with an appropriate level of biogenic amine. In this study, tyramine was the predominant amine among the biogenic amines and showed correlation with the mutagenicity of the fermented sausages (r=0.54). Badolo et al. (1998) have reported the mutagenicity and in vitro toxicity of biogenic amines, and the effects were analyzed based on the cytotoxicity of individual compounds. Bozkurt and Erkmen (2004) reported that sausages containing food preservatives, such as ascorbic acid (500 ppm) and α-tocopherol (200 ppm) significantly reduced biogenic amine formation. Especially, diaminoxidase activity of vitamins can degrade biogenic amine concentrations (Maintz et al., 2006). Therefore, high concentrations of biogenic amines in fermented meat and meat products may increase mutagenicity, and using antioxidants such as vitamins C and E may reduce the concentrations of biogenic amines and mutagenicity.
Certain starter cultures used in sausage fermentation can also retard the development of biogenic amines (Latorre-Moratalla et al., 2007). The microorganisms possess the decarboxylation ability may be used as starter cultures in fermented sausages (Spicka et al., 2002). S. xylosus and L. curvatus delay cadaverine and putrescine formation during the fermentation and storage of dry fermented sausages (Bover-Cid et al., 2001). S. carnosus, S. xylosus, and L. sakei have also been used during the manufacture of dry sausages and were found to inhibit the accumulation of biogenic amines (Bover-Cid et al., 2001). Combined starter cultures induce a synergistic effect for the reducing biogenic amines (Hu et al., 2007). The use of combined starter cultures results in a pH reduction (Hu et al., 2007), which may be an another factor that contributes to reducing the accumulation of biogenic amines. Due to increased decarboxylase activity of bacteria against acidic pH, biogenic amine concentration can be increased in the low pH condition (Bover-Cid et al., 2001). In this study, pH values of fermented sausages with six different starter cultures were 5.08, 4.92, 5.24, 4.96, 5.07, and 5.66 for T1, T2, T3, T4, T5, and T6, respectively. However, the highest biogenic amine concentration was observed in T4, which showed significantly lower pH than other treatments (p<0.05). This means that although pH may affect the formation of biogenic amines, other factors must also be considered. Lactobacilli are also capable to reduce biogenic amines. Dapkevicius et al. (2000) reported the ability of lactic acid bacteria to degrade biogenic amines. Therefore, the use of bacteria which has oxidizing enzymes or amine-oxidizing activity can reduce biogenic amine concentrations when it is difficult to control biogenic amine concentration and remove already formed biogenic amines in fermented meat products. For that reason, treatment with vitamins C and E could be useful for reducing biogenic amine levels in various sausages fermented with starter cultures.
Komprda et al. (2004) reported that the sum of all biogenic amines in fermented sausages produced with P. pentosaceus and S. carnosus was higher than that in fermented sausages produced with other starter cultures (p<0.05). In this study, various starter cultures were used to manufacture fermented sausages, and they showed a wide range of biogenic-amine reduction effects. As a result, the use of S. carnosus and L. sakei as starter cultures increased the mutagenicity and biogenic amine concentrations in the fermented sausages, whereas the combination of S. xylosus and L. plantarum starter cultures was the most effective in decreasing the mutagenicity and biogenic amines concentrations in the fermented sausages.
Conclusion
In this study, the fermented sausages containing S. xylosus and L. plantarum showed the lowest mutagenicity value. In addition, the fermented sausages treated with vitamin E alone decreased mutagenicity to a greater extent than the fermented sausages treated with vitamin C and a combination of vitamins C and E. The levels of biogenic amines decreased after the addition of vitamins C and E (p<0.05). In particular, vitamin E was more effective in decreasing the concentration of biogenic amines than vitamin C. The correlation between starter culture and concentrations of tyramine (r=0.85) was higher than that of the other parameters (p<0.05). In conclusion, we recommend the use of S. xylosus and L. plantarum as starter cultures for fermented sausages with vitamins, especially vitamin E, to reduce potential meat mutagens.













