ARTICLE
Kinetic Behavior of Salmonella on Low NaNO2 Sausages during Aerobic and Vacuum Storage
Jimyeong Ha1, Eunji Gwak1, Mi-Hwa Oh2, Beomyoung Park2, Jeeyeon Lee1, Sejeong Kim1, Heeyoung Lee1, Soomin Lee1, Yohan Yoon1, Kyoung-Hee Choi3,*
Author Information & Copyright ▼
1Department of Food and Nutrition, Sookmyung Women’s University, Seoul 04310, Korea
2National Institute of Animal Science, RDA, Wanju 55365, Korea
3Department of Oral Microbiology, College of Dentistry, Wonkwang University, Iksan 54538, Korea
*Corresponding author: Yohan Yoon, Department of Food and Nutrition, Sookmyung Women's University, Seoul 04310, Korea. Tel: +82-2-2077-7585, Fax: +82-2-710-9479, E-mail:
yyoon@sookmyung.ac.kr; Kyoung-Hee Choi, Department of Oral Microbiology, College of Dentistry, Wonkwang University, Iksan 54538, Korea. Tel: +82-63-850-6911, Fax: +82-63-850-6911, E-mail:
kheechoi@wku.ac.kr
Copyright © 2016, Korean Society for Food Science of Animal Resources. This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: Jan 17, 2016 ; Revised: Mar 01, 2016 ; Accepted: Mar 02, 2016
Published Online: Apr 30, 2016
Abstract
This study evaluated the growth kinetics of Salmonella spp. in processed meat products formulated with low sodium nitrite (NaNO2). A 5-strain mixture of Salmonella spp. was inoculated on 25-g samples of sausages formulated with sodium chloride (NaCl) (1.0%, 1.25%, and 1.5%) and NaNO2 (0 and 10 ppm) followed by aerobic or vacuum storage at 10℃ and 15℃ for up to 816 h or 408 h, respectively. The bacterial cell counts were enumerated on xylose lysine deoxycholate agar, and the modified Gompertz model was fitted to the Salmonella cell counts to calculate the kinetic parameters as a function of NaCl concentration on the growth rate (GR; Log CFU/g/h) and lag phase duration (LPD; h). A linear equation was then fitted to the parameters to evaluate the effect of NaCl concentration on the kinetic parameters. The GR values of Salmonella on sausages were higher (p<0.05) with 10 ppm NaNO2 concentration than with 0 ppm NaNO2. The GR values of Salmonella decreased (p<0.05) as NaCl concentration increased, especially at 10℃. This result indicates that 10 ppm NaNO2 may increase Salmonella growth at low NaCl concentrations, and that NaCl plays an important role in inhibiting Salmonella growth in sausages with low NaNO2.
Keywords: Salmonella; kinetic model; NaCl; NaNO2; sausages
Introduction
Salmonella is an invasive and facultative intracellular pathogen, and is mainly transmitted through contaminated food (Wagner et al., 2011). Especially, pork can be easily contaminated with Salmonella (Davies et al., 2000; Uyttendaele et al., 1999). Since Salmonella is highly resistant to acidic or dry conditions (Bearson et al., 1997), the pathogen may survive during sausage manufacturing (Bonnet and Montville, 2005), during which acidic conditions are created by fermentation and dry conditions are created by sodium addition. Therefore, Salmonella outbreaks related to sausages have been reported in many countries (Long et al., 2002; Nichols and De Louvois, 1995).
To enhance the flavor and water holding capacity of meat products, sodium chloride (NaCl) is added to processed meat products, usually up to 2.5% (Rhee and Ziprin, 2001). NaCl has also been used as a preservative to control foodborne pathogens in combination with sodium nitrite (NaNO2) (Aguilera and Karel, 1997). NaNO2 in meat products play a major role in coloring agents and fat deterioration (Horsch et al., 2014). In particular, the formation of Clostridium botulinum spores is controlled and the growth of pathogenic bacteria is inhibited by the addition of NaNO2 at anaerobic environmental conditions (Krause et al., 2011). However, NaNO2 is a precursor that changes into N-nitroso at the low pH conditions in the stomach (Sugimura, 2000). N-nitroso is a compound toxic to the human body, thus consumers prefer to purchase low NaNO2 meat products. However, when NaNO2 concentrations decrease, problems with microbial safety emerge (Sindelar et al., 2007).
To describe the kinetic behavior of foodborne pathogens, predictive microbiology has been used with mathematical equations (Whiting and Buchanan, 1997). The results from kinetic models can be used to ensure food safety in advance by blocking the possible intrinsic and extrinsic factors that can affect food (Yoon, 2010). Primary models are used to calculate kinetic parameters such as growth rate (GR; Log CFU/g/h) and lag phase duration (LPD; h). Secondary models are used to evaluate the effects of various factors on the kinetic parameters.
Therefore, the objective of this study was to describe the kinetic behavior of Salmonella in low NaNO2 sausage formulated with various NaCl concentrations, using mathematical equations.
Materials and Methods
Inoculum preparation
Salmonella Typhimurium NCCP10812, Salmonella Agona NCCP12231, Salmonella Enteritidis NCCP12243, Salmonella enterica KACC11595, and Salmonella Montevideo NCCP10141 were cultured in 10 mL nutrient broth (NB; Becton, Dickinson and Company, USA) at 37℃ for 24 h. The 0.1 mL portions of each culture were transferred into 10 mL fresh NB for subculture at 37℃ for 24 h. The cultures of the five strains were then mixed. The mixture was centrifuged at 1,912 g for 15 min at 4℃, and the cell pellets were washed twice with phosphate buffered saline (PBS, pH 7.4; 0.2 g of KH2PO4, 1.5 g of Na2HPO4, 8.0 g of NaCl, and 0.2 g of KCl in 1 L of distilled water). Eventually, the cell pellet was diluted with PBS to 6-7 Log CFU/mL for use as inoculum.
Sausage manufacture and inoculation
To prepare the sausages, pork meat (60%), pork fat (20%), and ice (20%) were mixed. Phosphate (0.3%), isolated soy protein (1.0%), mixed spice (0.5%), sugar (0.5%), potassium sorbate (0.2%), NaNO2 (0 and 10 ppm), and NaCl (1.0%, 1.25%, and 1.5%) were added to the mix. Since commercial sausages had approximately 11.5 ppm of NaNO2 residual (Ham et al., 2004), 10 ppm of NaNO2 residual was chosen to be examined in this study. All samples were emulsified using a silent cutter (MSK 760 H II, Mado, Germany) for 6 min. The mixed pastes were then stored at 4℃ for 1 h, and 30-g portions were stuffed into the collagen casing (#260, NIPPI Inc., Japan; approximate 25 mm diameter) with an automatic sausage filler (Konti A50; Frey, Germany). The sausages were heated at 75℃ for 40 min in a smokehouse (MAXI 3501; Kerres, Germany), and the emulsion type sausages were then vacuum-packaged with polyethylene. After the sausages were dipped in ice water for 10 min, the sausages were stored at 4℃ until used (Choi et al., 2014). To inoculate Salmonella on sausages, the vacuum-packages were aseptically opened. Samples were cut into 25 g and inoculated by immersion into 500 mL Salmonella inoculum in a sterilized plastic container for 2 min. The sausages were transferred to petri dishes to allow attachment for 15 min, and then transferred into sample bags. The samples were sealed for storage in aerobic condition or vacuum-packaged for the vacuum condition. The samples were incubated at 10℃ and 15℃ for up to 816 h and 408 h, respectively, and Salmonella cell counts were enumerated on xylose lysine deoxycholate agar (XLD; Becton, Dickinson and Company).
Kinetic parameter calculation
The modified Gompertz model (Zwietering et al., 1990) were fitted to the Salmonella cell counts to calculate kinetic parameters such as GR and LPD with GraphPad PRISM version 4.0 (GraphPad Software, USA). The model used was as follows;
Modified Gompertz model:
Nt = A+C× exp(–exp(–B(t–M)))
GR = B×C⁄ e (e = 2.7182)
LPD = M–(1⁄B)
Nmax= A+C
where A is the lower asymptotic line of the growth curve as t decrease to zero, C is the difference between the upper asymptotic line of the growth curve and the lower asymptotic line, B is the relative maximum growth rate at time M, and M is the time at which the growth rate is maximum (Gibson et al., 1987).
The secondary model was developed to evaluate the effect of NaCl concentration on the kinetic parameters with the following equation;
GR = a0+a1×NaCl
where ai is a coefficient, and NaCl is the NaCl concentration.
Statistical analysis
The growth parameter (GR and LPD) was analyzed using the general linear model procedure in SAS® version 9.3 (SAS Institute, USA). All least squares means comparisons were performed using a pairwise t-test at p=0.05.
Results and Discussion
The parameters estimated by the modified Gompertz model for Salmonella spp. on sausages formulated with NaNO2 and NaCl aerobically and anaerobically stored at 10℃ and 15℃ are shown at Table 1 and Table 2. R2 values for fitting the modified Gompertz model to the Salmonella growth data ranged from 0.913 to 0.979, suggesting that the model was appropriate for describing the kinetic behavior of Salmonella in sausage (Table 1, 2).
Table 1.
The growth parameters estimated by the modified Gompertz model for Salmonella spp. on sausages as a function of NaNO2 and NaCl concentration at 10℃ and 15℃ in aerobic conditions
| Storage Temperature (℃) |
NaNO2 (ppm) |
NaCl (%) |
LPD1) (h) |
GR2) (Log CFU/g/h) |
N03) (Log CFU/g) |
Nmax4) (Log CFU/g) |
R2 |
| 10 |
0 |
1 |
48.83±22.51ABCD |
0.042±0.03CD |
3.5±0.5 |
8.0±0.1 |
0.962 |
| 1.25 |
58.76±35.53ABC |
0.024±0.00D |
3.5±0.5 |
7.9±0.4 |
0.942 |
| 1.5 |
67.17±46.76A |
0.023±0.00D |
3.6±0.5 |
8.2±0.0 |
0.958 |
|
| 10 |
1 |
22.22±0.95BCD |
0.080±0.05ABC |
3.6±0.1 |
8.2±0.3 |
0.962 |
| 1.25 |
24.14±0.69BCD |
0.038±0.01CD |
3.7±0.0 |
8.2±0.6 |
0.964 |
| 1.5 |
62.90±8.42AB |
0.028±0.02D |
3.5±0.2 |
8.1±0.3 |
0.944 |
| 15 |
0 |
1 |
9.22±11.53D |
0.064±0.02BCD |
3.4±0.1 |
7.2±0.0 |
0.957 |
| 1.25 |
11.76±10.33D |
0.063±0.02BCD |
3.4±0.2 |
7.1±0.1 |
0.956 |
| 1.5 |
19.93±12.25CD |
0.037±0.02CD |
3.4±0.1 |
7.2±0.4 |
0.927 |
|
| 10 |
1 |
8.93±3.35D |
0.117±0.01A |
3.9±0.4 |
8.2±0.4 |
0.934 |
| 1.25 |
15.89±6.58D |
0.091±0.02AB |
3.6±0.2 |
7.8±0.8 |
0.923 |
| 1.5 |
16.65±6.48CD |
0.091±0.02AB |
3.7±0.7 |
7.8±1.1 |
0.973 |
Download Excel Table
Table 2.
The growth parameters estimated by the modified Gompertz model for Salmonella spp. on sausages as a function of NaNO2 and NaCl concentration at 10℃ and 15℃ in vacuum condition
| Storage Temperature (℃) |
NaNO2 (ppm) |
NaCl (%) |
LPD1) (h) |
GR2) (Log CFU/g/h) |
N03) (Log CFU/g) |
Nmax4) (Log CFU/g) |
R2 |
| 10 |
0 |
1 |
24.86±7.41CDE |
0.023±0.00CD |
3.7±0.1 |
7.9±0.2 |
0.949 |
| 1.25 |
26.23±6.33CDE |
0.022±0.01CD |
3.8±0.4 |
8.2±0.0 |
0.979 |
| 1.5 |
33.29±0.33ABC |
0.022±0.00CD |
3.7±0.1 |
8.2±0.3 |
0.974 |
|
| 10 |
1 |
28.60±5.54BCD |
0.043±0.03BCD |
3.5±0.1 |
7.2±0.0 |
0.950 |
| 1.25 |
39.15±0.00AB |
0.018±0.00D |
3.4±0.0 |
6.9±0.0 |
0.975 |
| 1.5 |
43.65±4.45A |
0.020±0.01CD |
3.4±0.1 |
6.9±0.2 |
0.962 |
| 15 |
0 |
1 |
16.64±4.23EF |
0.045±0.00BC |
3.7±0.4 |
8.1±0.4 |
0.977 |
| 1.25 |
20.73±8.77DE |
0.036±0.00BCD |
3.8±0.5 |
8.2±0.8 |
0.978 |
| 1.5 |
21.51±8.34DE |
0.027±0.01CD |
4.0±0.7 |
8.4±0.6 |
0.948 |
|
| 10 |
1 |
6.88±2.03F |
0.098±0.02A |
3.8±0.1 |
7.2±0.2 |
0.952 |
| 1.25 |
8.59±0.00F |
0.062±0.00B |
3.7±0.0 |
6.7±0.0 |
0.924 |
| 1.5 |
16.12±0.00EF |
0.049±0.00BC |
3.9±0.0 |
6.8±0.0 |
0.913 |
Download Excel Table
The LPD values were 22.22-67.17 h at 10℃ and 6.88-21.51 h at 15℃. The values at 15℃ were generally lower than at 10℃ (Table 1, 2). In general, NaNO2 inhibits bacterial growth in sausages (Junttila et al., 1989). However, in this study, GR was higher (p<0.05) in 10 ppm NaNO2 than in 0 ppm NaNO2 at 1% NaCl, when the sausages were stored at 15℃ in both aerobic and vacuum conditions (Table 1, 2). In addition, the highest GR values were observed with 10 ppm NaNO2 at 1% NaCl for all temperatures in both aerobic and vacuum storage (Table 1, 2). This result indicates that Salmonella may have more growth 10 ppm NaNO2 than 0 ppm NaNO2, which was not expected. Recent studies showed that Salmonella produces a nitrite reductase (Gilberthorpe and Poole, 2008; Mills et al., 2008) and flavohemoglobin (Hmp), which confer tolerance to NO and nitrosoactive stress (Poole and Hughes, 2000). However, Seong et al. (2010) and Birk et al. (2015) showed that 100 ppm NaNO2 combined with a low concentration (62 g/kg) of salt completely inhibited Salmonella growth. Taken together, it can be suggested that 10 ppm of NaNO2 was below the threshold needed to destroy Salmonella cells, and thus, Salmonella can resist the low concentration of NaNO2 because of flavohemoglobin and nitrite reductase, which break down NaNO2. The nitrogen produced by breaking down NaNO2 may subsequently be used as a nitrogen source for Salmonella growth (Page and Solberg, 1980), which was higher with 10 ppm NaNO2 than with no NaNO2. In addition, although N0 values were 3.4-3.9 Log CFU/g, Nmax values were higher (7.8-8.2 Log CFU/g) in 10 ppm NaNO2 treated samples than in 0 ppm NaNO2 treated samples at 15℃, but not at 10℃ under aerobic storage. This phenomenon, however, was not observed under vacuum storage (Table 1, 2).
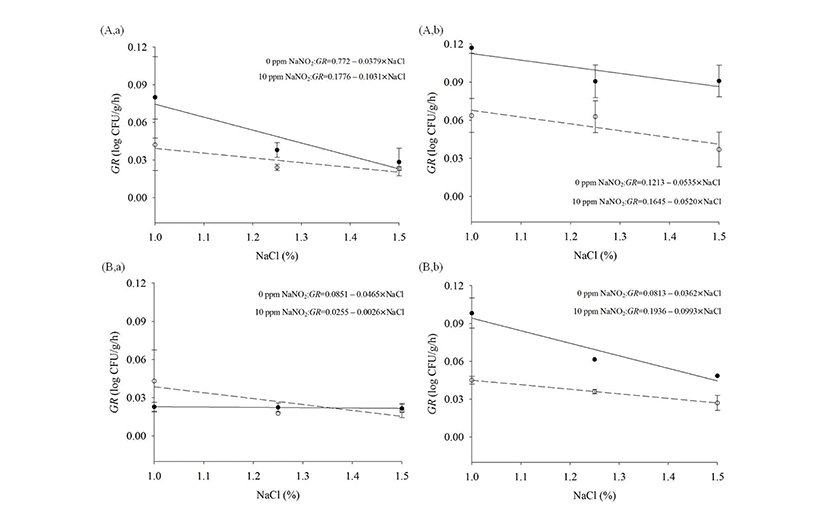
To evaluate the effects of NaCl and NaNO2 on the GR of Salmonella, a linear equation was fitted to the GR values to describe the effect of NaCl and NaNO2 (Fig. 1). At 10℃, the GR was higher with 10 ppm NaNO2 than with 0 ppm NaNO2 at 1% NaCl, but the GR values with 10 ppm NaNO2 rapidly decreased as NaCl concentration increased. The values became similar to the values with 0 ppm NaNO2 at 1.5% NaCl, regardless of atmospheric conditions (Table 1, 2, Fig. 1). This result indicates that 10 ppm of NaNO2 may increase the GR of Salmonella, compared to 0 ppm NaNO2, but the increase of NaCl in combination with NaNO2 can decrease the GR (Fig. 1). Jo et al. (2014) also showed that Pseudomonas spp. growth in processed meat was inhibited by a combination of NaNO2 and NaCl. At 15℃ in aerobic condition, the GR with 10 ppm NaNO2 was higher (p<0.05) than with 0 ppm NaNO2 at 1% NaCl, but the GR with 10 ppm NaNO2 did not become the same as the GR with 0 ppm as NaCl concentration increased, which were different from the results at 10℃(Fig. 1).
Fig. 1.
Effect of NaCl on growth rate (GR) according to the amount of NaCl under (A) aerobic conditions, (B) vacuum storage at (a) 10℃ and (b) 15℃. 0 ppm: -----, 10 ppm: −−−.
Download Original Figure
In conclusion, 10 ppm NaNO2 may increase Salmonella growth in processed meat products, and thus, sufficient NaCl must be combined with NaNO2 to improve food safety, especially for low NaNO2 products.
Acknowledgements
This work was carried out with the support of the “Cooperative Research Program for Agriculture, Science & Technology Development (Project No. PJ009237)” by the Rural Development Administration, Republic of Korea.
References
Aguilera J. M., Karel M. Preservation of biological materials under desiccation. Crit. Rev. Food Sci. Nutr. 1997; 37:287-309.

Bearson S., Bearson B., Foster J. W. Acid stress responses in enterobacteria. FEMS Microbiol. Lett. 1997; 147:173-180.

Birk T., Henriksen Müller, Hansen T. B., Aabo S. Growth potential of exponential- and stationary-phase Salmonella Typhimurium during sausage fermentation. 2015 (in press).

Bonnet M., Montville T. J. Acid-tolerant Listeria monocytogenes persist in a model food system fermented with nisin-producing bacteria. Lett. Appl. Microbiol. 2005; 40:237-242.

Choi Y. S., Kim H. W., Hwang K. E., Song D. H., Choi J. H., Lee M. A., Chung H. J., Kim C. J. Physicochemical properties and sensory characteristics of reducedfat frankfurters with pork back fat replaced by dietary fiber extracted from makgeolli lees. Meat Sci. 2014; 96:892-900.

Davies R., Paiba G., Evans S., Dalziel B. Surveys for Salmonella in pigs, cattle and sheep at slaughter in Great Britain. Vet. Rec. 2000; 147:695.

Gibson A. M., Bratchell N., Roberts T. A. The effect of sodium chloride and temperature on the rate and extent of growth of Clostridium botulinum type A in pasteurized pork slurry. J. Appl. Bacteriol. 1987; 62:479-490.

Gilberthorpe N. J., Poole R. K. Nitric oxide homeostasis in Salmonella typhimurium roles of respiratory nitrate reductase and flavohemoglobin. J. Biol. Chem. 2008; 283:11146-11154.

Ham H., Hong I., Lim H., Yang Y., Choi Y., Kim C., Kweon T., Lee J. Nitrites contents on processed meat products (ham, sausage etc) in market during 2000-2003. Korean J. Vet. Serv. 2004; 27:115-120.

Horsch A. M., Sebranek J. G., Dickson J. S., Niebuhr S. E., Larson E. M., Lavieri N. A., Wilson L. A. The effect of pH and nitrite concentration on the antimicrobial impact of celery juice concentrate compared with conventional sodium nitrite on Listeria monocytogenes. Meat Sci. 2014; 96:400-407.

Jo H., Park B., Oh M., Gwak E., Lee H., Lee S., Yoon Y. Probabilistic models to predict the growth initiation time for Pseudomonas spp. in processed meats formulated with NaCl and NaNO2. Korean J. Food Sci. An. 2014; 34:736-741.

Junttila J., Hirn J., Hill P., Nurmi E. Effect of different levels of nitrite and nitrate on the survival of Listeria monocytogenes during the manufacture of fermented sausage. J. Food. Protect. 1989; 52:158-161.

Krause B. L., Sebranek J. G., Rust R. E., Mendonca A. Incubation of curing brines for the production of readyto- eat, uncured, no-nitrite-or-nitrate-added, ground, cooked and sliced ham. Meat Sci. 2011; 89:507-513.

Long S. M., Adak G. K., O'Brien S. J., Gillespie I. A. General outbreaks of infectious intestinal disease linked with salad vegetables and fruit, England and Wales, 1992- 2000. Commun. Dis. Public Health. 2002; 5:101-105.

Mills P. C., Rowley G., Spiro S., Hinton J. C., Richardson D. J. A combination of cytochrome c nitrite reductase (NrfA) and flavorubredoxin (NorV) protects Salmonella enterica serovar Typhimurium against killing by NO in anoxic environments. Microbiology. 2008; 154:1218-1228.

Nichols G. L., De Louvois J. The microbiological quality of raw sausages sold in the UK. PHLS Microbiol. Dig. 1995; 12:236-242.

Page G. V., Solberg M. Nitrogen assimilation by Salmonella typhimurium in a chemically defined minimal medium containing nitrate, nitrite, or ammonia. J. Food Sci. 1980; 45:75-76.

Poole R. K., Hughes M. N. New functions for the ancient globin family: Bacterial responses to nitric oxide and nitrosative stress. Mol. Microbiol. 2000; 36:775-783.

Rhee K. S., Ziprin Y. A. Pro-oxidative effects of NaCl in microbial growth-controlled and uncontrolled beef and chicken. Meat Sci. 2001; 57:105-112.

Seong P. N., Kim J. H., Cho S. H., Kang D. W., Kang G. H., Park B. Y., Lee J. M., Jung J. H., Kim D. H. The effects of salt and NaNO2 on fatty acid composition, free amino acids, microbial counts and sensory characteristics of dry-cured ham processed under Korean environment. Korean J. Food Sci. An. 2010; 30:435-442.

Sindelar J. J., Cordray J. C., Sebranek J. G., Love J. A., Ahn D. U. Effects of varying levels of vegetable juice powder and incubation time on color, residual nitrate and nitrite, pigment, pH, and trained sensory attributes of ready-to-eat uncured ham. J. Food Sci. 2007; 72:S388-S395.

Sugimura T. Nutrition and dietary carcinogens. Carcinogenesis. 2000; 21:387-395.

Uyttendaele M., De Troy P., Debevere J. Incidence of Salmonella, Campylobacter jejuni, Campylobacter coli, and Listeria monocytogenes in poultry carcasses and different types of poultry products for sale on the Belgian retail market. J. Food Protect. 1999; 62:735-740.

Wagner C., Hensel M. Adhesive mechanisms of Salmonella enterica. Adv. Exp. Med. Biol. 2011; 715:17-34.

Whiting R. C., Buchanan R. L. Development of a quantitative risk assessment model for Salmonella enteritidis in pasteurized liquid eggs. Int. J. Food Microbiol. 1997; 36:111-125.

Yoon Y. Principal theory and application of predictive microbiology. Food Sci. Ind. 2010; 43:70-74.

Zwietering M. H., Jongenburger I., Rombouts F. M., Van't Riet K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990; 56:1875-1881.