Introduction
Fruits are rich source of bioactive compounds such as phenolics, ascorbic acid and carotenoids that have direct influence over the radical-scavenging potential as well as antimicrobial properties. The mode of action of most of these phyto-bioactive ingredients in alleviating the oxidative stress has been ascribed to their radical-scavenging activity. Public awareness, nutritional requirements as well as health consciousness has escalated the demand of alternative, newer, economical, nutritional and multifunctional natural dietary antioxidant and antimicrobial mainly sourced from various fruits, herbs and their agro-industry byproducts. Huge quantities of fruits are wasted annually due to lack of proper preservation, storage and processing. Thus by adopting suitable novel harvesting technology, these could be efficiently used leading to profitable returns to the industry and controlling environmental hazards.
Sapota, a popular tropical fruit remain underutilized as huge quantity of the fruit is wasted globally due to early ripening, softening and chilling injuries during cold storage. The processing of sapota remains a challenge due to change in colour, flavour and high cost involve in peeling skin and removal of seeds. Sapota is an excellent source of sugars (12–18%), dietary fibre (2.6%), ascorbic acid (6.0 mg/100 g), phenolic compounds (15.35 mg GAE/100 g) and minerals such as calcium (28 mg/100 g), iron (2.0 mg/100 g), phosphorous (27 mg/100 g), copper (0.086 mg/100 g) and potassium (193 mg/100 g) (Ugalat et al., 2012). Sapota is widely used as laxative and regularization of bowl movements due to high fibre content, presence of simple sugars and bioactive compounds (Siddiqui et al., 2014). Sapota fruit has been reported to have highest antioxidant activity among tropical fruits, mainly due to presence of free radical scavenging compounds such as ascorbic acid, carotenoids and polyphenolic compounds such as D-quercitol, quercitin, myricitrin, gallic acid, epigenin etc. The antioxidant potential of edible part of sapota fruits have been reported to very high (404.75 µm Trolox equivalent /100 g) (Moo-Huchin et al., 2014; Ribeiro da Silva et al., 2014).
Meat and meat products are inherently low in antioxidant compounds and are prone for lipid peroxidation and microbial spoilage due to unsaturated fatty acids coupled with high moisture and nutrients (Chatli et al., 2015; Kumar et al., 2015a,b). The lipid peroxidation deteriorates the nutritive value and compromises organoleptic properties due to production of off-odours, changes in colour and appearances. Packaging has a very crucial role in extending storage life of meat products by inhibiting microbial growth; avoid contamination, maintenance and stability of desired colour, minimization of water loss and marketing. There is an ever increasing preference of natural antioxidants and antimicrobial compounds over synthetic compounds in food products due to health concerns. Thus, sapota could be a suitable alternative to synthetic antioxidants and antimicrobial compounds in various food products. Better utilization of sapota powder (SP) in food processing would also ensure in profitable returns to farmers and overall integrated development of agro-meat industry.
Thus, present study was envisaged to assess antioxidant and antimicrobial efficacy of SP in pork patties under aerobic and MAP at refrigeration temperature as indicated by various physico-chemical, lipid peroxidation and microbiological characteristics.
Materials and Methods
Sapota fruits were procured from local market of Ludhiana, India and washed with potable water to remove any adhering extraneous material. Drying of sapota was done as per Ganjyal et al. (2003) with suitable modifications. After removal of seeds with the help of sharp blade, these were sliced into thin cuts of circular shape (5 mm thick) and heated in microwave oven at low frequency (914 Hz) for 12 minutes. It was further dried in hot-air oven till the moisture reached below 12%. The dried sapota chunks were ground in a grinder (Inalsa) to make fine powder.
Lean pork was obtained by scientifically slaughter of castrated Large White Yorkshire pigs (Age- 8 months, weighing 75–90 kg) in the departmental slaughter house by following standard protocol. The carcass was immediately chilled under refrigeration followed by manual deboning and was subsequently stored in a deep freezer at –18±2°C in low density polyethylene (LDPE) packages till use. The frozen deboned meat was thawed, cut into small chunks of uniform size and minced twice through meat mincer (MADO Eskimo Mew 714, Spain). Four groups of pork emulsions (control (C) and treatments viz. T1, T2, and T3 with varying combinations of SP/lean meat as 2.0/70.5, 4/68.5, and 6/66.5%, respectively) were prepared. The other ingredients incorporated during preparation of pork emulsion were animal fat (lard) (5%), ice flakes (10%), whole egg liquid (2.5%), salt (1.7%), sodium tetra-polyphosphate (STPP) (0.3%), condiment mix (Onion, garlic and ginger, 3:1:1) (3.0%), spice mix (2.0%), refined wheat flour (3.0%) and sodium nitrite (0.012%).
Minced pork with salts was chopped in a bowl chopper (Model:TC11, Scharfen, Germany) for 1 min followed by addition of ice flakes and again chopping for 1.5 min. Ground pork fat was slowly added and further chopping was done for 2–2.5 min. The remaining ingredients were added and chopping was continued for 1 min. The emulsion was filled in moulds (80×20 mm) and cooked in pre-heated hot air oven at 180±2°C for 20 min with regular turning after 10 min for uniform cooking and better colour. Cooked product were tempered at room temperature and evaluated for various quality parameters and optimum level of sapota powder incorporation was selected.
The selected as well as control patties were packaged in 140 gauge LDPE bags and Polyester/Polyethylene laminated plastic pouches (100/100 μm) for lipid peroxidation and microbial quality analysis under aerobic and MAP (70% CO2, 30% N2) (Ramon Packaging Machine: VP-580 A Model, type 19/S/CL, Germany), respectively. The samples were stored at refrigeration temperature (4±1°C) and drawn at weekly interval till 42 days for analysis of physico-chemical, lipid peroxidation, microbial count and sensory attributes.
The pH was recorded by using digital pH meter (SAB 5000, LABINDIA, Mumbai), water activity (aw) was measured with potable digital water activity meter (Rotronix HYGRO Palm AW1 Set, Rotronix Instrument (UK) Ltd., UK) and emulsion stability was evaluated as per Townsend et al. (1968).
The moisture (Oven drying), fat (Soxhlet method) and protein (Kjeldahl distillation) and ash (muffle furnace) of pork patties were determined as per Association of Official Analytical Chemists (AOAC, 2000). Total calories content were calculated by using Atwater values for protein (4.02 kcal/g), carbohydrate (4 kcal/g) and fat (9 kcal/g).
Cooking yield was determined by taking into account the difference in the product weight before and after cooking-
Cooking yield (%)=[Weight of uncooked pork patties/Weight of cooked pork patties]×100
Various dimensional parameters such as decrease in diameter and increase in height of developed products were measured by using vernier caliper at three different places and average value was taken-
Instrumental color profile of pork patties were measured using Lovibond Tintometer (Lovibond RT-300, Reflectance Tintometer, United Kingdom). The Hue and chroma were calculated by using following formulas.
Texture profile analysis of pork patties were measured by using a Texture Analyzer (TMS-PRO, Food Technology Corporation, Maries Road, Suite 120 Sterling, USA) as per Bourne (1978).
Various lipid oxidation parameters such as thiobarbituric acid reactive substances (TBARS) (Witte et al., 1970), free fatty acids (FFA) and peroxide value (PV) (Koniecko, 1979) were determined to assess the antioxidant efficacy of SP in pork patties. The ability to scavenge 2,2′-diphenyl-1-picrylhydrazyl (DPPH) radical by added antioxidants in samples was estimated following the method of Kato et al. (1998). The polyphenol content was quantified by Folin-Ciocalteau’s reagent assay and expressed as Gallic acid equivalents (mg/g) (Yuan et al., 2005). The spectrophotometric analysis of 2,2’-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid cationic ion (ABTS) radical scavenging activity was determined according to method of Shirwaikar et al. (2006).
Microbiological quality viz. standard plate count, coliforms count, psychrophilic count and yeast and mold count of pork patties were determined as per method given by APHA (1984). Sample preparation and serial dilution were performed under aseptic conditions, near flame in pre-sterilized horizontal laminar flow apparatus (Model Rescholar, M. No. RH-58-C3, Ambala Cantt, India).
A panel of twelve experienced members (6 male and 6 female) evaluated pork patties samples for appearance and colour, texture, flavour, juiciness and overall acceptability on 8-point descriptive scale (Keeton, 1983), where 8=extremely liked and 1=extremely unliked. Samples were warmed (40–45°C) using a microwave oven (LG Electronics India Pvt. Ltd., Mumbai) for 1.5 min before serving to panelist in sensory laboratory of the Department. The samples were blind-coded by using 2-digit numbers and presented to the panelists in random order on white colour glass plates. Aiming to check reliability of the results, control sample was introduced in evaluations two times, randomized among other samples. The panelists rated samples for appearance and colour, flavour, juiciness, tenderness and overall acceptability.
Data obtained during various experiments were analyzed on SPSS-20.0 software packages, IBM Corporation, USA (Snedecor and Cochran, 1989). Duplicate samples were drawn for each parameter and whole set of experiment was replicated six times (n=6). Means between periods of storage were compared by two-way analysis of variance (ANOVA). The statistical significance was estimated at 5% level (p<0.05) and evaluated with Duncan’s Multiple Range Test.
Results and Discussion
Antioxidant potential of SP was measured by carotene content, ascorbic acid, total phenolics, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS). ABTS+ radical scavengering capacity (% inhibition), DPPH (% inhibition) and total phenolics (GAE mg/g) contents of SP were found to be 91.26±0.24, 12.05±0.24 and 3.49±0.17, respectively. The pH and aw value of SP was recorded 5.53±0.52 and 0.40±0.01, respectively. The SP had appreciable amount of carbohydrates (28.45±0.43), protein (8.06±0.52), fat (3.17±0.06) and minerals (0.80±0.01). Siddiqui et al. (2014) and Woo et al. (2013) also reported similar findings.
Various physico-chemical parameters as well as proximate composition of pork patties incorporated with various levels of SP as well as control are presented in Table 1. The pH of both raw emulsion and cooked pork patties decreased significantly (p<0.05) with the increasing levels of SP. This could be attributed to lower pH (5.53) of SP. The increased pH of product than raw emulsion might be due to higher degree of protein denaturation and production of imidazolium during cooking. An increase (p<0.05) in emulsion stability in treated patties were recorded with highest value for T3. The higher emulsion stability in turn resulted in higher cooking yield and lower cooking losses. The cooking yield was significantly (p<0.05) higher for patties incorporated with 6% SP (T3) than other treated products as well as control. This might be due to formation of stable emulsion with improved water holding capacity and water binding properties of dietary fibre present in SP (2.60 g/100 g). The increase in emulsion stability and cooking yield upon incorporation of various plant powders enriched in fibres have been reported such as finger millet flour in chevon patties (Kumar et al., 2015a), emu meat nuggets (Chatli et al., 2015) and sweet potato powder in pork patties (Verma et al., 2015a), carrot and broccoli powder in meat cutlets (Singh et al., 2015a; Singh et al., 2015b). Water activity (aw) of SP incorporated patties was recorded lower than control and followed an increasing (p<0.05) trend with the increase in level of SP. It might be due to the water binding properties of SP, leading to higher moisture retention in the cooked product.
Proximate composition of cooked patties observed a significant (p<0.05) decrease in protein, fat and ash content in treatments than control, whereas a corresponding increase in carbohydrate content was noticed. The moisture content of emulsion followed a decreasing trend with the increasing levels of SP incorporation, whereas for cooked products, moisture content of control and treatments were comparable. This might be due to replacement of pork with SP, containing less moisture. The protein content of control patties was significantly higher (p<0.05) than treatments whereas estimated carbohydrate content showed an increasing trends with increasing SP levels. Moisture protein ratio (M:P ratio) was significantly higher (p<0.05) in treated products than control and noted increasing trends with the increase in the level of SP. The trends were well correlated to higher cooking yield and moisture retention in the treated products. The ash content of treatment products decreased significantly (p<0.05) with the increasing SP levels. The dimensional parameters viz. increase in height (%) and decrease in diameter (%) were better retained in the treated patties than control patties. The better retained dimensional parameters in treated products could be due to higher moisture retention, cooking yield and emulsion stability of these products attributed to inherent stabilizing and binding properties of SP containing high fibre content. Similar findings were also reported by Bhat et al. (2015) in chicken nuggets incorporated with Aloe vera gel.
SP incorporation had significantly (p<0.05) affected instrumental colour attributes of pork patties (Table 2). Lightness (L*) increased, while redness (a*) and yellowness (b*) values decreased significantly (p<0.05) upon increasing level of SP incorporation. This might be due to non-enzymatic browning reaction (Maillard reaction) between sugars of SP and meat proteins during cooking. A lower pH in treated products facilitates conversion of metmyoglobin to heamochromogen upon cooking (Thomas et al., 2016). Yellowness (b* values) decreased significantly (p<0.05) in treatments as compared to control, and lowest value was recorded for T3 whereas values for T1 and T2 were comparable.
All textural attributes of SP added pork patties was significantly (p<0.05) lower than control and a decreasing trends was observed with the increasing level of SP in treated products (Table 2). This might be due to lower pH of emulsion and formation of good quality gel matrix due to lower muscle protein denaturation, as well as better moisture and fat retention and water binding properties of treated products due to incorporation of SP (Thomas et al., 2016).
All the sensory attributes viz. appearance and colour, flavour, juiciness, tenderness and overall acceptability improved upto 4% SP (T2) incorporation afterward scores for all sensory parameters decreased at 6% level (T3) (Table 2). The appearance score was recorded highest for control, which was comparable to T1 and T2, which in turn was significantly higher (p<0.05) than T3. The flavour score of T2 were recorded highest and it was comparable to T1 and control. This could be due to generation of volatile compounds and controlled release of these compounds. The juiciness scores were recorded highest for T2 amongst the treated products and control. This could be due to the moisture retention and water binding properties of SP as depicted in Table 1. By incorporation of SP, tenderness was found to be improved (p<0.05) in T2 and T3. These results indicated that incorporation of SP enhanced tenderness of patties was also in accordance with decreased in hardness values. The pork patties incorporated with 4% SP (T2) was rated highest for overall acceptability by the sensory panelists. The starch present in SP had positive effect on the sensory quality such as flavour, juiciness, tenderness and overall acceptability of various meat products such as pork patties (Verma et al., 2015a) etc.
Thus based on the sensory attributes, pork patties with 4% incorporation of SP (T2) was found optimum and selected for further storage studies with control under aerobic (CA, TA) and MAP (CM, TM) at refrigeration temperature (4±1°C) for 42 days.
During refrigerated storage, physico-chemical, fat peroxidation, microbiological and sensory attributes were assessed at regular interval of 7 days till 42nd day of storage. The main criteria for spoilage of samples were flavour emanating at the time of opening of packets for analysis. Any abnormal development in colour and sliminess were also given due consideration.
The pH was comparable between treatments in both aerobic and MAP packaging conditions on 1st day and it showed increasing trends upon advancement of storage period (Table 3). The pH of treated patties was lower than control and the same trends continued throughout the storage period under aerobic as well as modified atmosphere packaging. The increase in pH of pork patties upon subsequent storage could be due to increase in microbial load leading to protein degradation of pork patties and accumulation of metabolites (Kyrana et al., 1997; Lawrie, 1998). The critical analysis of pH revealed that variation in the pH of MAP packaged patties (CM and TM) was at slower rate due to buffering capacity of carbonic acid produced due to influx of CO2 gas as well as lower microbial load in the package.
Mean±standard error with different superscripts row wise (small alphabet) and column wise (capital alphabet) differ significantly (p<0.05). n=6 for each treatment except for sensory n=72.
CA, control product under aerobic packaging, TA, product with 4% Sapota powder under aerobic packaging; CM, control product under modified atmosphere packaging; TM, product with 4% Sapota powder under modified atmosphere packaging; ND, not detected.
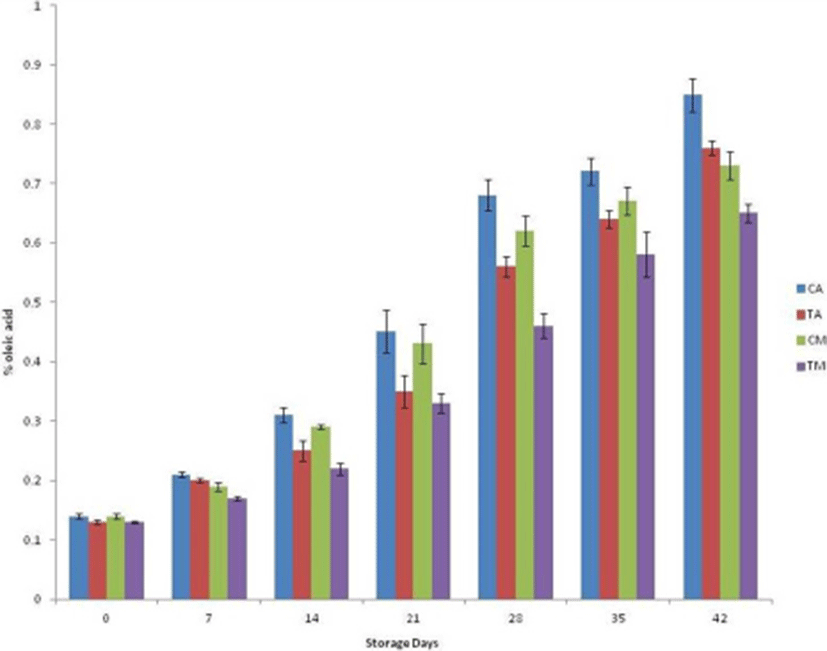
Patties with 4% SP had lower TBARS values on the day of processing compared to the control (Fig. 1) and increased upon subsequent storage. The rate of increase of TBARS value under MAP was lower as compared to aerobic packaging. This could be attributed to the increased rate of lipid oxidation with increasing levels of microbial population with elapse of storage time, as a positive correlation between microbial load and TBARS number has been reported (Raja et al., 2014). Throughout storage, TBARS value of CA was recorded highest and TMAP was recorded lowest among all samples. This could be attributed to presence of antioxidant compounds in SP exerting antioxidant activity.
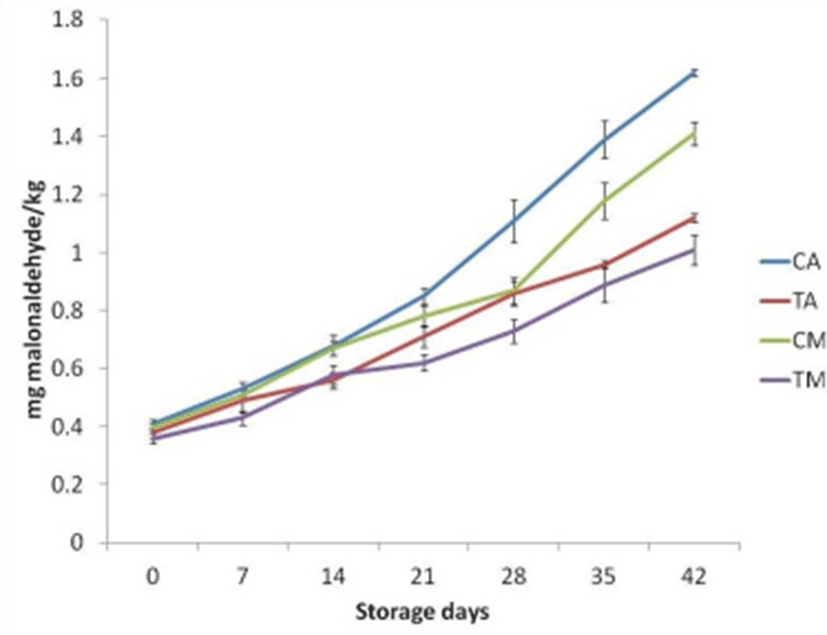
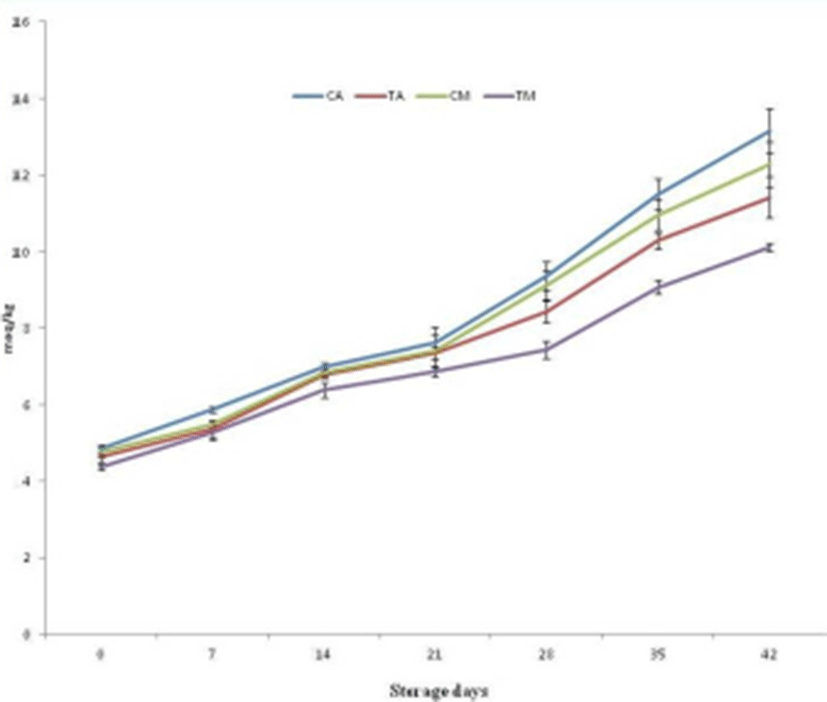
Free fatty acid values of pork patties recorded increasing trends with advancement of storage period and FFA value of control sample (CA) was recorded highest than other samples (Fig. 3). Under aerobic storage, FFA value increased with the advancement of storage period from day 1, whereas for MAP, FFA values were remain comparable for 7th day and afterward, recorded significantly (p<0.05) increased value upto 42th day of storage. FFA value was recorded lower for MAP patties than aerobic packaged patties throughout the storage period. The PV was noted significantly lower (p<0.05) in treated pork patties under MAP storage than control under aerobic storage throughout the storage period (Fig. 2). It might be due to inhibition of lipid peroxidation, attributed to polyphenolic compounds in SP acting as antioxidants by terminating free radical chain-type reactions. Further PV was recorded lower for the MAP products than aerobically packaged (AP) products due to the absence of the O2 in MAP, regarded as chelating agent for lipid oxidation (Ahn et al., 2004) as well lower microbial count due to antimicrobial activity of CO2.
Addition of SP at 4% level has resulted in significantly lower (p<0.05) microbial count in treated patties (Table 3). SPC increased significantly (p<0.05) in all samples throughout storage period with elapse of time, but incorporation of SP significantly (p<0.05) decreased the SPC in treated samples. This might be due to the direct inhibitory action of lower pH on microbial growth by causing initial cell injury and presence of polyphenolic compounds in SP possessing anti-microbial properties by prolonging lag phase. Leong and Shui (2002) also reported sapota as one of the fruit rich in polyphenolic compounds. Ma et al. (2003) noted methyl chlorogenate, dihydromyricetin, quercitrin, myricitrin, (+)-catechin, (–)-epicatechin, (+)-gallocatechin, and gallic acidas major polyphenolic compounds isolated from sapota fruit exerting antimicrobial and antifungal effects. Sliminess was noticeable in AP control samples on day 35 while in MAP control group on day 42. The spoilage of meat products is noticed when bacterial counts are recorded 108 cells/cm2 or more (Jay, 1996), however, in our study, spoilage of pork nuggets was noticed at bacterial counts below 7 Log CFU/g. The lower microbial count in MAP products might be due to the addition of 70% CO2 gas in the package, which act on lag phase of bacterial growth cycle and produces carbonic acid, inhibiting bacterial growth of package. Coliforms, an indicator of post-processing contamination, were detected during storage studies on 14th day in control samples, 21st day in TA and 28th day in TM. MAP samples reported lower coliforms count than aerobically packaged samples on same day. Narender et al. (2017) also reported significant antimicrobial and antifungal effect of sapota peel oon some common food spoilage and food borne microorganisms including E. coli, Staphylococcus aureus, Lactobacillus, Proteus vulgaris and Saccharomyces cerevisiae.
Psychrophills were detected on the day of processing in control samples whereas on day 14 in treated samples and thereafter, recorded increasing trends in all samples. The initial absence of psychrophilic count during storage could be due to lower metabolic rate of these microbes at low pH, retarding log phase. It was lower (p<0.05) in SP incorporated patties than control in both, aerobic as well as MAP conditions. On the last day of storage, lowest count was noted in TM and highest in CA. Staphylococcus aureus were detected on 14th day in CA, 21st day in TA, 28th day in CM and 35th day in TM. The yeast and mold count were detected on 14th day of storage irrespective of treatments and packaging conditions and remain comparable upto 21 day and thereafter, significantly lower counts were recorded for treated samples as compared to control samples. This could be due to lower moisture content, lower pH as well as presence of polyphenolic compounds such as flavonoids, sterols and prenylflavones in SP exerting antifungal properties (Verma et al., 2015).
The sensory evaluation was not conducted for CA on 28th day onward and CM on 35th day onwards (Table 3). The appearance scores were recorded higher for treated samples than control and followed decreasing trends with advancement of storage period. This might be due to better colour development due to controlled non enzymatic reactions in SP incorporated treatments. Bhat et al. (2015) also noted decreased colour and appearance score of chicken meat nuggets during storage and attributed it to the pigment and lipid oxidation, resulting in non-enzymatic browning. The MAP treated products were recorded highest flavour scores due to retardation of oxidation of lipids leading to development of off-flavour. These results are in correlation with higher microbial load and lipid oxidative parameters (TBARS, PV and FFA). The gradual decrease in flavour score could be attributed to increasing lipid oxidation due to microbial growth, production of FFA and oxidative rancidity. Juiciness and tenderness scores was higher for control than treated patties and followed decreasing trend with the increase in the storage period irrespective of packaging conditions. The rate of decrease was higher in AP samples than MAP samples due to prevention of moisture loss from double layered laminated films. This reduction in juiciness and tenderness scores might be due to increasing protein denaturation leading to change in disulphide bonds due to higher microbial load and lower moisture and fat retention properties at low pH due to incorporation of SP.
Gradual decrease in overall acceptability scores of pork patties with elapse of storage days could be due to reduction in the value of other sensory attributes. Overall acceptability of the pork patties followed the same pattern observed for that of flavour and a significant decrease (p<0.05) in overall acceptance of all samples were observed towards the end of storage period. However, the sensory attributes of pork patties incorporated 4% SP was found in the close to moderately acceptable category (5.95 in AP and 5.91 in MAP packets). Thus treated samples extended storage life upto 35 and 42 days under AP and MAP, respectively due to better antioxidant and antimicrobial properties attributed due to SP incorporation.
Conclusions
SP is a rich source of bioactive compounds exerting antioxidant and antimicrobial effects. It can be effectively utilized in the development of pork patties with enhanced oxidative stability and microbial quality. The developed patties were recorded safe during refrigeration (4±1°C) storage for 35 days in aerobic and 42 days in modified atmosphere packaging conditions based on sensory attributes and microbiological quality.













