Introduction
Propolis is a product produced by honey bees from a mixture of wax compounds, β-glucosidase enzymes, and resins from plant parts, shoots, and exudates derived from bee saliva (Rocha et al., 2012). For decades, the chemical composition and properties of propolis have been extensively studied and research data have also been published in various scientific papers around the world. From 1998 until now, more than 5,000 articles on propolis have been published in Science Direct. The chemical composition of propolis varies according to geographic area, climate, environmental conditions, and harvest season (López et al., 2014). More than 420 chemical compounds have been identified in propolis from various geographic regions of the world (Bankova, 2005; Milojković Opsenica et al., 2016). Propolis has a reputation as a natural product in the world. In recent decades, it has been widely accepted in many countries as a dietary supplement for promoting health and preventing disease. In general, propolis influences human health (Azemin et al., 2018; Farooqui and Farooqui, 2012).
The health-promoting properties of propolis come from its chemical composition, including antimicrobial and antiviral (Bankova et al., 2014; das Neves et al., 2016; Nolkemper et al., 2010), antioxidant properties (Azemin et al., 2018; Mello and Hubinger, 2012; Sun et al., 2015), anticancer (Markiewicz-Zukowska et al., 2013; Xuan et al., 2014), anti-inflammatory and cytostatic (Corrêa et al., 2017; Kismet et al., 2017), immunostimulants (Nassar et al., 2012), and anti-allergic (Yasar et al., 2016). The rich bioactive components are useful in their application in various fields such as medicine and dentistry, pharmaceuticals, cosmetics, and the food industry.
Research data related to antimicrobial properties and the application of propolis extract to livestock products are quite widely published at the national and international levels. The results of research on the antimicrobial properties of propolis extract have been widely published and quite a lot related to its application. The antimicrobial properties of propolis are effective against the type of bacteria, both gram-positive and negative, molds, and fungi (gram-positive bacteria: Staphylococcus aureus, Bacillus cereus, Listeria monocytogenes, Enterococcus faecalis; gram-negative bacteria: Salmonella enteritidis, Shigella sonnei, Klebsiella pneumoniae, Escherichia coli O157, Proteus mirabilis, Enterobacter aerogenes, Pseudomonas aeruginosa; molds: Rhodotorula mucilaginosa, Candida albicans, Candida krusei, Saccharomyces cerevisiae; and fungi: Colletotrichum gloeosporoides, Alternaria solani, Fusarium solani, Rhizopus stolonifer, Botrytis cinerea, Cladosporium cladosporoides, Aspergillus niger, Aspergillus ochraceus, Mucor mucedo, Penicillium expansum, Penicillium chrysogenum; Pobiega et al., 2019a). Meanwhile, several studies stated that the antimicrobial properties of propolis extract were applied to livestock products (such as fermented meat sausage, beef patties, fresh oriental sausage, sausage, Tuscan sausage, milk, and ice cream) with various concentrations of propolis extract added (Pobiega et al., 2019b). Preliminary research was also carried out related to the use of propolis extract as food preservation in beef products stored for 24 hours. The result data shows that the higher the concentration used, the better the quality given (Andre et al., 2021). However, in this study, there is not enough data to produce comprehensive information. Thus, it is necessary to conduct a study using meta-analysis to produce information related to problems in the field of livestock products.
Regulation of propolis on the production in the food sector are grouped under the category of health products. However, the regulation depends on region on each with the requirements of registration are different. Now, the legal regulations regarding the use of propolis has been introduced in various areas, such as Brazil, the USA, European Union, Australia, Canada, China, Japan, and Korea (Berretta et al., 2017). Propolis in it is classified as a dietary supplement and has been listed on the group Directive 2002/46/EC, as a source of concentrated nutrients with physiological effects (European Parliament and Council of the European Union, 2002). However, everything depends on the stage of production and the method of extraction used [European Food Safety Authority (EFSA), 2010]. Aspects of the commercialization of propolis, especially in my region it was reported that as many as 3,957 kg/year production propolis is produced from a total of four farmer groups (Utama et al., 2021). Given the magnitude of the results of such production, along with groups from different areas and regions, propolis enough potential to be commercialized synergy with the resulting production.
A meta-analysis, according to Anwar (2005), is a statistical technique for quantitatively combining two or more original studies (a statistical procedure for combining data from various studies). Meta-analyses have been carried out in the fields of medicine, pharmacy, education, psychology, criminology, business, marketing, economics, management, nutrition, and food (Donker et al., 2014). This study aims to obtain comprehensive information through quantitative data analysis using meta-analysis. In addition to providing information related to a meta-analysis on food, especially in the livestock sector, this study aims to evaluate the effectiveness of the potential of propolis as food preservation by considering the results of the analysis of the antimicrobial compound content of propolis extract and its application to the chemical and microbiological characteristics of livestock products. Therefore, this study is expected to provide information related to natural preservatives as a solution to problems in the field of livestock products.
Materials and Methods
The meta-analysis in this study was divided into two groups which were analyzed separately using the same method. This is due to the limitations of several studies. The division of these groups, the antimicrobial activity of propolis extract, and the application of propolis extract to chemical and microbiological properties are presented in Table 1.
| Group 1 | Group 2 |
|---|---|
| (antimicrobial activity of propolis extract) | (application of propolis extract on chemical and microbiological characteristics) |
| Subgroup | |
|---|---|
| Pathogenic microbes | Chemical characteristics |
| TBARs | |
| Microbiology | |
| Microbial test | |
The stages carried out in this study were identification, selection, suitability check, and the final included articles were selected to be used in the meta-analysis. The identification stage included the number of journals produced by scientific database search engines such as Science Direct, Springer, and others using certain keywords and search results on other sources. The selection of articles was done using reference software such as Zotero and Mendeley to read the titles and abstracts of the journals and then eliminated the duplicate journals. The conformity assessment stage was carried out by viewing the full article and selecting it based on its suitability with the topic under study. The data required included complete control and experimental treatment with the average value, number of samples, and SD or SE. Articles that meet these requirements were then used for meta-analysis. The meta-analysis data processing was carried out using Microsoft Excel 2019 on the recap data of the selected article data extraction results. The articles were obtained from electronic databases, namely Science Direct and Google Scholar. The effect size value used in this study used Hedge’s d.
Results and Discussion
Initial search results on antimicrobial activity from databases of international journals and Google search engine obtained 522 articles. After removing the articles with the same content and selecting the title and abstract contents, 9 complete articles were selected which were assessed for their suitability for meta-analysis. Complete articles were excluded from 101 articles as many as 92 due to inappropriate substances such as incomplete and inappropriate information. The following 9 articles that were used are presented in Table 2. Furthermore, the search results for the application of propolis extract on livestock products from the database of international journals and the google search engine are 216 articles. After removing articles with the same content and selecting titles and abstracts, 12 complete articles were selected which were assessed for their suitability for meta-analysis. Thirty eight complete articles were excluded due to inappropriate content; 109 articles were excluded with 50 selected articles from 159 duplicated articles. The following 12 articles that were used in this study are presented in Table 3 and diagram of the meta-analysis of screening, inclusion, and exclusion of articles are presented in Fig. 1.
| Source | Study location | Extract method | N | EPc | Control | Bacterial species |
|---|---|---|---|---|---|---|
| Abdullah et al. (2020) | Brunei Darussalam | Ethanol | 55 | 2 g/L | Negative | Basillus subtilis and Staphylococcus aureus |
| Abdullah et al. (2019) | Brunei Darussalam | Ethanol | 18 | 2 mg/mL; 8 mg/mL | Negative | Staphylococcus aureus, and Pseudomonas aeruginosa |
| Seibert et al. (2019) | Brazil | Ethanol, hexane, ethyl acetate | 36 | 50 mg/mL | Negative | Staphylococcus saprophyticus, Listeria monocytogenes, and Enterococcus faecalis |
| Khodayari et al. (2019) | Iran | Ethanol | 4 | 2% | Negative | Escherichia coli |
| Rezaeigolestani et al. (2017) | Iran | Ethanol | 12 | 2% | Negative | Staphylococcus aureus, and V. parahaemolyticus |
| Kujumgiev et al. (1999) | Bulgaria | Ethanol | 6 | 0.1% | Negative | Streptococcus aureus |
| Oliveira et al. (2017) | Portugal | Ethanol | 12 | 20 uL | Negative | Staphylococcus aureus, and Salmonella Typhimurium |
| Airen et al. (2018) | India | Ethanol | 60 | 5%; 20% | Negative | L. acidophilus, and Streptococcus mutans |
| Tosi et al. (2007) | Argentina | Ethanol | 10 | 1.4 mg | Negative | Escherichia coli |
| Source | Study location | Product | N | EPc | Treatment | Output |
|---|---|---|---|---|---|---|
| Mehdizadeh and Mojaddar Langroodi (2019) | Iran | Chicken breast meat | 18 | 1% | Storage at 4°C for 16 days | Inhibits oxidative activity and prolongs the shelf life. |
| Pedonese et al. (2019) | Italia | Milk and whey cheese | 48 | 2% and 5% | Cultivation of pasteurized milk products at 37°C for 24 hours and storage for 28 days on cheese products | Inhibits the growth of Bacillus cereus, Pseudomonas fluorescens, and Staphylococcus aureus and prolongs shelf life. |
| Vargas-Sánchez et al. (2019) | Mexico | Beef patties | 4 | 2% | Storage at 2°C for 16 days | Inhibits the growth of Staphylococcus aureus and prolongs shelf life. |
| Kisa et al. (2018) | Turkey | Turkish dry-fermented sausage | 96 | 1% and 2% | Storage at 4°C for 30 days | Inhibits the occurrence of fat oxidation and the growth of mesophilic bacteria and extends the shelf life. |
| dos Reis et al. (2017) | Brazil | Burger meat | 6 | 0.3 g/kg | Storage at −16°C for 28 days | Inhibits fat oxidation reactions and prolongs shelf life. |
| Viera et al. (2016) | Brazil | Tuscan sausage | 24 | 0.5% | Storage at 4°C for 56 days | Inhibits the growth of mesophilic, psychotropic, and Staphylococcus bacteria and prolongs the shelf life. |
| Gutiérrez-Cortés and Suarez Mahecha (2014) | Colombia | Sausage | 6 | 0.8 mg/mL | Storage for 24 days | Inhibits fat oxidation. |
| Vargas-Sánchez et al. (2014) | Mexico | Beef patties | 6 | 2% | Refrigerator temperature storage for 8 days | Inhibits the growth of psychotropic bacteria. |
| El-Mossalami and Abdel-Hakeim (2013) | Egypt | Fresh Egyptian sausage | 12 | 400 and 600 mg/kg | Storage at 5°C for 21 days | Inhibits fat oxidation reactions and prolongs shelf life. |
| Ali et al. (2010) | Egypt | Fresh oriental sausage | 6 | 0.6% | Storage at 5°C for 21 days | Inhibits fat oxidation reactions and prolongs shelf life. |
| Kunrath et al. (2017) | Brazil | Salami Italian | 12 | 0.01 and 0.05% | Storage at 18°C for 35 days | Inhibit oxidation reactions and prolongs shelf life. |
| Coró et al. (2020) | Brazil | Jerked beef | 12 | 200 and 400 ppm | Storage at 25°C for 60 days | Inhibits oxidation reaction and prolongs shelf life. |
| Andre et al. (2021) | Indonesia | Beef slice | 18 | 1% and 2% | Storage at 25°C for 24 hours | Inhibits oxidation reaction. |
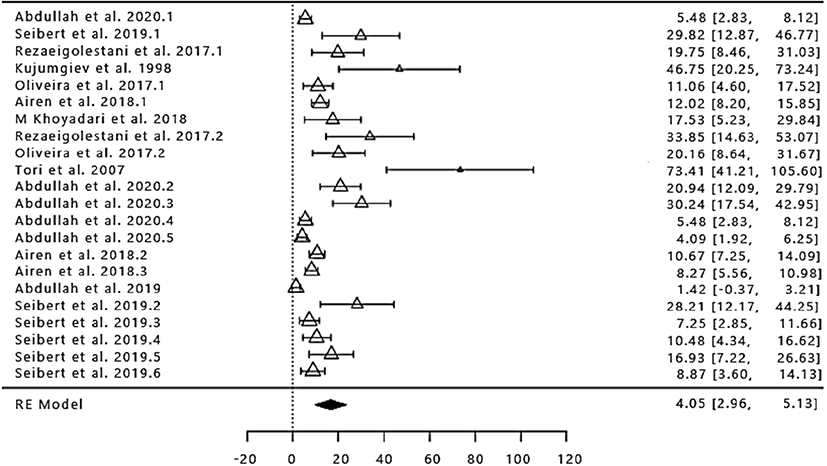
The number of study data used in the meta-analysis of the difference in antimicrobial activity through the inhibition zone diffusion method at various concentrations of propolis extract was 22 with a range of 1998 to 2020 (Fig. 2). The total number of samples/ replications in the bacterial inhibition zone of propolis extract was 182. Based on the results of the calculation with random-effect model values obtained SMD/d+ overall by 4.05 with Cl95% (2.96, 5.13; Table 4), because the confidence interval does not contain 0 (zero), then the treatment given to the experimental group different from the control group in terms of inhibiting the microbial activity. The results of the analysis showed (Table 4), then in this case the true effect size is not equal to 0. It indicates that there is a significant relationship between propolis extract with the results of the antimicrobial activity (p<0.05). However, before drawing any conclusions based on the random-effect model this would be accurate if it is proved that all research results in true effect size that is different in the population so that the need for the test of heterogeneity. Analysis of the heterogeneity of the impact on antimicrobial activity of propolis extract showed the presence of variability that occurs in all of the research (p<0.05; Table 4). Then the assumption of homogeneity needs to be rejected and accept the assumption of heterogeneity, namely the variability that occurs not more caused by sampling error (Yi = μ + τi + εi). It was also evidenced by the high percentage of I2 (inconsistency) that is equal to 97.38% (Table 4). Thus it can be concluded that propolis extract is effective in inhibiting the growth of microbes.
Further evaluation of the validation of the effectiveness of propolis extract in inhibiting the growth of microbes test is required validation against the bias of the publication using the test provided by Kendall’s test. The results of the analysis show that the p-value on the method (rank correlation test for Funnel plot Asymmetry) is symmetrical or in other words did not happen or finding evidence of a bias publication (p>0.05). Negative rank correlation (−0.096) in Table 4 indicates that research with a big sample isn’t included in the study sample meta-analysis. So the conclusion made based on the random-effect model on the effectiveness of antimicrobial activity of propolis extract is valid is indicated by the inconsistency is high and the bias publication of the resulting low through the test provided by Kendall’s (p<0.05; Table 4).
The potency of propolis extract in various studies shows that there is an activity from the influence of antimicrobial compound content with different concentrations. This is evidenced by the variability that occurs in all the studies analyzed. The variability that occurs is no longer caused by sampling error. The results showed that all propolis extracts showed strong antimicrobial activity against, namely B. subtilis, S. aureus, and P. aeruginosa (Kharsany et al., 2019; Okińczyc et al., 2020). Several alternatives can complement or can be used as a substitute for the use of synthetic preservatives, propolis provides antimicrobial effects in several studies that have been carried out (Aga et al., 1994; Farnesi et al., 2009). Furthermore, propolis has been tested as a food preservative because of its activity that can inhibit various bacteria and is safe (Tosi et al., 2007). The potential of propolis can show that propolis is economically feasible by introducing safe additive compounds as preservatives in food technology.
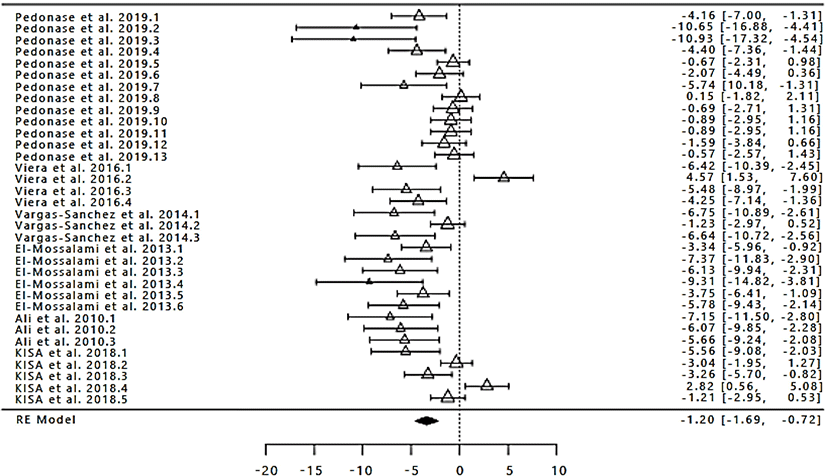
The number of study data used in the meta-analysis of differences in product microbial test results with the addition of different concentrations of propolis extract was 34 with the range from 2014 to 2019 (Fig. 3). The total number of samples/ replications in the microbial test was 188. Based on the results of the calculation with random-effect model values obtained SMD/d+ overall by −1.20 with Cl95% (−1.69, −0.72; Table 4), because the confidence interval does not contain 0 (zero), then the treatment given to the experimental group different from the control group in terms of inhibiting pathogenic microbes on products. These values indicate that the test microbes with the treatment of the addition of propolis extract on various products lower than the control. It is shown through the summary effect (the difference value is negative). The results of calculations showed (Table 4), then in this case the true effect size is not equal to 0. It indicates that there is a significant relationship between the addition of propolis extract with the results of the test microbes on the farm (p<0.05). However, before drawing any conclusions based on the random-effect model this would be accurate if it is proved that all research results in true effect size that is different in the population so that the need for the test of heterogeneity. Analysis of the heterogeneity against test microbes of the product indicates the presence of variability that occurs in all of the research (p<0.05; Table 4). Then the assumption of homogeneity needs to be rejected and accept the assumption of heterogeneity, namely the variability that occurs not more caused by sampling error. It was also evidenced by the high percentage of I2 (inconsistency) that is equal to 84.10% (Table 4). Thus it can be concluded that the addition of propolis extract is effective in inhibiting the growth of microbial pathogens in livestock products.
Further evaluation of the validation of the effectiveness of propolis extract in inhibiting the growth of pathogenic microbes necessary validation test against the bias of the publication using the test provided by Kendall’s test. The results of the analysis show that the p-value on the method (rank correlation test for Funnel plot Asymmetry) is symmetrical or in other words did not happen or finding evidence of a bias publication (p>0.05). The rank correlation value is negative (−0.179) in Table 4 indicate that research with a large sample is not included in the sample of the study meta-analysis, more dominant research with a small sample size. So the conclusion made based on the random-effect model about the effectiveness of the extract of propolis in its application as a natural preservative in products of livestock is valid is indicated by the inconsistency is high and the bias publication of the resulting low through the test provided by Kendall’s test (p>0.05; Table 4).
Propolis in terms of its use has been quite promising as a natural preservative in various food products, such as juices, fruit, and vegetables even in various livestock products (meat and milk) due to its antimicrobial and antioxidant properties (Bankova et al., 2016). The antimicrobial effect of propolis has been extensively studied, as it was reported that the use of propolis as a preservative was able to inhibit mesophilic and psychotropic bacteria in beef patties (Vargas-Sánchez et al., 2014), inhibit micrococcaceae, molds, and yeats on sausage surfaces (Ozturk, 2015), inhibit the activity of pathogenic microbes (Gutiérrez-Cortés and Suarez Mahecha, 2014), inhibit L. monocytogenes (Thamnopoulos et al., 2018), inhibit S. aureus (El-Bassiony et al., 2012), and increase the shelf life of yogurt (Özer, 2020). In this case, propolis can be used as an enrichment in food products, both as a natural additive, improving food quality, and as a natural preservative (Pobiega et al., 2019b; Seibert et al., 2019).
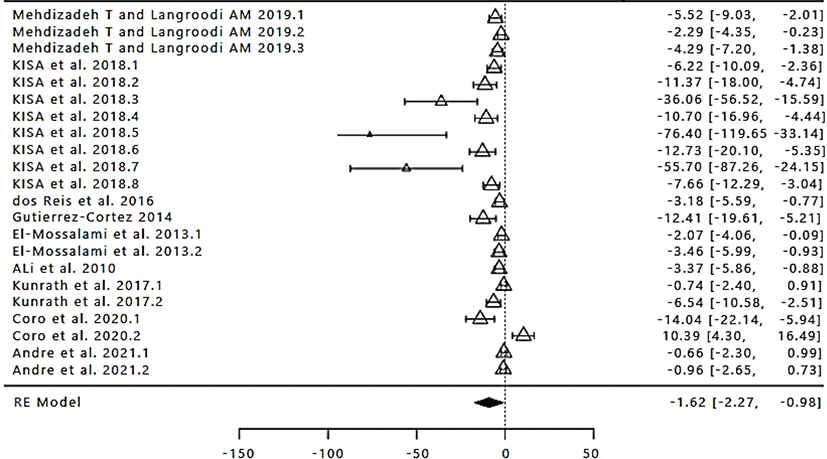
The number of study data used in the meta-analysis of the difference in TBARs values in products with the addition of various concentrations of propolis extract was 22 with the range from 2010 to 2021 (Fig. 4). The total number of samples/ replicates on the TBARs value of the product with the addition of propolis extract was 132. Based on the results of the calculation with a random-effect model value obtained SMD/d+ overall by −1.62 with Cl95% (−2.27, −0.98; Table 4), because the confidence interval does not contain 0 (zero), then the treatment given to the experimental group different from the control group in terms of inhibiting the oxidation of lipids on the farm with the addition of propolis extract as a natural preservative. These values indicate that the TBARs value with the addition of propolis extract on various products lower than the control treatment. It is shown through the summary effect (the difference value is negative). The results of the analysis showed (Table 4), then in this case the true effect size is not equal to 0. It indicates there is a significant relationship between the addition of propolis extract with the TBARs value produced on products (p<0.05). However, before drawing any conclusions based on the random-effect model this would be accurate if it is proved that all the research produces true effect size differences in the population so that the need for the test of heterogeneity. The analysis of heterogeneity on the value of TBARs product indicates the presence of variability that occurs in all of the research (p<0.05; Table 4). Then the assumption of homogeneity needs to be rejected and accept the assumption of heterogeneity, namely the variability that occurs not more caused by sampling error. This was evidenced by the high percentage of I2 (inconsistency) that is equal to 99.32% (Table 4). Thus it can be concluded that the addition of propolis extract is effective in inhibiting the oxidation reaction on the product as indicated by TBARs value.
Further evaluation of the validation of the effectiveness of propolis extract in inhibiting the oxidation reaction required a validation test against the bias of the publication using the test provided by Kendall’s test. The results of the analysis show that the p-value on the method (rank correlation test for Funnel plot Asymmetry) is symmetrical or in other words did not happen or finding evidence of a bias publication (p>0.05). The positive rank correlation (0.048) in Table 4 indicates that research with a large sample is included in the study sample meta-analysis. So the conclusion made based on the random-effect model about the effectiveness of the extract of propolis in its application as a natural preservative to inhibit the oxidation reaction on the product is the result of breeding is valid is indicated by the inconsistency is high and the bias publication of the resulting low through the test provided by Kendall’s test (p>0.05; Table 4).
Fat oxidation is one of the main causes of food spoilage. This is indicated by the resulting TBARs value. Several cases reported that propolis can inhibit the reduction of fat oxidation in sausage products (Ali et al., 2010), beef patties (Vargas-Sánchez et al., 2014), and research articles used in this study. Preliminary research also reported that giving propolis extract to beef products stored at room temperature for 24 hours was able to inhibit fat oxidation, the greater the concentration of propolis extract added, the smaller the TBARs value produced (Andre et al., 2021).
The chemical composition of propolis is composed of resin (flavonoids and phenolic compounds) by 42% to 58%, candles and oil (oleic acid and palmitic acid fiber of essential oils and aromatic) by 33% to 47%, polen (protein, free amino acids, vitamins, and minerals) of 3% to 5%, and other components (ketones, lactones, steroids, and sugars) by 2% to 5% (Barlak, 2009; Burdock, 1998; Değirmencioğlu, 2018).
The difference of opinion related to the mechanism of action of flavonoids in inhibiting the growth of bacteria. Flavonoids cause damage to the permeability of the bacterial cell wall, microsomes, and lysosomes as a result of the interaction between flavonoids with DNA of bacteria (Bryan, 1982; Wilson, 1982). Flavonoids are able to release energy tranduksi against the cytoplasmic membrane of bacteria it also inhibits the motility of bacteria (Mirzoeva et al., 1997). A different mechanism is also reported that the hydroxyl groups contained in the structure of the flavonoid compounds cause changes in the organic component and the transport of nutrients which will eventually lead to the onset of toxic effects against bacteria (Di Carlo et al., 1999; Estrela et al., 1995). Although the mechanism of the detail of the antibacterial activity of propolis is still unknown (Santos et al., 2002), the possibility it is related to the compound polar and phenolic lipophilic namely flavonoid compounds. The compound has a carbonyl electronegative, amen, imina, sulfid, thiol, metoksil, and hydroxyl groups are very polar and lipophilic, and is responsible for contact with the bacterial cells and induce damage to the structure of the cell wall and membrane, causing cell lysis and death (Cushnie and Lamb, 2005; Cushnie et al., 2003; Echeverría et al., 2017; Kim and Chung, 2011; Sanpa et al., 2015).
Conclusion
The effectiveness of propolis extract as a natural preservative in products of livestock indicates the presence of a significant relationship between the addition of propolis extract at various concentrations of the antimicrobial activity as well as test microbes and the value of TBARs in its application in a variety of storage. Based on the random-effect model on the effectiveness of antimicrobial activity of propolis extract and its application as a natural preservative against the characteristics of the chemical and microbiological analysis on products of livestock is valid and not the discovery of bias publication that is produced through the test provided by kendall’s. Propolis in this case effectively used as natural preservatives in the products of livestock.













