Introduction
Recently, with increase in world population, problems related to environmental pollution as well as food supply and demand due to industrialization have been rising (van Huis et al., 2013). To respond to these changes in the food production industry, edible insects are attracting attention as an innovative protein source. In 2013, United Nations’ Food and Agriculture Organization (FAO) designated insects as a future food resource (Lee et al., 2017; van Huis, 2013). Edible insects have a short growing period and generate a small amount of biological waste, thus serving as an eco-friendly and economical future food resource (Nam and Sim, 2021; Oonincx and de Boer, 2012; Yun and Hwang, 2016). In addition, it contains more essential amino acids than soybean, indicating their great potential for use as an alternative to plant protein (Yi et al., 2013). In particular, edible insects are highly regarded for their nutritional value because they can supply amino acids lacking in the living body and contain abundant nutrients, such as unsaturated fatty acids and minerals (Nam and Sim, 2021; Park and Choi, 2020; Yun and Hwang, 2016). Moreover, they are suitable feed ingredients as an alternative protein source for broiler (Biasato et al., 2018; Bovera et al., 2016; De Marco et al., 2015; Ramos-Elorduy et al., 2002) and pigs (Cho et al., 2020; Jin et al., 2016; Yoo et al., 2019). Accordingly, a number of studies are underway worldwide, aimed at exploring the application of edible insects as an important alternative food for protein supply to livestock.
Tenebrio molitor L. (Yellow mealworm), belonging to the Tenebrionidae family of the Coleoptera order is one of the most common edible insects. These insects exhibit a strong adaptability to harsh environments, such as drought and temperature (high and low), and are considered among the edible insects that can be readily industrialized due to the ease of mass breeding. These insects need not be bred at a large scale and are widely used as a biological research model due to easy handling and reproduction (Jung et al., 2014; Yang et al., 2015). In addition, their safety as a raw material for food production has been determied, and the European Food Safety Authority (EFSA) has validated mealworms as edible insects in 2021 (EFSA Panel on Nutrition et al., 2021). In particular, Tenebrio molitor L. are protein-rich edible insects, with over 50% crude protein yield, and contain abundant essential amino acids, such as threonine, valine, histidine, and lysine, as well as unsaturated fatty acids and minerals, such as calcium and magnesium (Baek et al., 2017; Yoo et al., 2013). The safety of Tenebrio molitor L. powder (TMlp) as food has been proven through in vivo experiments, which revealed no obvious toxicity in Sprague Dawley (SD) rats of both sexes fed 3,000 mg/kg/day TMlp for 28 days (Han et al., 2014). In addition, in weaned pigs fed Tenebrio molitor L., growth performance and protein utilization were improved, and Tenebrio molitor L. could be used as a protein source in monogastric animal (poultry and pig) feed, with comparable protein quality to soybean meal (Ramos-Elorduy et al., 2002; Hong et al., 2020).
Polystyrene (PS) is a common petroleum-based plastic produced through the polymerization of styrene monomer. It is used in various packaging and building structures, such as in plastic cups, packaging materials, egg trays, disposable products, and building insulation (Farrelly and Shaw, 2017). In recent years, with increase in plastic consumption following industrial development, a large amount of plastic waste is being generated, and due to its light weight, inertness, durability, and strong structural safety, biodegradation and natural decomposition of PS are extremely challenging (Gautam et al., 2007; Pushpadass et al., 2010). PS is widely used in the form of expanded polystyrene (EPS) and extruded polystyrene, with Styrofoam being the most common form of EPS. They were widely used in the production of foam insulation and packaging materials (Matyja et al., 2020; Peng et al., 2019). EPS materials are primarily manufactured for one-time use, which increases the accumulation of EPS waste due to the contrasting patterns of high durability and short consumption, leading to significant environmental problems (Barnes et al., 2009; Kale et al., 2015). Currently, most EPS waste is disposed of in landfills or incinerated. In addition to being energy-intensive, EPS incineration generates toxic substances, such as dioxins (Faravelli et al., 2001; Matyja et al., 2020). Moreover, due to its structural safety, EPS is dispersed in natural systems, such as soil, rivers, lakes, and seas, upon when decomposing, leading to microplastic accumulation (Hidalgo-Ruz et al., 2012; Wu et al., 2017). In particular, the endocrine-disrupting chemicals released from these synthetic substances exhibited hormonal activity similar to estrogen, a female hormone, in vivo and can adversely affect the human body by disrupting homeostasis, reproduction, development, and behavior (Qiang et al., 2020; Swanson et al., 1995).
In addition to the mechanical, chemical, and thermal treatments of PS to solve these problems, various efforts are underway to use edible insects, such as red flour beetles (Tribolium castaneum; Fabreag and Familara, 2019), superworm (Zophobas morio L.) larvae (Yang et al., 2020), dark mealworm (Tenebrio obscurus L.) larvae and yellow mealworm (Tenebrio molitor L.) larvae (Bae et al., 2021), in an environmentally friendly and economic way (Maharana et al., 2007). Tenebrio molitor L. from 22 countries could biodegrade PS, reducing its mass, and depolymerization/cleavage of long-chain structures was observed (Peng et al., 2019). Furthermore, low-molecular-weight residues and functional groups indicative of oxidative transformation were detected in the extract of mealworm frass (insect excrement). Therefore, EPS biodegradation ability may be a universal characteristic of these insects regardless of their geographical origin (Yang et al., 2018). Given their ability to alter the chemical and physical properties of PS through biodegradation and mineralization, Tenebrio molitor L. is proposed as an innovative solution to environmental problems caused by the accumulation of EPS waste.
In this context, the application of Tenebrio molitor L. used for EPS biodegradation as a protein source in livestock feed can serve as an economical means to mitigate the problems of environmental pollution caused by the accumulation of PS waste. Moreover, it is possible to stabilize protein supply and demand through edible insects in an environmentally friendly and economical way. Therefore, in the present study, in vitro and in vivo toxicological safety evaluations were undertaken to investigate the industrial applicability of Tenebrio molitor L. used for EPS biodegradation as livestock feed in the powder form.
Materials and Methods
All experimental procedures were performed in accordance with the Animal Experimental Guidelines provided by the Kongju National University Institutional Animal Care and Use Committee (KNU-IACUC), Korea. The experimental protocol was approved by the KNU-IACUC (Approval number: KNU_2021-08).
The breast cancer cell line MCF-7 used in the experiment was purchased in the frozen form from the Korean Cell Line Bank (Seoul, Korea), thawed in a 75 cm2 cell culture flask, and cultured. MCF-7 cells were grown in RPMI 1640 (Welgene, Gyeongsan, Korea) containing 5% fetal bovine serum (Gibco BRL, Grand Island, NY, USA) and 1% streptomycin/penicillin (Gibco BRL) at 37°C under 5% CO2. The medium was replaced every 2–3 days. Upon reaching 80%–90% confluency in the flask, the cell cultures were washed with phosphate-buffered saline (PBS; Biosesang, Seongnam, Korea) and passaged following treatment with trypsin-EDTA (Gibco BRL). The reagents used in this experiment, 17-β estradiol, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
The preparation of TMlp was supported by the Inc. MCE (Daejeon, Korea). Tenebrio molitor L. (yellow mealworm) were purchased from KEIL (Cheongju, Korea). The insects were housed and bred in a dark room at 26±2°C under 65±10% relative humidity in HDPE boxes (270 W×450 L×100 H mm). In the control group, 6–8-week-old Tenebrio molitor L. were fed wheat bran. In the experimental group, 85% of the total intake was substituted with EPS feed blocks (FBs) and the remaining 15% constituted ordinary feed, such as Chinese cabbage and lettuce, to replenish moisture. In the expanded polystyrene-fed (W/ eps) experimental group, EPS FBs (bead method, type 1 insulation material no. 3) were cut into 20×20×10 mm sheets weighing 0.08 g and fed to the Tenebrio molitor L. for 8 wk. Thereafter, the remaining EPS in the intestine was completely discharged through feeding only bran for 4 d. Following 2 d of fasting, TMlp was prepared from 14-week-old Tenebrio molitor L., which is the normal age at which they are typically shipped. The powder was sterilized at 120°C for 10 min using a high-pressure steam sterilizer (MK-50S, Komachine, Yongin, Korea) and then heat-dried in a hot-air oven (J-300S, Komachine) at 80°C–85°C for 8 h. Thereafter, oil was extracted using a low-temperature compressor (LOP-G3, Lequip, Seoul, Korea). The residue was pulverized through a 100 mesh using a pulverizer, and the samples were stored in a −20°C freezer until further use.
TMlp and 70% ethyl alcohol were leached for 3 d at a ratio of 3:7 (w/v). Then, the solution was concentrated under reduced pressure at 45°C–50°C for 1 d using a rotary vacuum concentrator (EYELA N-1000, Rikakikai, Tokyo, Japan). The concentrated samples in dimethylsulfoxide (DMSO, Duksan, Ansan, Korea) were used as the final extract. The concentration of the TMlp extract was selected at the high concentration of 250 mg/mL, which is the maximum dose that can be dissolved in DMSO as a solvent. Thereafter, the TMlp extract was gradually diluted depending on the treatment concentration.
Cell viability was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylthtrazolium bromide (MTT) assay. MCF-7 cells were dispensed at a density of 2×104 cells/mL in a 96-well plate and cultured in an incubator maintained at 37°C under 5% CO2 for 24 h. Next, the cells were treated with different concentrations of 17β-estradiol (E2) (0, 10–10, 10–9, 10–8, 10–7, 10–6, and 10–5 M). The concentration showing the greatest increase in the cell proliferation rate compared to the control value was selected as the positive control group. MCF-7 cells were treated with TMlp W/ eps and TMlp W/o eps extract at concentrations of 0, 50, 100, 150, 200, and 250 μg/mL and incubated for 24 h. Then, 40 μL of MTT solution dissolved in PBS at 1 mg/mL was added to each well, and the cells were further incubated for 2 h. Thereafter, the MTT reagent was removed, and 100 μL DMSO was added to each well to dissolve the produced formazan. Finally, absorbance was measured at 595 nm using an ELISA reader (Bio-Rad Laboratories, Hercules, CA, USA).
All animals used in the experiment were bred in stainless steel wire mesh breeding boxes (260 W×350 L×210 H mm) in a room (A323) in the animal facility of Kongju National University, Korea, at 22±3°C under 30.0%–70.0% relative humidity, 150–300 lux illuminance, 10–15 times/h ventilation frequency, and 12/12 h light/dark cycle. Four-week-old male SD rats were purchased from Nara Biotech (Seoul, Korea). Upon arrival of the animals, visual inspection was performed, body weight was measured with an electronic scale, and general symptoms were observed once a day during the 1-week acclimatization period; during this time, normal feed and water were provided ad libitum through a stainless steel feeder and polycarbonate bottles.
The male SD rats were classified following the randomized complete block design based on similar average weights and stratified into a control group fed only distilled water (DW) and experimental groups fed the test substance (TMlp). In previous studies, Tenebrio molitor L. could be added up to 10% level to broiler feed without negatively affecting growth performance and carcase characteristics, and could completely replace SBM in broiler feed without deleterious effects (Hong et al., 2020). Therefore, referring to previous studies, the experimental group was further classified into four groups based on TMlp concentration: normal- (W/o eps 10%), low-dose (W/ eps 5%), middle-dose (W/ eps 10%), or high-dose concentration (W/ eps 15%) groups (n=5 in each group). The doses of TMlp administered orally were set to be 5%, 10%, and 15% of the recently measured daily feed intake, and the TMlp was administered by dissolving in DW, which was used as the solvent for the control group. The dosing volume, 10 mL/kg, was based on the most recent body weight. Before administration, the rats fasted overnight (12 h) to minimize the burden on the stomach. Drinking water was provided ad libitum. The TMlp was administered orally once a day for 5 weeks using a syringe tube equipped with a zonde.
During this study period, changes in the clinical symptoms of rats were observed once a day at a specific time (around 4–5 pm KST). Mortality was checked twice a day. Body weight and daily food intake were measured before the start of the study and twice a week during the study. To calculate daily food consumption, the food ration for each cage was measured the day before the weight measurement, and the remaining amount on the day of the weight measurement was measured. Food intake per rat was recalculated according to the average food consumption (g/rat/day) for each animal.
After completion of the study, the absolute and relative weights of the heart, lung, thymus, liver, spleen, kidney, adrenal, and testis were measured through autopsy. In the case of bilateral organs, the weights of the left and right organs were measured and the average value was calculated.
After completion of tests, all animals were anesthetized with ether, and blood was collected from the abdominal aorta into EDTA-containing tubes. Red blood cell (RBC), white blood cell (WBC), hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean cell hemoglobin concentration (MCHC), platelet, lymphocyte, neutrophil, eosinophils, basophils, monocytes, red cell distribution width (RDW), and mean platelet volume (MPV) were examined for hematological testing. For serum biochemical analysis, a part of the collected blood was allowed to stand at room temperature (20°C–22°C) for 30 min, coagulated, and then centrifuged (840×g for 30 min). Alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), albumin, globulin, total bilirubin (T-Bili), blood urea nitrogen (BUN), gamma glutamyl transpeptidase (γ-GTP), creatinine (Crea), glucose, total protein (TP), chloride (Cl), sodium (Na), and potassium (K) were measured.
After weight measurement, all organs were fixed in 10% neutral formalin solution. Following sufficient fixation for at least 2 weeks, the samples were embedded using a paraffin embedding machine (Tissue-Tek VIP, Sakura, Tokyo, Japan). A rotary microtome (Microm HM340E, Thermo Scientific, Walldorf, Germany) was used to obtain 2–3-μm-thick sections. The sections were stained with hematoxylin and eosin (H&E) and observed under an optical microscope to determine toxicity.
All data are presented as mean±standard deviation (SD). Differences between the mean values of TMlp W/ eps group and W/o eps group were analyzed by one-way analysis of variance (ANOVA) to determine statistical significance. When the F-test and variance test revealed equal variance (significance level=0.05), t-test was performed (significance level=0.05). A p<0.05 was considered statistically significant.
Results
To determine whether the composition of TMlp W/ eps was suitable for its use as a feed material for livestock, a component analysis was performed. The powder contained 1.22% moisture, 65.41% crude protein, 19.92% crude fat, 9.32% crude fiber, and 5.48% crude ash. In particular, proteins accounted for a relatively high proportion compared with the other components (Table 1).
| General components | Compositional average (%) |
|---|---|
| Moisture | 1.22 |
| Crude protein | 65.41 |
| Crude fat | 19.92 |
| Crude fiber | 9.32 |
| Crude ash | 5.48 |
| Mycotoxin | Contents (μg/kg) |
|---|---|
| Total aflatoxins (aflatoxin B1, B2, G1, G2, and ochratoxin A) | ND |
| Heavy metal | Contents (ppm) |
|---|---|
| Lead (Pb) | 0.01 |
| Cadmium (Cd) | ND |
| Arsenic (As) | 0.01 |
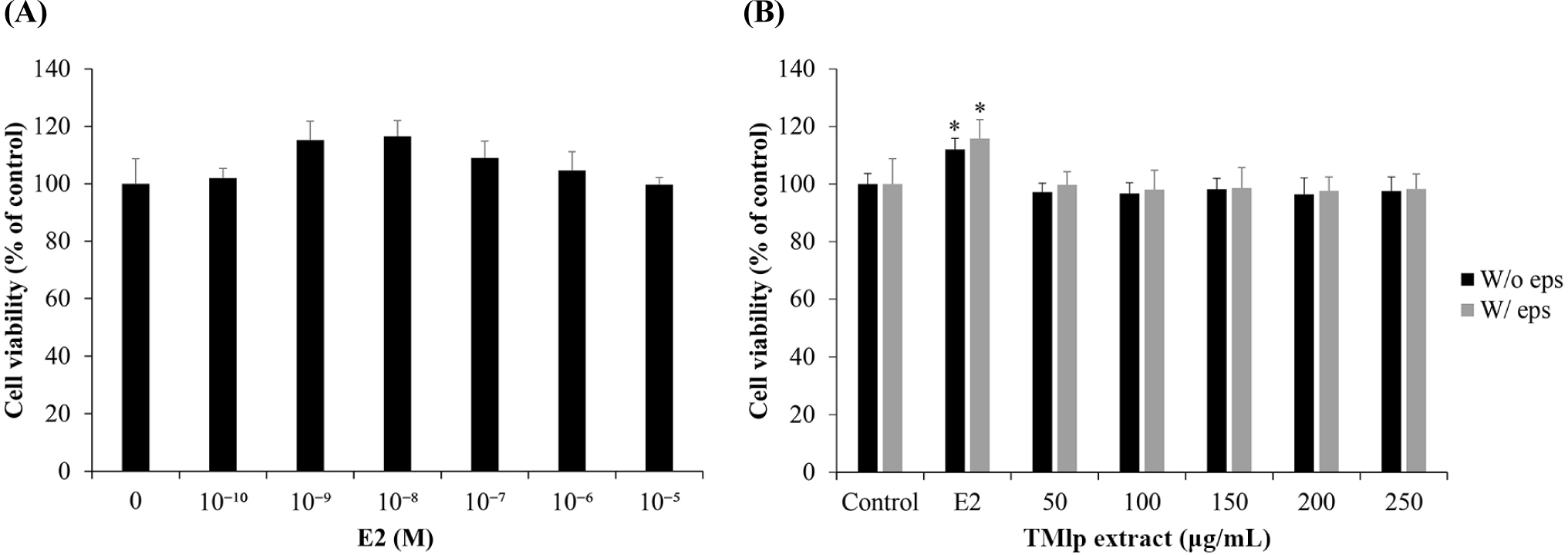
The MTT assay was performed to evaluate the effects of TMlp extract with (W/ eps) and without (W/o eps) W/ eps on MCF-7 cell viability (Fig. 1). First, to assess viability in the presence of E2, the estrogen-dependent cell line MCF-7 was treated with 0, 10–10, 10–9, 10–8, 10–7, 10–6, and 10–5 M E2 for 24 h and subjected to the MTT assay. The concentration maximizing the proliferation of MCF-7 cells was found to be 10–8 M; thus, in subsequent experiments, 10–8 M was set as the positive control. To determine the estrogen activity of W/ eps TMlp extract, MCF-7 cells were treated W/ eps TMlp extract and W/o eps TMlp extract at concentrations of 0, 50, 100, 150, 200, and 250 μg/mL and subjected to the MTT assays. There were no significant differences in proliferation rate between the two experimental groups and between the experimental and control groups.
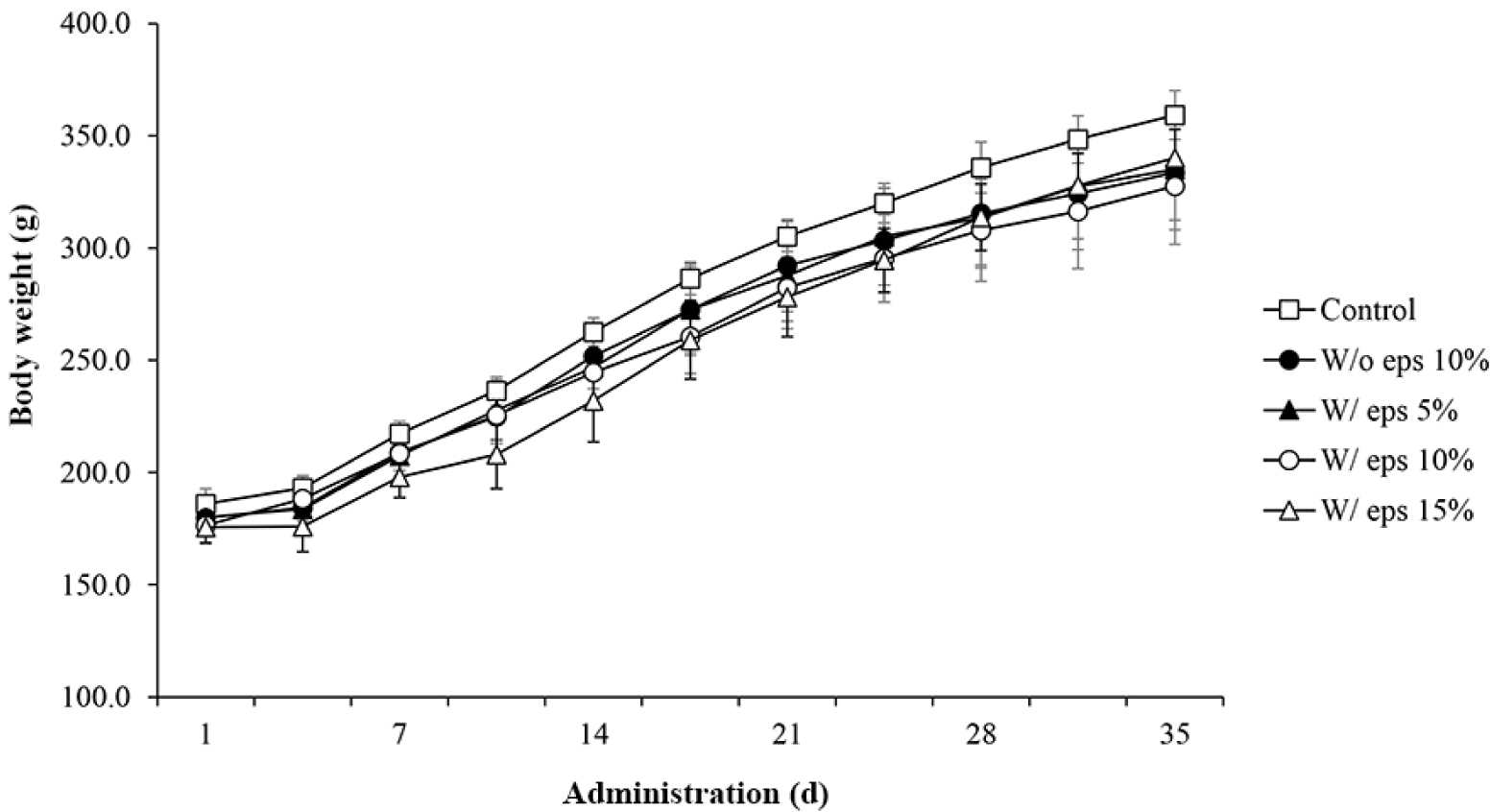
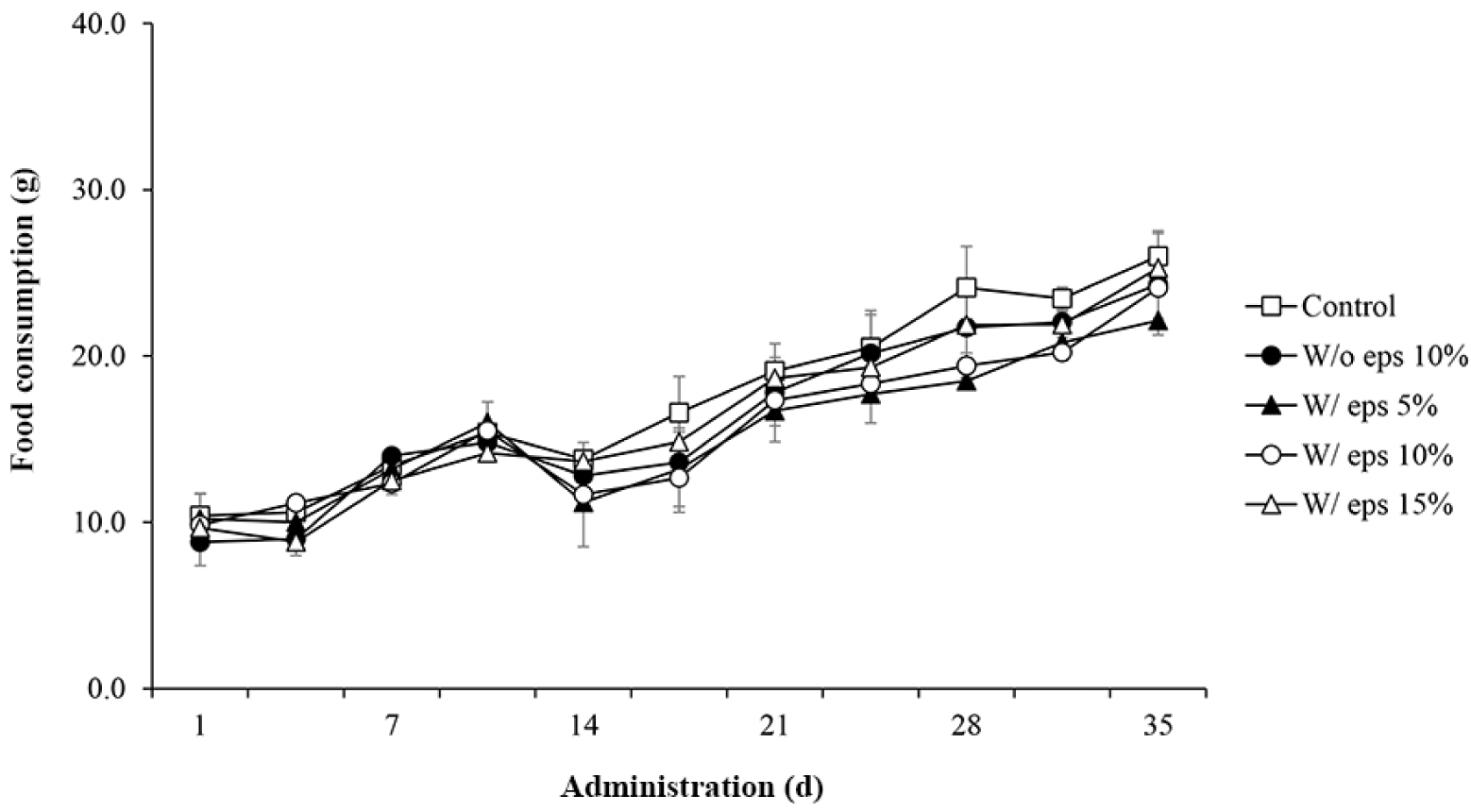
To determine the effects of W/ eps TMlp on the feed intake and body weight of rats, the corresponding factors were measured. No specific weight loss was noted in the control versus W/o eps 10% group and W/o eps 10% versus W/ eps 10% group (Fig. 2). However, feed intake tended to decrease in all experimental groups on the 14th day, although it gradually increased thereafter (Fig. 3). Overall, there were no significant differences in feed intake between the control versus W/o eps 10% group and W/o eps 10% versus W/ eps 10% group throughout the study period.
To determine the effects of W/ eps TMlp on the in vivo of rats, autopsy was performed after the completion of administration test to measure organ weights. The liver, kidney, adrenal gland, heart, thymus, testes, lung, and spleen were extracted, and their absolute (g) and relative (%) weights were measured (Table 2). The absolute weights of all organs, except the heart and thymus, tended to decrease in the W/o eps 10% group compared with those in the control group. However, there were no significant differences in terms of relative weights except for the spleen. Moreover, There were no significant differences in absolute and relative weights of all organs between the W/o eps 10% group and the W/ eps 10% group.
Table 3 presents the results of hematological examination of blood collected from the abdominal aorta at the time of autopsy after the completion of the study. We assessed hematological differences between the TMlp W/o eps 10% group and the W/ eps 10% group. Neutrophils tended to increase but lymphocytes tended to decrease in the W/ eps 10% group compared with that in the W/o eps 10% group. In addition, serum components were analyzed through biochemical tests, and the results are presented in Table 4. Specifically, there were no significant differences between the W/o eps 10% group and the W/ eps 10% group in terms of AST, ALT, and ALP, which are common indicators of liver function. Furthermore, T-Bili was below the measurement range in the control, W/ eps 5%, and W/ eps 15% groups.
TMlp, Tenebrio molitor larvae powder; W/o eps, without expanded-polystyrene-fed; W/ eps, expanded-polystyrene-fed; WBC, white blood cell; RBC, red blood cell; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean cell hemoglobin concentration; RDW, red cell distribution width; MPV, mean platelet volume.
TMlp, Tenebrio molitor larvae powder; W/o eps, without expanded-polystyrene-fed; W/ eps, expanded-polystyrene-fed; TP, total protein; T-Bili, total bilirubin; BUN, blood urea nitrogen; Crea, creatinine; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; γ-GTP, gamma glutamyl transpeptidase.
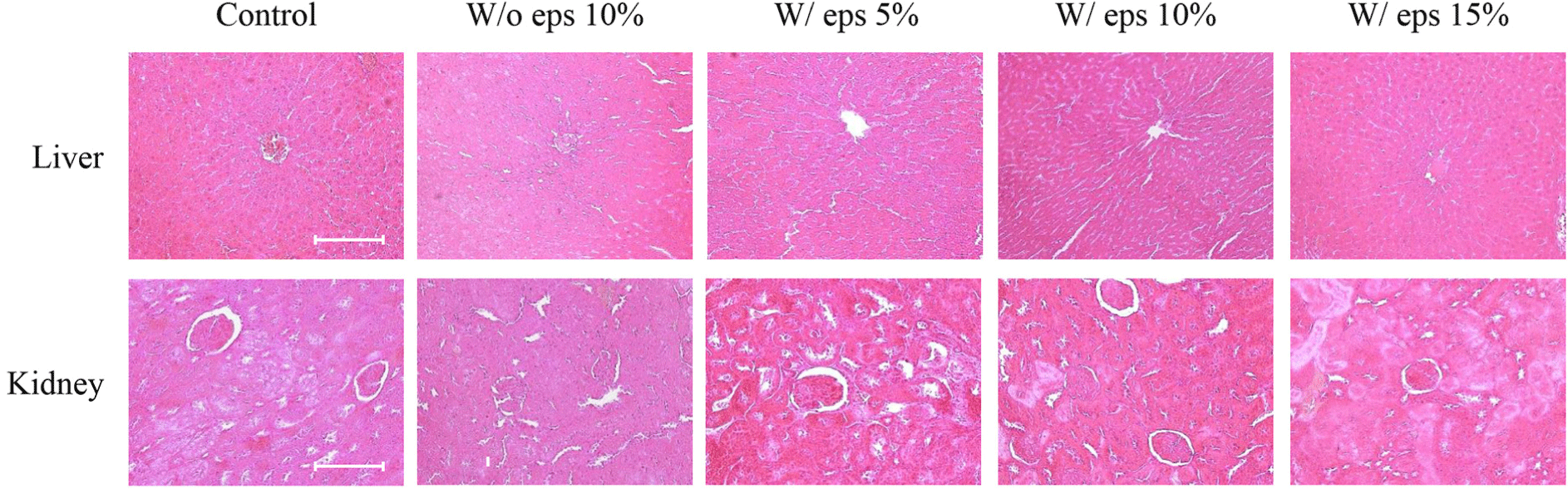
To evaluate the long-term toxicity of W/ eps TMlp administration, liver and kidneys were collected from rats autopsied after the end of the experiment and were stained with H&E (Fig. 4). Light microscopy revealed no histopathological abnormalities in the liver and kidneys of rats.
Discussion
Tenebrio molitor L. (yellow mealworm) are protein-rich edible insects (crude protein >50%), which are considered to be of great nutritional value as they contain abundant essential amino acids, minerals, and unsaturated fatty acids (Baek et al., 2017; Yoo et al., 2013). In addition, Tenebrio molitor L. can biodegrade EPS, a form of PS that has been causing environmental problems in recent years, and these insects are, therefore, proposed as an innovative solution to environmental problems caused by the accumulation of EPS waste (Bae et al., 2021; Yang et al., 2015). Moreover, Tenebrio molitor L. have been proven a suitable alternative protein source for broiler and pig feed (Biasato et al., 2018; Bovera et al., 2016; Cho et al., 2020; De Marco et al., 2015; Ramos-Elorduy et al., 2002; Jin et al., 2016; Yoo et al., 2019). Therefore, if Tenebrio molitor L. used for EPS biodegradation can be applied in livestock feed, it can serve as an economic alternative protein source and alleviate the problem of environmental pollution. In the present study, we performed component analysis to evaluate the feasibility of using the powder of Tenebrio molitor L. used for EPS biodegradation as a feed material for livestock. Further, in order to observe the effect of this powder on the living body, toxicological safety evaluations were performed through in vitro and in vivo experiments.
Composition analysis revealed that the W/ eps TMlp contained 1.22% moisture, 65.41% crude protein, 19.92% crude fat, 9.32% crude fiber, and 5.48% crude ash, with proteins accounting for a relatively high proportion compared with the other components. Yoo et al. (2013) reported that the yellow mealworm larvae powder contained 2.90% water, 50.32% crude protein, 33.70% crude fat, and 3.73% crude ash. Nam and Sim (2021) reported that the Korean yellow mealworm larvae powder contained 4.10% water, 68.50% crude protein, 13.50% crude fat, and 4.01% crude ash. Thus, the ratios of different components show slight variations, which can be attributed to differences in the growth and manufacturing environment of larvae. In the case of mycotoxins, neither total aflatoxin (the sum of B1, B2, G1, and G2) nor ochratoxin A was detected, and the content of heavy metals was also trace, indicating values consistent with the lowest standard for heavy metals of edible insects (MFDS, 2022). Moreover, the TMlp acquired from W/ eps Tenebrio molitor L. contains a large amount of protein, so it can be used as a protein substitute or as an economical material for other biological purposes. However, the potential toxic effects of TMlp W/ eps should be further investigated in terms of animal species, sex differences, and induction of allergic reactions before application to livestock feed.
In the in vitro experiment, the MTT assay was performed to observe the effect of E2 and TMlp extract on MCF-7 cell viability. E2 is an estrogen receptors (ER) and exhibits transcriptional activity, which is regulated by the binding of an agonist or antagonist ligand. E2 affects cell growth and differentiation and is involved in the proliferation of estrogen-dependent breast cancer cells (Colborn, 1995; Colborn et al., 1993; Harrison et al., 1997). Furthermore, endocrine-disrupting substances released from synthetic substances exhibit estrogen-like hormonal activity in the living body, producing adversely effects by disrupting homeostasis, reproduction, development, or behavior (Swanson et al., 1995; Wu et al., 2017). Therefore, in this study, E2 was used as a positive control to evaluate the validity of the experiments and experiments were conducted to observe the release of estrogen, an endocrine disrupting substance, from TMlp W/eps and TMlp W/o eps extracts depending on whether the EPS was supplied using ER-positive cells, MCF-7 (Soule et al., 1973). First, to assess the cell proliferation, MCF-7 cells were treated with E2 at concentrations of 0, 10–10, 10–9, 10–8, 10–7, 10–6, and 10–5 M for 24 h and then subjected to MTT assays. The E2 concentration of 10–8 M was found to maximize the proliferation of MCF-7 cells and was set as the positive control in subsequent experiments. Next, to assess the estrogen activity of W/ eps and W/o eps TMlp extract in MCF-7 cells, the proliferation rate was evaluated following treatment. The cell viability increased after E2 treatment, confirming the validity of the experiment. In addition, there were no significant differences in the cell viability and proliferation rate between the W/ eps and W/o eps TMlp extract. These results suggested that the W/ eps TMlp extract is non-toxic to cells and does not mediate an estrogen-induced response.
In the in vivo experiment, oral toxicity test was conducted using male SD rats. No animals died or showed clinical symptoms during the study period. Body weight tended to increase in all experimental groups, but there was no significant differences. In the case of daily feed intake, there were no significant differences between the W/o eps 10% and the W/ eps 10% groups. Weight tended to decrease in all experimental groups on the 14th day, but gradually increased thereafter. Acclimatization to administration may be attributed to the constant trend of body weight until the 14th day. Therefore, There was no significant difference in body weight and feed intake between the W/o eps 10% and the W/ eps 10% groups, which were the main comparators. Five weeks after the start of administration of the test substance, autopsy was performed, and the absolute (g) and relative (%) weights of organs were measured. The relative weights of all organs, except the spleen, did not significantly differ between the W/o eps and the control groups. In particular, the absolute weight of testis decreased significantly, but there was no significant difference in its relative weights. Therefore, these trends likely depend on the body weight of individual rats. However, in the case of the spleen, both absolute and relative organ weight significantly differed between the W/o eps 10% and the W/ eps 10% groups. These results were similar to those of toxicological assays of Han et al. (2016), in which lyophilized mealworm powder was administered to SD rats for 90 days. Long-term weight change is a useful indicator in toxicity studies. However, the change in spleen weight was weakly correlated with histopathological findings and appears to be due to individual differences in physiological factors (Nirogi et al., 2014). To confirm changes in the organ weight of rats fed with the W/ eps and the W/o eps TMlp, the organ weights of rats in the W/o eps 10% and W/ eps 10% groups were compared, but there were no significant differences. All blood and serum biochemical tests showed values within the normal range. Specifically, neutrophils tended to increase but lymphocytes tended to decrease in the W/ eps 10% group compared with that in the W/o eps 10% group. However, in previous studies on rodents, both neutrophils (10%–20%) and lymphocytes (75%–90%) were within the normal range (Doeing et al., 2003; Mestas and Hughes, 2004). These discrepancies in analytical results may be attributed to individual differences. Finally, the liver and kidneys were observed through H&E staining but no histopathological abnormalities were noted. In a study by Yang et al. (2015), the depolymerization/cleavage of long-chain PS structure was noted, and low-molecular-weight fragments were newly formed in the stomachs of Tenebrio molitor L. EPS is likely biodegraded by the intestinal microbes of Tenebrio molitor L.; thus, the W/ eps TMlp does not cause toxicological and pathological abnormalities in vivo. Considering the above results, it was observed that W/ eps TMlp did not cause in vivo toxicity in male SD rats under our experimental conditions.
In conclusion, our toxicological safety evaluations through in vitro and in vivo experiments revealed that the W/ eps TMlp did not mediate an estrogen-induced response in MCF-7 cells and did not cause toxicity in male SD rats under the experimental conditions of this study. Thus, TMlp W/ eps, acquired from W/ eps Tenebrio molitor L., can be used as an alternative protein source for livestock feed or as an economical material for other biological purposes.













