ARTICLE
Multiclass Method for the Determination of Anthelmintic and Antiprotozoal Drugs in Livestock Products by Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry
Hyunjin Park1
,
Eunjung Kim1,*
,
Tae Ho Lee1
,
Sihyun Park1
,
Jang-Duck Choi1
,
Guiim Moon1
Author Information & Copyright ▼
1Pesticide and Veterinary Drug Residues Division, National Institute of Food and Drug Safety Evaluation, Ministry of Food and Drug Safety, Osong 28159, Korea
*Corresponding author : Eunjung Kim, Pesticide and Veterinary Drug Residues Division, National Institute of Food and Drug Safety Evaluation, Ministry of Food and Drug Safety, Osong 28159, Korea, Tel: +82-43-719-4211, Fax: +82-43-719-4200, E-mail:
ejkim81@korea.kr
© Korean Society for Food Science of Animal Resources. This is an Open-Access article distributed under the terms of the
Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits
unrestricted non-commercial use, distribution, and reproduction in any
medium, provided the original work is properly cited.
Received: Jun 02, 2023 ; Revised: Jul 19, 2023 ; Accepted: Jul 22, 2023
Published Online: Sep 01, 2023
Abstract
The objective of this study was to establish a multi-residue quantitative method for the analysis of anthelmintic and antiprotozoal drugs in various livestock products (beef, pork, and chicken) using ultra-high-performance liquid chromatography–tandem mass spectrometry. Each compound performed validation at three different levels i.e., 0.5, 1, and 2× the maximum residue limit according to the CODEX guidelines (CAC/GL 71-2009). This study was conducted according to the modified quick, easy, cheap, effective, rugged, and safe procedure. The matrix-matched calibrations gave correlation coefficients >0.98, and the obtained recoveries were in the range of 60.2%–119.9%, with coefficients of variation ≤32.0%. Furthermore, the detection and quantification limits of the method were in the ranges of 0.03–3.2 and 0.1–9.7 μg/kg, respectively. Moreover, a survey of residual anthelmintic and antiprotozoal drugs was also carried out in 30 samples of beef, pork, and chicken collected in Korea. Toltrazuril sulfone was detected in all three samples. Thus, our results indicated that the developed method is suitable for determining the anthelmintic and antiprotozoal drug contents in livestock products.
Keywords: anthelmintic; antiprotozoal; livestock products; ultra-high-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS); multi-class analysis
Introduction
With the consumption of livestock products increasing annually, veterinary drugs are being increasingly employed to promote growth and prevent and treat disease (Zeleny et al., 2006). If these drugs or their metabolites are not fully excreted, consuming the derived animal products can lead to potential health risks for the consumers. To address this issue, the residual tolerance standards of such compounds are strictly regulated (Beyene, 2016). Generally, such residues are generated because of excessive use or noncompliance with the withdrawal period (Danaher et al., 2007; Delatour et al., 1981; Whittaker and Faustman, 1992). Veterinary medicines include antibiotics, synthetic antibacterial agents, nervous system drugs, hormones, anticoccidial drugs, antimicrobial agents, and anthelmintics (Rana et al., 2019). Despite their advantages, antibiotics have been reported to lead to the generation and propagation of resistant bacteria, in addition to the induction of hypersensitivity reactions, tumor induction, abnormal physical development, and teratogenesis (Abbas et al., 2011; González-Díaz et al., 2005). Thus, maximum residue limits (MRLs) have been set for 193 substances in Korea, including 26 banned substances. For example, the number of MRLs of the anthelmintic and antiprotozoal drugs are 26 and 23, respectively (MFDS, 2023).
As one example drug class, anthelmintic drugs are used to treat parasites (Danaher et al., 2007). More specifically, the benzimidazoles (e.g., albendazole, cambendazole, carbendazim, febantel, flubendazole, oxfendazole, oxibendazole, mebendazole, thiabendazole, and triclabendazole) are widely used in agriculture (Cano et al., 1987). In addition, avermectin is a macrocyclic lactone anthelmintic agent produced by Streptomyces avermitiles. To broaden its therapeutic range, the original structure of avermectin has been modified by substitution to give abamectin, ivermectin, doramectin, and eprinomectin. In this group of compounds, abamectin is used as an insecticide, and its side effects include psychosis, respiratory failure, and hypotension (Wang et al., 2009). In addition, ivermectin is a hydrogenated version of abamectin that is effective in treating onchocerciasis, despite causing various side effects, such as a rash, swelling, headache, and dizziness (Hoyos et al., 2017).
In contrast, antiprotozoal drugs are used to treat protozoan infections. In particular, coccidiostats are used to prevent or treat coccidiosis, which is a disease caused by protozoan parasites that parasitize and attack the digestive tract of animals, causing diarrhea and secondary infections such as enteritis (Roila et al., 2019; Rusko et al., 2019).
According to previous studies, anthelmintic and antiprotozoal drugs frequently exceed the MRL. For example, Lee et al. (2017) confirmed MRL violations in pigs treated with mebendazole, while Escribano et al. (2012) confirmed an excess of ivermectin in the liver and milk of cattle, sheep, pigs, and rabbits. Moreover, Cooper et al. (2012) reported that the MRLs of rafoxanide and doramectin were violated in pigs. Thus, the development of novel methods with high sensitivities and resolutions is required due to the frequent occurrences of anthelmintic and antiprotozoal drugs in animal samples. In this paper shows excellent sensitivity compared to other studies (Clarke et al., 2013; Kang et al., 2014; Kang et al., 2015). To ensure domestic food safety in Korea, The Ministry of Food and Drug Safety is preparing to introduce a positive list system (PLS). The PLS Program for veterinary drugs covers livestock and fishery products produced in 2024 or beyond. Previously, the CODEX guidelines (CAC/GL 71-2009) were applied in cases where no MRL had been previously established in Korea; alternatively, the lowest MRL established for similar products was employed. However, with the introduction of the PLS, a limit of 10 μg/kg is applied if a Korean MRL is unavailable. Therefore, a rapid, highly sensitive, and reliable analytic method is required to prepare for the introduction of the PLS. Among the various analytical techniques reported to date, ultra-high-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) has become a popular technique for analyzing veterinary drugs owing to its ability to analyze a wide range of compounds at low levels quickly (Moloney et al., 2012). In addition, according to recent study trends, the quick, easy, cheap, effective, rugged, and safe (QuEChERS) method has been used to develop multi-residue analytical approaches for analyzing pesticides and veterinary drugs in various matrices. The QuEChERS approach is flexible and can be modified depending on the matrix and the properties of the analyte. This method is beneficial because it minimizes the time required to complete the extraction and cleanup processes, while also reducing the cost of analysis (Chen et al., 2021; Kang et al., 2014; Stubbings and Bigwood, 2009; Ye et al., 2022).
Thus, by applying a modified QuEChERS approach, this study aims to increase the extraction efficiency by adding anhydrous magnesium sulfate (MgSO4) and sodium chloride (NaCl) to remove moisture and interfering substances from the sample. This is followed by the separation of the extraction solution and the aqueous layer using the salting-out method. Furthermore, during the dispersive solid-phase extraction (d-SPE) step, MgSO4, primary secondary amine (PSA), and C18 are used for matrix cleanup. Consequently, this study aims to verify the sensitivity and quantitation of 54 anthelmintic and antiprotozoal drugs that are commonly present in livestock products, and this will be achieved using a modified QuEChERS extraction and purification approach, followed by UHPLC-MS/MS analysis.
Materials and Methods
Chemicals and reagents
The following standards were purchased from Sigma-Aldrich (St. Louis, MO, USA): 5-hydroxy thiabendazole, albendazole sulfoxide, bithionol, carbendazim, fluazuron, keto triclabendazole, isometamidium, ternidazole, thiophanate, and toltrazuril sulfone. Arprinocide, benznidazole, diethylcarbamazine, and halofuginone were purchased from Toronto Research Chemicals (Toronto, ON, Canada). Emamectin b1a (emamectin) and ornidazole were purchased from ChemService (West Chester, PA, USA) and StordSynthesis (Hebei, China), respectively. The rest of the 42 compounds (abamectin, albendazole, albendazole sulfone, etc.) were purchased from Dr. Ehrenstorfer (Augsburg, Germany). Methanol (MeOH) and acetonitrile (MeCN) were purchased from Merck (Darmstadt, Germany). Dimethyl sulfoxide (DMSO), formic acid, MgSO4, and NaCl were purchased from Sigma-Aldrich, and PSA was purchased from Agilent Technologies (Santa Clara, CA, USA). Ammonium formate was purchased from Alfa Aesar (Ward Hill, MA, USA) and C18 (55–105 μm, 125 Å) was purchased from Waters (Milford, MA, USA). A syringe filter from Teknokroma (Barcelona, Spain) was used by incorporating it into polytetrafluoroethylene (PTFE) membrane filters (0.2 μm). For albendazole, albendazole sulfone, albendazole sulfoxide, buquinolate, flubendazole, oxfendazole, oxfendazole sulfone, oxibendazole, mebendazole, mebendazole amine, standard solutions (1,000 μg/mL) were prepared in MeOH/DMSO (1:1, v/v). Similarly, MeCN was used as the solvent to prepare a standard stock solution of guaifenesin (1,000 μg/mL), while DMSO was used to prepare the stock solutions for fenbendazole, 5-hydroxy mebendazole, methylbenzoquate, and nicarbazin (1,000 μg/mL). The corresponding standard stock solutions (1,000 μg/mL) were prepared at MeOH in all other compounds. All standard stock solutions were stored in amber bottles at −20°C.
Sample collection and preparation
Beef (n=10), pork (n=10), and chicken (n=10) were purchased from local markets in Korea. Each sample was homogenized and stored in a freezer (−20°C) until required for further use. Thus, each homogenized sample (2 g) was weighed into a 50 mL centrifuge tube and then extracted using 0.1% formic acid in MeCN/MeOH (95:5, v/v, 10 mL) and water (10 mL) under shaking for 5 min. Subsequently, MgSO4 (4 g) and NaCl (1 g, original QuEChERS salt) were added to the sample. After, shaken for 5 min, and subjected to centrifugation at 4,700×g (4°C, 10 min). The supernatant was then transferred to a 50 mL centrifuge tube containing C18 (150 mg), PSA (150 mg), and MgSO4 (900 mg). And then, the obtained mixture was shaken for 5 min and centrifuged at 4,700×g (4°C, 5 min). The obtained supernatant (5 mL) was transferred to a new centrifuge tube, DMSO (20 μL) was added, and the solvent was evaporated under a stream of N2 at 40°C. Afterwards, the residue was dissolved in a mixture of MeOH and water (1:1, v/v, 1 mL), and the extract was subsequently filtered through a 0.2 μm PTFE filter before analysis (Kim et al., 2021).
Ultra-high-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) conditions
Separation was conducted on a Shimadzu UHPLCMS 8060 triple quadrupole mass spectrometer (MS, Shimadzu, Kyoto, Japan) equipped with a Waters X-SELECT HSS C18 column (2.1 mm×150 mm, 3.5 μm particle size, Waters, Dublin, Ireland). Data processing used LC solution software version (5.99) from Shimadzu. Gradient separation was performed using a binary gradient composed of water containing 0.1% formic acid and 2 mM ammonium formate (mobile phase A) and MeCN containing 0.1% formic acid (mobile phase B). The gradient profile was as follows: 0 min, 15% B; 2 min, 15% B; 12.5 min, 95% B; 17.0 min, 95% B; 17.1 min, 15% B; 20.0 min, 15% B. The injection volume was 5 μL, and a flow rate of 0.3 mL/min was used under argon gas. The MS source settings were as follows: capillary voltages=4.0 kV (positive) and 2.8 kV (negative); capillary temperature=350°C, auto-sampler temperature=15°C, column temperature=40°C, and cone voltage=30 kV. The MS instrument was operated in the electrospray ionization (ESI) mode with positive and negative switching modes, and scheduled multiple reaction monitoring (MRM) was employed for all target compounds.
Method validation
The linearity, accuracy, precision, limit of detection (LOD), and limit of quantification (LOQ) of the developed method were determined according to the CODEX guidelines (CAC/GL71-2009) and the Ministry of Food and Drug Safety (MFDS) of Korea guidelines (FAO and WHO, 2009; MFDS, 2016). More specifically, the accuracy and precision were determined by analyzing negative samples at three different concentrations, i.e., spiking at 0.5, 1, and 2× the MRL. In addition, the analysis included the determination of toltrazuril (toltrazuril sulfone), emamectin (emamectin B1a), and nicarbazin [N,N’-bis(4-nitrophenylurea)], based on the specified marker residues. The matrix-matched standards for the calibration curves were prepared using a six-point range of target concentrations (i.e., 0.25, 0.5, 1, 2, 4, and 8× the MRL). The LODs and LOQs were defined as the concentrations at which the signal-to-noise ratios were ≥3 and ≥10, respectively.
Matrix effect
To determine the degree of the matrix effect for each system, the matrix-matched curve of a post-extraction spiked sample and the solvent standard curve were compared at the same concentration (in the case set MRL value; 0.25, 0.5, 1, 2, 4, and 8× the MRL and in the case not set MRL value; 2.5, 5, 10, 20, and 40 μg/kg), as outlined in Eq. (1) below. In general, the matrix components of a sample can either increase or decrease ionization efficiency due to interfering substances, i.e. salts, lipids, and peptides (Antignac et al., 2005).
Results and Discussion
Ultra-high-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) optimization
The MS parameters were determined using individual standard solutions and were optimized based on the mass spectra of all compounds. Using the ESI mode with positive and negative switching and MRM, bithionol, chlorfluazuron, oxyclozanide, keto triclabendazole, nicarbazine, niclosamide, and toltrazuril sulfone were detected as their corresponding [M−H]− species, semduramyicin was detected as [M+NH]+, and all other compounds were detected as [M+H]+ mode. Using standard solutions diluted in MeOH/water (1:1, v/v), the MS parameters were optimized using a cone voltage of 30 V. The parent and daughter ions were selected by optimizing the collision energy. Furthermore, daughter ions with higher intensities and better peak shapes were selected as quantitative ions. Most compounds possessed one parent ion and either one or two daughter ions. The optimized precursor ions, daughter ion collision energies, and retention times of all compounds are listed in Table 1. A reversed-phase X-SELECT HSS C18 column was used to separate the various veterinary drugs examined herein. This column was selected because the multi-residue analysis was previously performed using a C18 column (Dasenaki and Thomaidis, 2015). It was found that mobile phase A improved sensitivity and reduced peak tailing (Chang et al., 2019; Clarke et al., 2013; Frenich et al., 2014), while mobile phase B produced a better peak shape than a mixture of MeOH and 0.1% formic acid in MeCN (Zrnčić et al., 2014). Supplementary Fig. S1 shows the extracted ion chromatograms of the target compounds. These chromatograms were observed by injecting an aliquot (5 μL) of the desired standard solution into the beef sample at a concentration of 100 μg/kg (fenbantel and isometamidium) or 10 μg/kg, which corresponds to spiking of 1× the MRL.
Table 1.
UHPLC-MS/MS parameters of the 54 target compounds
| Class |
Compounds |
ESI (+/−) |
Molecular weight (m/z) |
Precursor ion (m/z) |
Production (m/z) |
Collision energy (eV) |
Retention time (min) |
| Anthelmintic |
Abamectin |
+ |
872.5 |
895.0 |
327.3a |
20 |
13.8 |
| 449.3 |
20 |
| 751.4 |
20 |
| Albendazole |
+ |
265.1 |
266.0 |
234.0a |
20 |
8.00 |
| 191.1 |
20 |
| 159.2 |
20 |
| Albendazole sulfoxide |
+ |
281.1 |
282.0 |
208.1a |
16 |
5.12 |
| 240.1 |
19 |
| 159.2 |
19 |
| Albendazole sulfone |
+ |
297.1 |
298.0 |
159.2a |
20 |
6.19 |
| 224.1 |
20 |
| 266.1 |
20 |
| 2-Amino albendazole sulfone |
+ |
239.1 |
240.0 |
133.2a |
17 |
2.84 |
| 198.2 |
16 |
| 105.2 |
30 |
| Benznidazole |
+ |
260.1 |
261.0 |
91.2a |
20 |
6.51 |
| 107.3 |
18 |
| 65.3 |
17 |
| Bithionol |
− |
353.9 |
352.0 |
161.1a |
−25 |
12.43 |
| 192.0 |
−25 |
| 125.1 |
−42 |
| Cambendazole |
+ |
302.1 |
303.0 |
217.0a |
20 |
6.15 |
| 261.0 |
20 |
| 190.1 |
24 |
| Carbendazim |
+ |
191.1 |
192.0 |
132.2a |
20 |
2.78 |
| 105.2 |
30 |
| 160.1 |
30 |
| Carnidazole |
+ |
244.1 |
245.0 |
118.2a |
30 |
6.51 |
| 75.2 |
14 |
| 47.2 |
26 |
| Chlorfluazuron |
− |
539.0 |
538.0 |
518.0a |
−15 |
13.1 |
| 355.0 |
−22 |
| 175.1 |
−42 |
| Cymiazole |
+ |
218.1 |
219 |
171.2a |
15 |
6.00 |
| 144.1 |
20 |
| 77.3 |
23 |
| Derquantel |
+ |
479.6 |
481 |
405.2a |
20 |
7.10 |
| 462.1 |
20 |
| 148.3 |
30 |
| Diethylcarbamazine |
+ |
199.2 |
200.0 |
100.0a |
20 |
1.79 |
| 72.0 |
20 |
| 44.2 |
20 |
| Emamectin |
+ |
885.5 |
886.0 |
158.1a |
30 |
10.98 |
| 82.2 |
34 |
| 159.2 |
32 |
| Febantel |
+ |
446.1 |
448.0 |
384.1a |
19 |
10.50 |
| 416.2 |
14 |
| 281.2 |
33 |
| Fenbendazole |
+ |
299.1 |
299.9 |
268.0a |
20 |
8.90 |
| 159.0 |
35 |
| 131.3 |
46 |
| Fluazuron |
+ |
505.0 |
506.0 |
158.2a |
22 |
12.50 |
| 141.2 |
49 |
| 351.1 |
21 |
| Flubendazole |
+ |
313.1 |
314.0 |
282.1a |
21 |
7.90 |
| 123.2 |
35 |
| 95.2 |
50 |
| 2-Amino flubendazole |
+ |
255.1 |
256.0 |
123.2a |
34 |
6.00 |
| 95.2 |
34 |
| 123.2 |
17 |
| Levamisole |
+ |
204.1 |
205.0 |
91.0a |
20 |
2.91 |
| 123.1 |
20 |
| 178.0 |
20 |
| Mebendazole |
+ |
295.1 |
295.9 |
264.0a |
20 |
7.55 |
| 105.2 |
30 |
| 77.2 |
20 |
| Mebendazole amine |
+ |
237.1 |
238.0 |
105.2a |
16 |
5.71 |
| 133.3 |
30 |
| 77.2 |
16 |
| 5-Hydroxy mebendazole |
+ |
297.1 |
298.0 |
266.0a |
20 |
5.88 |
| 79.2 |
20 |
| 160.2 |
20 |
| Morantel |
+ |
220.1 |
221.0 |
123.0a |
20 |
5.58 |
| 164.0 |
20 |
| 111.0 |
21 |
| Niclosamide |
− |
326.0 |
325.0 |
171.1a |
−20 |
11.22 |
| 289.0 |
−18 |
| 135.1 |
−21 |
| Ornidazole |
+ |
219.0 |
220.0 |
128.2a |
10 |
5.29 |
| 82.3 |
20 |
| 42.2 |
10 |
| Oxantel |
+ |
216.1 |
217.0 |
91.1a |
20 |
3.08 |
| 118.3 |
20 |
| 131.3 |
29 |
| Oxfendazole |
+ |
315.1 |
316.0 |
159.2a |
19 |
6.23 |
| 284.2 |
21 |
| 191.2 |
21 |
| Oxfendazole sulfone |
+ |
331.1 |
332.0 |
300.1a |
19 |
7.23 |
| 159.2 |
12 |
| 131.3 |
12 |
| Oxibendazole |
+ |
249.1 |
250.0 |
176.0a |
20 |
6.57 |
| 218.0 |
20 |
| 148.2 |
18 |
| Oxyclozanide |
− |
398.9 |
397.0 |
362.0a |
−19 |
11.08 |
| 202.0 |
−24 |
| 176.2 |
−27 |
| Praziquantel |
+ |
312.2 |
313.0 |
159.0a |
20 |
8.97 |
| 131.3 |
21 |
| 174.2 |
19 |
| Pyrantel |
+ |
206.1 |
207.0 |
109.0a |
20 |
4.25 |
| 150.0 |
34 |
| 136.0 |
34 |
| Ternidazole |
+ |
185.1 |
186.0 |
82.2a |
30 |
2.87 |
| 128.2 |
12 |
| 42.2 |
12 |
| Tetramisole |
+ |
204.1 |
205.0 |
178.0a |
20 |
2.91 |
| 91.0 |
20 |
| 123.1 |
20 |
| Thiabendazole |
+ |
201.0 |
202.0 |
121.2a |
19 |
3.20 |
| 175.0 |
20 |
| 5-Hydroxy thiabendazole |
+ |
217.0 |
218.0 |
131.0a |
20 |
1.92 |
| 65.2 |
26 |
| 191.1 |
16 |
| Thiophanate |
+ |
370.1 |
371.0 |
147.2a |
16 |
9.11 |
| 81.3 |
15 |
| 151.0 |
20 |
| Triclabendazole |
+ |
358.0 |
359.0 |
273.9a |
35 |
10.99 |
| 343.9 |
30 |
| 171.1 |
53 |
| Keto triclabendazole |
− |
328.0 |
326.9 |
182.1a |
−25 |
9.63 |
| 146.1 |
−34 |
| 118.0 |
−40 |
| Antiprotozoal |
Arprinocid |
+ |
277.1 |
278.0 |
142.9a |
20 |
6.01 |
| 107.2 |
16 |
| 108.2 |
19 |
| Buquinolate |
+ |
361.2 |
362.0 |
204.0a |
20 |
10.05 |
| 316.1 |
21 |
| 148.2 |
25 |
| Diaveridine |
+ |
260.1 |
261.0 |
123.1a |
20 |
2.84 |
| 81.3 |
20 |
| 245.0 |
19 |
| Guaifenesin |
+ |
198.1 |
199.0 |
121.2a |
15 |
5.30 |
| 125.3 |
25 |
| Halofuginone |
+ |
413.0 |
415.8 |
100.2a |
29 |
6.19 |
| 120.2 |
20 |
| 138.3 |
20 |
| Imidocarb |
+ |
348.2 |
349.0 |
188.2a |
20 |
1.54 |
| 162.2 |
13 |
| 97.7 |
24 |
| Isometamidium |
+ |
460.2 |
461.0 |
299.2a |
10 |
5.70 |
| 313.2 |
16 |
| 314.3 |
30 |
| Methylbenzoquate, Nequinate |
+ |
365.2 |
366.0 |
334.1a |
20 |
9.88 |
| 91.0 |
22 |
| 201.1 |
26 |
| Monensin |
+ |
692.4 |
693.0 |
675.4a |
24 |
16.60 |
| 461.4 |
24 |
| 479.3 |
24 |
| Nicarbazine |
− |
302.1 |
301.0 |
136.9a |
−20 |
9.74 |
| 107.1 |
−30 |
| 46.0 |
−20 |
| Semduramicin |
+ |
872.5 |
890.0 |
629.4a |
34 |
14.49 |
| 647.4 |
20 |
| 727.5 |
34 |
| Tinidazole |
+ |
247.1 |
248.0 |
121.2a |
19 |
4.30 |
| 82.2 |
11 |
| 128.2 |
11 |
| Toltrazuril sulfone |
− |
457.1 |
456.0 |
41.8a |
−22 |
9.96 |
| 399.1 |
−22 |
Download Excel Table
Sample preparation
This study was conducted according to the modified QuEChERS procedure. The sample extraction and clean-up conditions were optimized based on a previously used method for multiclass drug analysis (Kim et al., 2021). Kim et al. used a modified QuEChERS type extraction method and added the concentration step. The original QuEChERS method consists of two steps an extraction/partitioning step with the addition of salts, and a clean-up step that uses d-SPE. More specifically, sample extraction was carried out using 0.1% formic acid with MeCN/MeOH (95:5, v/v), which had been previously demonstrated to yield a high recovery rate (Clarke et al., 2013; Lopes et al., 2012). In addition, MgSO4 was employed due to its good water absorbency properties, which permits salting-out, while NaCl was added to increase the polarity of the extraction solvent and enhance the extraction selectivity (Rejczak and Tuzimski, 2015). For sample clean-up, PSA was used to remove fatty acids and organic acids, MgSO4 was used to remove water, and a C18 absorbent was used to remove non-polar components (Anastassiades et al., 2003; Wilkowska and Biziuk, 2011). DMSO was added prior to sample concentration to enhance the sample recovery to act as a keeper during evaporation (Kim et al., 2021; Whelan et al., 2010).
Validation of the analytical method
Method validation was performed in terms of linearity, accuracy, precision, LOD, and LOQ. All compounds exhibited a best linearity, with correlation coefficients (r2) exceeding 0.98 at matrix-matched calibration at six points. The accuracy, expressed as the recovery, ranged from 60.2% to 119.9%, and the coefficients of variation (CV) ranged from 1.2% to 31.5% for the three determined levels. In the chicken samples, the average recovery of 2-amino albendazole sulfone was ~111.1%, which was considered unacceptable based on the recovery limit of 110% specified by the CODEX guidelines at a concentration of 200 μg/kg; all other compounds satisfied the CODEX guidelines. In addition, inter-laboratory (n=2) validation was conducted according to CODEX guidelines (CAC/GL-71) and the results were satisfied with the guideline. Table 2 lists the accuracies and precisions obtained of all compounds following their analyses in the three matrices. In addition, the LOD values ranged from 0.3 to 3 μg/kg, while the LOQ values ranged from 1 to 10 μg/kg, which are lower than the corresponding values of the Korean MRLs. It should be noted that, in general, the LOQ values were ~1 μg/kg; however, the corresponding values for imidocarb and pyrantel in beef were 10 μg/kg, respectively, while oxfendazole had a LOQ value of 10 μg/kg. The LOD, LOQ, and Korean MRL values for the three matrices (beef, pork, and chicken) are given in Table 3, wherein it can be deduced that the obtained values were satisfactory. Thus, the developed method appeared to demonstrate an acceptable analytical performance for residue control in livestock products.
Table 2.
Validation of the analytical method for the 54 target compounds (n=5)
| Compound |
Beef |
Pork |
Chicken |
| Target concentration levels (μg/kg) |
Rec. (%) |
CV (%) |
r2 |
Target concentration levels (μg/kg) |
Rec. (%) |
CV (%) |
r2 |
Target concentration levels (μg/kg) |
Rec. (%) |
CV (%) |
r2 |
| Abamectin |
5 |
97.3 |
27.9 |
0.9914 |
5 |
75.6 |
25.6 |
0.9865 |
5 |
94.1 |
22.8 |
0.9838 |
| 10 |
102.0 |
19.1 |
10 |
73.2 |
24.3 |
10 |
105.7 |
16.4 |
| 20 |
91.8 |
19.5 |
20 |
98.1 |
17.7 |
20 |
105.1 |
11.6 |
| Albendazole |
50 |
77.6 |
12.2 |
0.9990 |
50 |
90.3 |
7.9 |
0.9970 |
50 |
107.8 |
9.1 |
0.9952 |
| 100 |
73.8 |
12.3 |
100 |
97.0 |
9.0 |
100 |
111.2 |
11.7 |
| 200 |
93.2 |
6.4 |
200 |
94.6 |
11.5 |
200 |
109.0 |
12.6 |
| Albendazole sulfone |
50 |
109.4 |
13.4 |
0.9946 |
50 |
107.0 |
16.3 |
0.9944 |
50 |
109.0 |
5.9 |
0.9987 |
| 100 |
110.8 |
20.1 |
100 |
91.0 |
18.6 |
100 |
107.3 |
8.6 |
| 200 |
98.8 |
10.4 |
200 |
94.0 |
11.9 |
200 |
93.6 |
13.6 |
| Albendazole sulfoxide |
50 |
96.3 |
9.2 |
0.9985 |
50 |
85.5 |
11.2 |
0.9996 |
50 |
110.1 |
10.6 |
0.9996 |
| 100 |
92.2 |
10.0 |
100 |
93.8 |
8.3 |
100 |
110.0 |
9.1 |
| 200 |
85.9 |
3.9 |
200 |
93.3 |
1.2 |
200 |
107.2 |
4.3 |
| 2-Amino albendazole sulfone |
50 |
106 |
4.8 |
0.9979 |
50 |
100.1 |
3.6 |
0.9999 |
50 |
113.8 |
7.2 |
0.9998 |
| 100 |
100.8 |
7.5 |
100 |
100.5 |
9.7 |
100 |
111.7 |
5.8 |
| 200 |
85.7 |
18.0 |
200 |
99.7 |
2.8 |
200 |
111.1 |
7.0 |
| Arprinocid |
5 |
106.6 |
4.7 |
0.9974 |
5 |
92.0 |
5.2 |
0.9996 |
5 |
105.3 |
5.4 |
0.9984 |
| 10 |
87.4 |
8.2 |
10 |
98.3 |
5.1 |
10 |
111.6 |
3.0 |
| 20 |
88.9 |
6.6 |
20 |
90.5 |
6.6 |
20 |
109.3 |
5.7 |
| Benznidazole |
5 |
92.7 |
5.9 |
0.9969 |
5 |
98.5 |
5.2 |
0.9991 |
5 |
116.0 |
8.9 |
0.9996 |
| 10 |
92.4 |
7.8 |
10 |
96.5 |
5.6 |
10 |
115.9 |
6.2 |
| 20 |
92.8 |
6.4 |
20 |
93.2 |
6.0 |
20 |
112.0 |
6.5 |
| Bithionol |
5 |
71.0 |
6.4 |
0.9983 |
5 |
79.4 |
21.0 |
0.9966 |
5 |
104.2 |
16.1 |
0.9854 |
| 10 |
76.9 |
14.9 |
10 |
90.3 |
11.4 |
10 |
116.9 |
18.0 |
| 20 |
98.5 |
13.0 |
20 |
112.4 |
17.5 |
20 |
115.0 |
18.2 |
| Buquinolate |
5 |
93.5 |
16.2 |
0.9922 |
5 |
90.6 |
12.5 |
0.9960 |
5 |
90.8 |
7.9 |
0.9987 |
| 10 |
97.6 |
10.4 |
10 |
83.5 |
23.0 |
10 |
107.9 |
4.2 |
| 20 |
100.6 |
11.1 |
20 |
82.6 |
11.7 |
20 |
81.9 |
10.1 |
| Cambendazole |
5 |
99.8 |
10.3 |
0.9944 |
5 |
60.9 |
28.5 |
0.9944 |
5 |
105.9 |
16.2 |
0.9914 |
| 10 |
74.1 |
16.7 |
10 |
100.4 |
8.1 |
10 |
115.4 |
9.8 |
| 20 |
86.4 |
7.2 |
20 |
102.9 |
9.9 |
20 |
114.3 |
11.5 |
| Carbendazim |
5 |
107.2 |
10.5 |
0.9995 |
5 |
75.3 |
14.7 |
0.9976 |
5 |
110.8 |
8.3 |
0.9995 |
| 10 |
119.9 |
11.0 |
10 |
100.4 |
7.4 |
10 |
102.4 |
11.8 |
| 20 |
104.8 |
8.0 |
20 |
102.5 |
10.9 |
20 |
101.9 |
6.0 |
| Carnidazole |
5 |
99.8 |
9.2 |
0.9990 |
5 |
96.4 |
8.2 |
0.9995 |
5 |
101.6 |
8.9 |
0.9998 |
| 10 |
93.4 |
11.3 |
10 |
103.0 |
4.8 |
10 |
99.3 |
2.8 |
| 20 |
98.4 |
11.7 |
20 |
92.0 |
7.2 |
20 |
99.5 |
9.0 |
| Chlorfluazuron |
5 |
103.5 |
11.1 |
0.9946 |
5 |
118.6 |
11.9 |
0.9944 |
5 |
101.2 |
8.6 |
0.9962 |
| 10 |
89.7 |
25.0 |
10 |
81.6 |
6.3 |
10 |
103.5 |
19.0 |
| 20 |
89.5 |
14.2 |
20 |
75.7 |
19.5 |
20 |
75.6 |
7.3 |
| Cymiazole |
5 |
66.7 |
9.1 |
0.9972 |
5 |
73.6 |
4.0 |
0.9875 |
5 |
90.9 |
9.5 |
0.9948 |
| 10 |
71.6 |
7.3 |
10 |
83.8 |
12.4 |
10 |
79.0 |
23.4 |
| 20 |
84.5 |
7.9 |
20 |
75.8 |
15.3 |
20 |
85.7 |
21.9 |
| Derquantel |
5 |
102.7 |
11.0 |
0.9963 |
5 |
107.0 |
3.2 |
0.9996 |
5 |
105.1 |
9.4 |
0.9991 |
| 10 |
80.6 |
9.6 |
10 |
84.0 |
7.1 |
10 |
104.0 |
5.0 |
| 20 |
76.5 |
13.8 |
20 |
75.8 |
8.9 |
20 |
101.4 |
10.3 |
| Diaveridine |
5 |
113.3 |
5.8 |
0.9993 |
5 |
98.8 |
9.7 |
0.9987 |
5 |
99.4 |
3.8 |
0.9944 |
| 10 |
103.0 |
9.6 |
10 |
100.7 |
4.5 |
10 |
110.7 |
3.2 |
| 20 |
101.2 |
12.8 |
20 |
95.2 |
8.0 |
20 |
109.9 |
2.0 |
| Diethylcarbamazine |
5 |
94.5 |
5.8 |
0.9988 |
5 |
94.5 |
13.1 |
0.9993 |
5 |
94.4 |
20.7 |
0.9992 |
| 10 |
96.3 |
12.0 |
10 |
103.0 |
5.5 |
10 |
95.5 |
12.0 |
| 20 |
86.3 |
5.3 |
20 |
88.7 |
12.5 |
20 |
82.1 |
21.7 |
| Emamectin |
5 |
93.3 |
3.0 |
0.9971 |
5 |
68.9 |
10.1 |
0.9843 |
5 |
104.5 |
13.1 |
0.9985 |
| 10 |
101.5 |
13.8 |
10 |
74.8 |
11.3 |
10 |
95.7 |
7.8 |
| 20 |
106.6 |
5.6 |
20 |
86.0 |
13.6 |
20 |
84.2 |
18.9 |
| Febantel |
50 |
87.3 |
11.3 |
0.9945 |
50 |
87.8 |
4.9 |
0.9982 |
50 |
93.4 |
17.5 |
0.9987 |
| 100 |
76.6 |
8.2 |
100 |
88.2 |
9.4 |
100 |
93.7 |
2.5 |
| 200 |
74.9 |
8.9 |
200 |
77.5 |
5.6 |
200 |
92.2 |
8.8 |
| Fenbendazole |
50 |
73.6 |
14.3 |
0.9955 |
50 |
91.7 |
13.9 |
0.9926 |
50 |
107.3 |
8.2 |
0.9955 |
| 100 |
84.3 |
16.7 |
100 |
96.6 |
27.7 |
100 |
107.5 |
6.9 |
| 200 |
109.9 |
5.2 |
200 |
93.6 |
17.3 |
200 |
103.4 |
17.5 |
| Fluazuron |
100 |
116.5 |
7.2 |
0.9888 |
5 |
97.7 |
30.7 |
0.9592 |
5 |
94.2 |
15.0 |
0.9839 |
| 200 |
108.2 |
13.8 |
10 |
78.6 |
19.2 |
10 |
112.7 |
15.3 |
| 400 |
103.7 |
6.1 |
20 |
109.6 |
12.0 |
20 |
84.5 |
19.9 |
| Flubendazole |
5 |
100.2 |
8.0 |
0.9973 |
5 |
94.8 |
19.6 |
0.9978 |
5 |
110.9 |
13.6 |
0.9987 |
| 10 |
82.8 |
6.2 |
10 |
88.5 |
8.1 |
10 |
98.6 |
10.6 |
| 20 |
78.1 |
6.7 |
20 |
70.4 |
14.7 |
20 |
88.5 |
12.0 |
| 2-Amino flubendazole |
5 |
79.7 |
10.4 |
0.9944 |
5 |
93.9 |
9.4 |
0.9985 |
5 |
107.5 |
7.7 |
0.9972 |
| 10 |
87.4 |
20.8 |
10 |
99.5 |
7.6 |
10 |
102.4 |
9.9 |
| 20 |
99.3 |
9.2 |
20 |
94.7 |
12.8 |
20 |
101.5 |
6.4 |
| Guaifenesin |
5 |
111.1 |
29.5 |
0.9936 |
5 |
80.9 |
25.3 |
0.9884 |
5 |
82.9 |
20.8 |
0.9961 |
| 10 |
102.9 |
20.3 |
10 |
86.0 |
20.0 |
10 |
103.3 |
16.2 |
| 20 |
101.8 |
13.1 |
20 |
83.7 |
13.9 |
20 |
108.6 |
15.1 |
| Halofuginone |
5 |
84.9 |
13.6 |
0.9976 |
5 |
118.4 |
7.2 |
0.9973 |
5 |
113.2 |
8.6 |
0.9990 |
| 10 |
90.3 |
13.5 |
10 |
89.6 |
10.5 |
10 |
97.2 |
6.9 |
| 20 |
81.4 |
6.5 |
20 |
71.4 |
4.0 |
20 |
95.3 |
15.3 |
| Imidocarb |
150 |
99.2 |
11.9 |
0.9844 |
5 |
104.6 |
11.0 |
0.9983 |
5 |
103.9 |
17.0 |
0.9916 |
| 300 |
88.8 |
15.6 |
10 |
106.0 |
9.0 |
10 |
103.4 |
17.3 |
| 600 |
91.5 |
9.2 |
20 |
97.5 |
15.5 |
20 |
80.4 |
16.1 |
| Isometamidium |
50 |
114.5 |
7.7 |
0.9987 |
5 |
91.5 |
16.2 |
0.9883 |
5 |
83.4 |
12.1 |
0.9974 |
| 100 |
106.5 |
14.8 |
10 |
74.2 |
19.6 |
10 |
65.5 |
8.4 |
| 200 |
95.4 |
9.5 |
20 |
76.4 |
19.0 |
20 |
78.4 |
18.1 |
| Levamisole |
5 |
111.8 |
2.2 |
0.9978 |
5 |
102.4 |
5.6 |
0.9994 |
5 |
95.3 |
12.0 |
0.9994 |
| 10 |
104.3 |
7.5 |
10 |
90.2 |
12.1 |
10 |
98.1 |
3.6 |
| 20 |
88.8 |
8.9 |
20 |
90.1 |
10.5 |
20 |
94.8 |
10.0 |
| Mebendazole |
5 |
91.5 |
5.0 |
0.9995 |
30 |
91.3 |
5.4 |
0.9981 |
30 |
114.2 |
9.7 |
0.9995 |
| 10 |
78.7 |
11.6 |
60 |
97.1 |
9.9 |
60 |
111.3 |
3.8 |
| 20 |
100.4 |
7.3 |
120 |
90.5 |
6.3 |
120 |
103.6 |
6.9 |
| Mebendazole amine |
5 |
94.4 |
10.6 |
0.9802 |
30 |
84.3 |
8.0 |
0.9994 |
30 |
115.5 |
10.0 |
0.9993 |
| 10 |
110.9 |
5.0 |
60 |
84.5 |
5.4 |
60 |
107.2 |
10.7 |
| 20 |
104.0 |
12.3 |
120 |
85.1 |
6.4 |
120 |
97.8 |
7.4 |
| 5-Hydroxy mebendazole |
5 |
93.8 |
5.8 |
0.9993 |
30 |
95.5 |
8.0 |
0.9991 |
30 |
100.3 |
8.3 |
0.9962 |
| 10 |
84.6 |
8.3 |
60 |
89.0 |
7.1 |
60 |
103.8 |
6.8 |
| 20 |
78.5 |
19.2 |
120 |
83.1 |
4.8 |
120 |
102.6 |
4.9 |
| Methylbenzoquate |
5 |
78.1 |
6.6 |
0.9860 |
5 |
99.9 |
14.7 |
0.9911 |
5 |
88.8 |
11.5 |
0.9942 |
| 10 |
81.6 |
22.4 |
10 |
89.0 |
15.6 |
10 |
117.6 |
20.9 |
| 20 |
99.9 |
14.2 |
20 |
85.8 |
12.4 |
20 |
89.2 |
11.8 |
| Monensin |
25 |
73.5 |
7.1 |
0.9966 |
25 |
84.3 |
9.9 |
0.9945 |
25 |
110.9 |
15.1 |
0.9916 |
| 50 |
78.6 |
10.5 |
50 |
86.4 |
13.9 |
50 |
93.3 |
15.9 |
| 100 |
90.2 |
15.9 |
100 |
86.3 |
20.0 |
100 |
102.1 |
10.9 |
| Morantel |
5 |
114.7 |
6.6 |
0.9985 |
5 |
81.9 |
10.6 |
0.9983 |
5 |
96.1 |
7.1 |
0.9960 |
| 10 |
87.0 |
19.3 |
10 |
87.1 |
16.4 |
10 |
99.7 |
6.2 |
| 20 |
83.8 |
9.8 |
20 |
90.0 |
5.3 |
20 |
94.1 |
7.5 |
| Nicarbazin |
5 |
77.3 |
3.0 |
0.9936 |
5 |
66.5 |
7.5 |
0.9876 |
5 |
106.1 |
7.4 |
0.9999 |
| 10 |
77.6 |
9.3 |
10 |
74.4 |
15.0 |
10 |
106.9 |
6.7 |
| 20 |
90.6 |
10.2 |
20 |
83.6 |
12.2 |
20 |
98.6 |
9.6 |
| Niclosamide |
5 |
107.1 |
11.2 |
0.9853 |
5 |
75.7 |
8.4 |
0.9899 |
5 |
106.3 |
19.9 |
0.9988 |
| 10 |
87.8 |
25.8 |
10 |
87.8 |
31.5 |
10 |
98.7 |
15.2 |
| 20 |
84.1 |
13.9 |
20 |
93.2 |
18.4 |
20 |
106.0 |
20.5 |
| Ornidazole |
5 |
89.4 |
13.4 |
0.9994 |
5 |
78.2 |
26.6 |
0.9958 |
5 |
91.2 |
16.7 |
0.9912 |
| 10 |
79.6 |
15.0 |
10 |
108.6 |
9.0 |
10 |
101.9 |
11.0 |
| 20 |
89.5 |
11.0 |
20 |
106.3 |
10.1 |
20 |
105.0 |
14.4 |
| Oxantel |
5 |
93.4 |
4.7 |
0.9999 |
5 |
98.2 |
4.2 |
0.9997 |
5 |
99.5 |
6.0 |
0.9982 |
| 10 |
91.9 |
7.0 |
10 |
99.2 |
2.0 |
10 |
107.3 |
1.8 |
| 20 |
94.2 |
7.4 |
20 |
95.8 |
3.3 |
20 |
107.2 |
2.2 |
| Oxfendazole |
50 |
110.1 |
8.1 |
0.9998 |
50 |
87.9 |
9.3 |
0.9989 |
50 |
99.8 |
7.6 |
0.9974 |
| 100 |
106.7 |
4.5 |
100 |
102.7 |
7.8 |
100 |
99.3 |
8.3 |
| 200 |
105.1 |
8.0 |
200 |
105.9 |
4.7 |
200 |
96.3 |
5.9 |
| Oxfendazole sulfone |
50 |
99.3 |
2.3 |
0.9979 |
50 |
111.8 |
12.7 |
0.9992 |
50 |
104.3 |
6.9 |
0.9994 |
| 100 |
96.3 |
4.0 |
100 |
100.1 |
9.1 |
100 |
102.7 |
9.4 |
| 200 |
103.8 |
5.1 |
200 |
101.6 |
7.8 |
200 |
100.8 |
6.1 |
| Oxibendazole |
50 |
93.6 |
8.5 |
0.9942 |
50 |
84.6 |
9.1 |
0.9977 |
50 |
113.9 |
11.3 |
0.9994 |
| 100 |
86.6 |
10.4 |
100 |
88.0 |
6.3 |
100 |
110.3 |
2.6 |
| 200 |
92.3 |
8.8 |
200 |
85.6 |
10.4 |
200 |
106.8 |
6.6 |
| Oxyclozanide |
5 |
104.8 |
10.4 |
0.9970 |
5 |
93.0 |
9.4 |
0.9940 |
5 |
104.0 |
13.7 |
0.9959 |
| 10 |
91.8 |
3.7 |
10 |
98.2 |
14.2 |
10 |
111.6 |
6.5 |
| 20 |
94.1 |
5.3 |
20 |
104.3 |
12.8 |
20 |
113.5 |
11.1 |
| Praziquantel |
5 |
104.0 |
9.9 |
0.9992 |
5 |
91.8 |
9.4 |
0.9993 |
5 |
98.9 |
5.3 |
0.9953 |
| 10 |
86.4 |
17.9 |
10 |
110.4 |
12.3 |
10 |
114.1 |
11.2 |
| 20 |
90.6 |
7.6 |
20 |
114.3 |
8.6 |
20 |
116.1 |
8.2 |
| Pyrantel |
5 |
97.6 |
8.8 |
0.9989 |
5 |
111.8 |
9.1 |
0.9953 |
5 |
114.3 |
15.0 |
0.9995 |
| 10 |
100.5 |
4.7 |
10 |
98.0 |
5.9 |
10 |
112.7 |
6.3 |
| 20 |
97.3 |
12.3 |
20 |
88.3 |
9.3 |
20 |
95.1 |
15.1 |
| Semduramicin |
5 |
76.7 |
22.0 |
0.9930 |
5 |
80.8 |
25.6 |
0.9957 |
5 |
110.0 |
15.3 |
0.9974 |
| 10 |
79.1 |
20.6 |
10 |
95.7 |
24.7 |
10 |
85.7 |
13.0 |
| 20 |
96.5 |
21.7 |
20 |
112.4 |
10.5 |
20 |
94.5 |
14.8 |
| Ternidazole |
5 |
92.4 |
29.6 |
0.9807 |
5 |
91.4 |
22.9 |
0.9947 |
5 |
102.7 |
15.4 |
0.9843 |
| 10 |
107.0 |
9.1 |
10 |
107.9 |
8.5 |
10 |
112.1 |
8.2 |
| 20 |
84.6 |
20.7 |
20 |
80.9 |
14.9 |
20 |
108.1 |
6.0 |
| Tetramisole |
5 |
100.1 |
4.5 |
0.9997 |
5 |
90.8 |
3.1 |
0.9992 |
5 |
105.0 |
2.1 |
0.9999 |
| 10 |
100.3 |
5.5 |
10 |
97.4 |
4.1 |
10 |
100.5 |
4.5 |
| 20 |
98.8 |
5.7 |
20 |
95.4 |
2.9 |
20 |
102.0 |
2.0 |
| Thiabendazole |
5 |
101.6 |
7.6 |
0.9998 |
5 |
78.7 |
15.1 |
0.9966 |
5 |
101.4 |
10.4 |
0.9970 |
| 10 |
103.3 |
3.0 |
10 |
110.3 |
5.1 |
10 |
119.9 |
4.0 |
| 20 |
106.6 |
5.5 |
20 |
114.2 |
7.4 |
20 |
116.8 |
7.8 |
| 5-Hydroxy thiabendazole |
5 |
110.9 |
18.3 |
0.9931 |
5 |
93.1 |
14.4 |
0.9950 |
5 |
116.7 |
18.0 |
0.9946 |
| 10 |
90.7 |
11.4 |
10 |
116.9 |
7.2 |
10 |
105.6 |
18.7 |
| 20 |
76.4 |
9.9 |
20 |
111.8 |
7.9 |
20 |
72.0 |
17.7 |
| Thiophanate |
5 |
117.4 |
9.3 |
0.9908 |
5 |
84.1 |
10.0 |
0.9974 |
5 |
109.9 |
15.8 |
0.9941 |
| 10 |
84.1 |
16.2 |
10 |
103.9 |
20.9 |
10 |
92.4 |
4.2 |
| 20 |
90.2 |
4.2 |
20 |
93.4 |
4.8 |
20 |
94.3 |
10.9 |
| Tinidazole |
5 |
78.7 |
10.6 |
0.9889 |
5 |
96.3 |
12.0 |
0.9997 |
5 |
94.6 |
17.1 |
0.9985 |
| 10 |
103.3 |
9.0 |
10 |
93.0 |
9.3 |
10 |
86.2 |
9.6 |
| 20 |
100.9 |
9.3 |
20 |
73.7 |
9.2 |
20 |
92.5 |
6.9 |
| Toltrazuril sulfone |
50 |
98.3 |
8.1 |
0.9954 |
50 |
86.0 |
11.8 |
0.9996 |
50 |
111.5 |
7.0 |
0.9992 |
| 100 |
95.0 |
9.5 |
100 |
101.4 |
7.5 |
100 |
108.3 |
3.4 |
| 200 |
101.1 |
10.2 |
200 |
104.3 |
3.8 |
200 |
103.9 |
7.6 |
| Triclabendazole |
100 |
85.3 |
15.5 |
0.9832 |
5 |
86.8 |
13.1 |
0.9983 |
5 |
110.5 |
7.8 |
0.9970 |
| 200 |
92.4 |
8.1 |
10 |
83.0 |
15.5 |
10 |
109.5 |
12.3 |
| 400 |
107.1 |
12.2 |
20 |
82.6 |
19.7 |
20 |
104.8 |
6.6 |
| Keto triclabendazole |
100 |
82.5 |
7.4 |
0.9929 |
5 |
91.2 |
8.1 |
0.9899 |
5 |
104.6 |
25.2 |
0.9951 |
| 200 |
87.7 |
11.0 |
10 |
90.2 |
15.9 |
10 |
107.7 |
11.5 |
| 400 |
92.2 |
7.9 |
20 |
83.7 |
16.1 |
20 |
96.1 |
13.9 |
Download Excel Table
Table 3.
The LODs, LOQs (μg/kg) and Korean MRLs (μg/kg) for livestock products
| Compounds |
Beef |
Pork |
Chicken |
| MRL (μg/kg) |
LOD (μg/kg) |
LOQ (μg/kg) |
MRL (μg/kg) |
LOD (μg/kg) |
LOQ (μg/kg) |
MRL (μg/kg) |
LOD (μg/kg) |
LOQ (μg/kg) |
| Abamectin |
10 |
0.03 |
0.1 |
10 |
0.1 |
0.3 |
|
0.03 |
0.1 |
| Albendazole |
100 |
0.03 |
0.1 |
100 |
0.03 |
0.1 |
100 |
0.03 |
0.1 |
| Albendazole sulfone |
100 |
0.1 |
0.3 |
100 |
0.1 |
0.3 |
100 |
0.1 |
0.3 |
| Albendazole sulfoxide |
100 |
0.2 |
0.6 |
100 |
0.3 |
1 |
100 |
0.2 |
0.7 |
| 2-Amino albendazole sulfone |
100 |
0.1 |
0.2 |
100 |
0.1 |
0.4 |
100 |
0.1 |
0.4 |
| Arprinocid |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
| Benznidazole |
|
0.2 |
0.7 |
|
0.2 |
0.5 |
|
0.2 |
0.6 |
| Bithionol |
10 |
0.3 |
|
1 |
0.3 |
0.9 |
|
0.1 |
0.2 |
| Buquinolate |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
| Cambendazole |
|
0.1 |
0.2 |
|
0.1 |
0.2 |
|
0.1 |
0.2 |
| Carbendazim |
|
0.03 |
0.1 |
10 |
0.1 |
0.2 |
|
0.03 |
0.1 |
| Carnidazole |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
| Chlorfluazuron |
|
0.1 |
0.2 |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
| Cymiazole |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
| Derquantel |
|
0.1 |
0.4 |
|
0.1 |
0.3 |
|
0.1 |
0.2 |
| Diaveridine |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
50 |
0.1 |
0.3 |
| Diethylcarbamazine |
10 |
0.2 |
0.5 |
|
0.2 |
0.5 |
|
0.1 |
0.4 |
| Emamectin |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
| Febantel |
100 |
0.03 |
0.1 |
100 |
0.03 |
0.1 |
50 |
0.03 |
0.1 |
| Fenbendazole |
100 |
0.1 |
0.3 |
100 |
0.1 |
0.2 |
50 |
0.1 |
0.2 |
| Fluazuron |
200 |
0.2 |
0.5 |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
| Flubendazole |
|
0.03 |
0.1 |
10 |
0.03 |
0.1 |
200 |
0.03 |
0.1 |
| 2-Amino flubendazole |
|
0.03 |
0.1 |
10 |
0.1 |
0.3 |
200 |
0.1 |
0.2 |
| Guaifenesin |
10 |
0.8 |
2.4 |
10 |
1.3 |
3.8 |
10 |
1 |
3 |
| Halofuginone |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
| Imidocarb |
300 |
0.5 |
1.5 |
|
0.03 |
0.1 |
|
0.5 |
1.5 |
| Isometamidium |
100 |
0.1 |
0.2 |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
| Levamisole |
10 |
0.03 |
0.1 |
10 |
0.03 |
0.1 |
10 |
0.03 |
0.1 |
| Mebendazole |
|
0.03 |
0.1 |
60 |
0.03 |
0.1 |
60 |
0.03 |
0.1 |
| Mebendazole amine |
|
0.1 |
0.3 |
60 |
0.1 |
0.4 |
60 |
0.2 |
0.6 |
| 5-Hydroxy mebendazole |
|
0.2 |
0.5 |
60 |
0.1 |
0.3 |
60 |
0.2 |
0.5 |
| Methylbenzoquate |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
10 |
0.03 |
0.1 |
| Monensin |
50 |
0.03 |
0.1 |
50 |
0.03 |
0.1 |
50 |
0.03 |
0.1 |
| Morantel |
|
0.9 |
2.6 |
|
0.9 |
2.7 |
|
1.1 |
3.4 |
| Nicarbazin |
|
0.1 |
0.2 |
|
0.03 |
0.1 |
200 |
0.1 |
0.2 |
| Niclosamide |
|
0.2 |
0.5 |
|
0.1 |
0.3 |
|
0.1 |
0.2 |
| Ornidazole |
|
0.1 |
0.2 |
|
0.1 |
0.4 |
|
0.2 |
0.5 |
| Oxantel |
|
0.2 |
0.5 |
|
0.2 |
0.5 |
|
0.2 |
0.5 |
| Oxfendazole |
100 |
0.03 |
0.1 |
100 |
0.03 |
0.1 |
50 |
0.03 |
0.1 |
| Oxfendazole sulfone |
100 |
0.03 |
0.1 |
100 |
0.03 |
0.1 |
50 |
0.03 |
0.1 |
| Oxibendazole |
100 |
0.03 |
0.1 |
100 |
0.03 |
0.1 |
|
0.0 |
0.1 |
| Oxyclozanide |
|
0.2 |
0.7 |
|
0.2 |
0.5 |
|
0.1 |
0.4 |
| Praziquantel |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
|
0.0 |
0.1 |
| Pyrantel |
|
3.2 |
9.7 |
|
0.2 |
0.5 |
|
0.2 |
0.6 |
| Semduramicin |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
100 |
0.3 |
1 |
| Ternidazole |
|
0.1 |
0.2 |
|
0.1 |
0.3 |
|
0.1 |
0.3 |
| Tetramisole |
10 |
0.03 |
0.1 |
10 |
0.03 |
0.1 |
10 |
0.0 |
0.1 |
| Thiabendazole |
10 |
0.03 |
0.1 |
10 |
0.03 |
0.1 |
|
0.03 |
0.1 |
| 5-Hydroxy thiabendazole |
10 |
0.1 |
0.2 |
10 |
0.03 |
0.1 |
|
0.03 |
0.1 |
| Thiophanate |
|
0.03 |
0.1 |
10 |
0.03 |
0.1 |
|
0.03 |
0.1 |
| Tinidazole |
|
0.03 |
0.1 |
|
0.1 |
0.2 |
|
0.03 |
0.1 |
| Toltrazuril sulfone |
100 |
0.1 |
0.3 |
100 |
0.03 |
0.1 |
100 |
0.03 |
0.1 |
| Triclabendazole |
200 |
0.03 |
0.1 |
|
0.03 |
0.1 |
|
0.03 |
0.1 |
| Keto triclabendazole |
200 |
0.3 |
0.9 |
|
0.1 |
0.2 |
|
0.1 |
0.2 |
Download Excel Table
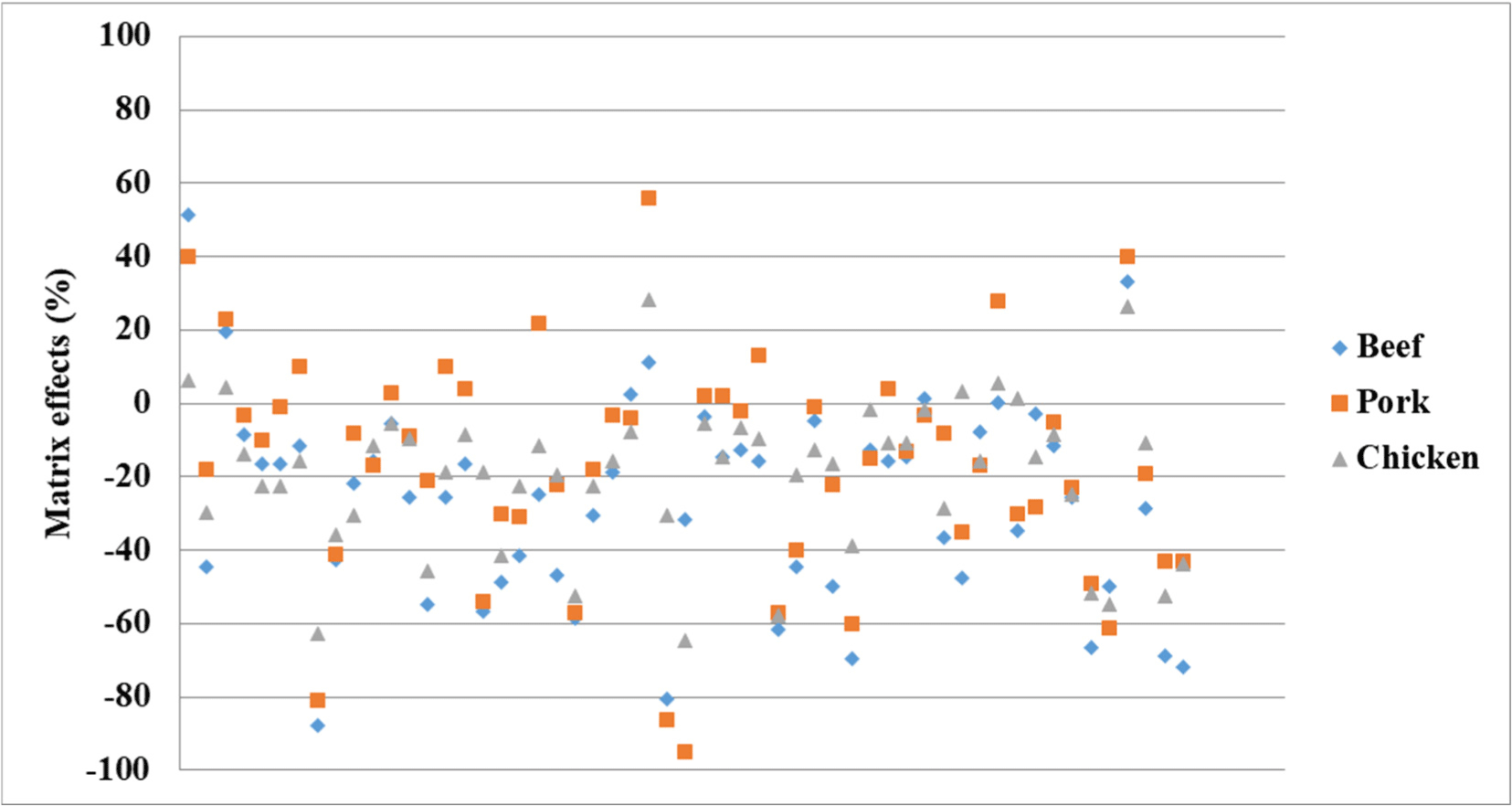
Matrix effects
The matrix effects observed for the various samples and compounds are presented in Fig. 1 and Table 4. Fig. 1 shown that the positive and negative matrix effects were observed for the livestock products examined herein. These effects were classified into five groups, namely high signal suppression (ME<−50%), moderate suppression (ME<−10% to −50%), no matrix effect (ME>−10% to <10%), moderate signal enhancement (ME>10% to <50%), and high signal enhancement (ME>50%; Chatterjee et al., 2016). It was found that the matrix effects varied in the range of −95%–56%, wherein high matrix effects were observed for 11 compounds (20.0%) in the beef matrix, nine compounds (16.4%) in the pork matrix, and seven compounds (12.7%) in the chicken matrix. No matrix effect was observed for 9 compounds (16.4%) in beef samples, 17 compounds (30.9%) in the pork samples, and 15 compounds (27.3%) in the chicken samples. In addition, moderate matrix effects were observed for 35 compounds (63.6%) in the beef matrix, 29 compounds (52.7%) in the pork matrix, and 33 compounds (60.0%) in the chicken matrix. Most compounds (beef, 47; pork, 40; chicken, 47) exhibited signal suppression, while a few (beef, 6; pork, 14; chicken, 7) exhibited signal enhancement. Pyrantel is neither in both suppression and signal enhancement in beef. The beef and chicken matrices were largely responsible for signal suppression, while the pork matrix led to both signal suppression and enhancement. The greatest suppression and enhancement were observed for the pork matrix, and these corresponded to −95% and 56% for isometamidium and halofuginone, respectively. These variable matrix effects are likely due to the complexity of the tissue matrix. Although the most effective means to compensate for matrix effects is to use an internal standard (Yin et al., 2016), internal standards are expensive, and the corresponding compounds for the various target compounds are often unavailable. Thus, the current study was performed using a matrix-matched standard curve.
Table 4.
Matrix effects of the 54 target compounds
| No. |
Compound |
Beef |
Pork |
Chicken |
| 1 |
Abamectin |
51 |
40 |
6 |
| 2 |
Albendazole |
−45 |
−18 |
−30 |
| 3 |
Albendazole sulfone |
19 |
23 |
4 |
| 4 |
Albendazole sulfoxide |
−9 |
−3 |
−14 |
| 5 |
2-Amino albendazole sulfone |
−17 |
−10 |
−23 |
| 6 |
Arprinocid |
−17 |
−1 |
−23 |
| 7 |
Benznidazole |
−12 |
10 |
−16 |
| 8 |
Bithionol |
−88 |
−81 |
−63 |
| 9 |
Buquinolate |
−43 |
−41 |
−36 |
| 10 |
Cambendazole |
−22 |
−8 |
−31 |
| 11 |
Carbendazim |
−16 |
−17 |
−12 |
| 12 |
Carnidazole |
−6 |
3 |
−6 |
| 13 |
Chlorfluazuron |
−26 |
−9 |
−10 |
| 14 |
Cymiazole |
−55 |
−21 |
−46 |
| 15 |
Derquantel |
−26 |
10 |
−19 |
| 16 |
Diaveridine |
−17 |
4 |
−9 |
| 17 |
Diethylcarbamazine |
−49 |
−30 |
−42 |
| 18 |
Emamectin |
−42 |
−31 |
−23 |
| 19 |
Febantel |
−25 |
22 |
−12 |
| 20 |
Fenbendazole |
−47 |
−22 |
−20 |
| 21 |
Fluazuron |
−59 |
−57 |
−53 |
| 22 |
Flubendazole |
−31 |
−18 |
−23 |
| 23 |
2-Amino flubendazole |
−19 |
−3 |
−16 |
| 24 |
Guaifenesin |
2 |
−4 |
−8 |
| 25 |
Halofuginone |
11 |
56 |
28 |
| 26 |
Imidocarb |
−81 |
−86 |
−31 |
| 27 |
Isometamidium |
−32 |
−95 |
−65 |
| 28 |
Levamisole |
−4 |
2 |
−6 |
| 29 |
Mebendazole |
−15 |
2 |
−15 |
| 30 |
Mebendazole amine |
−13 |
−2 |
−7 |
| 31 |
5-Hydroxy mebendazole |
−16 |
13 |
−10 |
| 32 |
Methylbenzoquate |
−62 |
−57 |
−58 |
| 33 |
Monensin |
−45 |
−40 |
−20 |
| 34 |
Morantel |
−5 |
−1 |
−13 |
| 35 |
Nicarbazin |
−50 |
−22 |
−17 |
| 36 |
Niclosamide |
−70 |
−60 |
−39 |
| 37 |
Ornidazole |
−13 |
−15 |
−2 |
| 38 |
Oxantel |
−16 |
4 |
−11 |
| 39 |
Oxfendazole |
−15 |
−13 |
−11 |
| 40 |
Oxfendazole sulfone |
1 |
−3 |
−2 |
| 41 |
Oxibendazole |
−37 |
−8 |
−29 |
| 42 |
Oxyclozanide |
−48 |
−35 |
3 |
| 43 |
Praziquantel |
−8 |
−17 |
−16 |
| 44 |
Pyrantel |
0 |
28 |
5 |
| 45 |
Semduramicin |
−35 |
−30 |
1 |
| 46 |
Ternidazole |
−3 |
−28 |
−15 |
| 47 |
Tetramisole |
−12 |
−5 |
−9 |
| 48 |
Thiabendazole |
−26 |
−23 |
−25 |
| 49 |
5-Hydroxy thiabendazole |
−67 |
−49 |
−52 |
| 50 |
Thiophanate |
−50 |
−61 |
−55 |
| 51 |
Tinidazole |
33 |
40 |
26 |
| 52 |
Toltrazuril sulfone |
−29 |
−19 |
−11 |
| 53 |
Triclabendazole |
−69 |
−43 |
−53 |
| 54 |
Keto triclabendazole |
−72 |
−43 |
−44 |
Download Excel Table
Application of our method to real samples
To demonstrate the applicability of our method, the livestock samples (n=30) collected from Korean local markets were analyzed. Among these samples, toltrazuril sulfone was detected in pork and chicken samples at concentrations of 1 μg/kg in pork (2 samples) and 5 μg/kg in chicken (1 sample); however, it should be noted that their concentrations were lower than the Korean MRL (Table 3). Toltrazuril is a triazine-based antiprotozoal that is commonly used in pigs and chicken turkeys (Mehlhorn et al., 1988). Although toltrazuril sulfone is reportedly more effective in smaller amounts than toltrazuril, it is highly toxic and can cause side effects if consumed by humans through the food chain (Franklin et al., 2003; Lindsay et al., 2000).
In a previous study, toltrazuril and toltrazuril sulfone were detected in frankfurter sausages at a concentration of 2 μg/kg (Martínez-Villalba et al., 2010). Indeed, the detection of anthelmintic and antiprotozoal drugs in livestock samples has been widely reported (Adesiyun et al., 2021; Pawar et al., 2021; Yoo et al., 2021). Ai et al. (2011) detected diclazuril in rabbit muscles (n=10), while monensin (1.4–22 ng/g, n=42) and ractopamine (0.6–64 ng/g, n=15) were detected in bovine liver, and monensin (0.8 and 1.1 ng/g, n=2) and ractopamine (1.8–6.3 ng/g, n=12) were detected in bovine muscle. Ractopamine (0.5–67 ng/g, n=7) was detected in bovine kidney, while monensin (2.0 ng/g, n=1), decoquinate (150 ng/g, n=1), lasalocid (1.5 and 14 ng/g, n=2), narasin (4 ng/g, n=1), and N,N′-bis(4-nitrophenylurea; 190 ng/g, n=1) were detected in chicken muscle (Matus and Boison, 2016). Furthermore, according to Kang et al. (2015), acetyl salicylic acid (12–576 μg/kg; n=28, 50–53 μg/kg; n=1) was detected in pigs and chickens, paracetamol (28–381 μg/kg, n=15) was detected in pigs, clopidol (9–4,614 μg/kg) was detected in chickens (n=28) and ducks (n=6), while diclazuril and amprolium were detected in chicken livers (104–525 μg/kg, n=8; and 195–196 μg/kg, n=2, respectively). Moreover, toltrazuril and its metabolites (toltrazuril sulphone and toltrazuril sulfoxide) were detected in chicken liver (n=29) at concentrations of 161–469, 67–1,822, and 209–760 μg/kg, respectively, while phenylbutazone and its metabolite (oxyphenylbutazone) were detected at levels of 247 and 15 μg/kg in cattle liver (n=1), respectively, and nicarbazin was detected at a concentration of 0.05 μg/kg in eggs (n=1; Kang et al., 2015).
Overall, this study shows that the detection amount is smaller than in previous studies (Ai et al., 2011; Matus and Boison, 2016). Therefore, monitoring results shows that livestock products are a safe level of residues. Therefore, the UHPLC-MS/MS method established in this study can be used as a reliable method for the detection of anthelmintic and antiprotozoal drug residues.
Conclusion
We herein reported the validation of an analytical method for the simultaneous quantification of anthelmintic and antiprotozoal drugs in livestock products (i.e., beef, pork, and chicken). This method exhibited an overall satisfactory performance in terms of its accuracy and precision, thereby indicating its applicability as a quantitative method. In addition, the current method achieved low limits of quantitation (0.1–9.7 μg/kg) for all target compounds in the beef, pork, and chicken. Following the successful analysis of 30 real samples obtained from markets in Korea, three samples gave detection rate of 10%; however, the residual concentrations did not exceed those of the Korean MRLs. Thus, the obtained the results confirm the suitability of this method for the detection of anthelmintic and antiprotozoal drugs in livestock products. Further study needs to increase the number of real samples and have to perform a risk assessment for the detected results. In addition, previous studies show the detection of residues in by-products (Kang et al., 2015). Therefore, it is necessary to conduct extended experiments on by-products. Nevertheless, the proposed method can be used to successfully perform the routine analysis of residues in livestock products, thereby significantly contributing to the development of multi-residue analysis and safety management in the future. Also, we expect to use the developed method to prepare for PLS program for veterinary drug in livestock and fishery products produced in 2024.
Acknowledgements
This study was supported by a grant (No. 21161MFDS382) from the Ministry of Food and Drug Safety of Korea in 2021.
References
Abbas RZ, Iqbal Z, Blake D, Khan MN, Saleemi MK. 2011; Anticoccidial drug resistance in fowl coccidian: The state of play revisited. Worlds Poult Sci J. 67:337-350


Adesiyun AA, Fasina FO, Abafe OA, Mokgoatlheng-Mamogobo M, Adigun O, Mokgophi T, Phosa M, Majokweni Z. 2021; Occurrence and concentrations of residues of tetracyclines, polyether ionophores, and anthelmintics in livers of chickens sold in the informal market in Gauteng Province, South Africa. J Food Prot. 84:655-663



Antignac JP, de Wasch K, Monteau F, De Brabander H, Andre F, Le Bizec B. 2005; The ion suppression phenomenon in liquid chromatography–mass spectrometry and its consequences in the field of residue analysis. Anal Chim Acta. 529:129-136


Beyene T. 2016; Veterinary drug residues in food-animal products: Its risk factors and potential effects on public health. J Vet Sci Technol. 7:1000285


Cano P, De la Plaza JL, Muñoz-Delgado L. 1987; Determination and persistence of several fungicides in postharvest-treated apples during their cold storage. J Agric Food Chem. 35:144-147


Chen D, Xu Q, Lu Y, Mao Y, Yang Y, Tu F, Xu J, Chen Y, Jiang X, Lu J, Yang Z. 2021; The QuEChERS method coupled with high-performance liquid chromatography-tandem mass spectrometry for the determination of diuretics in animal-derived foods. J Food Compost Anal. 101:103965


Delatour P, Parish RC, Gyurik RJ. 1981; Albendazole: A comparison of relay embryotoxicity with embryotoxicity of individual metabolites. Ann Rech Vet. 12:159-167.

Food and Agriculture Organization of the United Nations [FAO], World
Health Organization [WHO]. 2009. Guidelines for the design and implementation of National Regulatory Food
Safety Assurance Programme associated with the use of veterinary drugs in
food producing animals: CAC/GL 71-2009. Available from:
http://www.fao.org/input/download/ standards/11252/CXG_071e_2014.pdfAccessed at Mar 6, 2023.
Franklin RP, MacKay RJ, Gillis KD, Tanhauser SM, Ginn PE, Kennedy TJ. 2003; Effect of a single dose of ponazuril on neural infection and clinical disease in
Sarcocystis neurona-challenged interferon-gamma knockout mice. Vet Parasitol. 114:123-130



Frenich AG, Romero-González R, del Mar Aguilera-Luiz M. 2014; Comprehensive analysis of toxics (pesticides, veterinary drugs and mycotoxins) in food by UHPLC-MS. TrAC Trends Analyt Chem. 63:158-169


Kang J, Fan CL, Chang QY, Bu MN, Zhao ZY, Wang W, Pang GF. 2014; Simultaneous determination of multi-class veterinary drug residues in different muscle tissues by modified QuEChERS combined with HPLC-MS/MS. Anal Methods. 6:6285-6293


Kang J, Park HC, Gedi V, Park SJ, Kim MA, Kim MK, Kwon HJ, Cho BH, Kim TW, Lee KJ, Lim CM. 2015; Veterinary drug residues in domestic and imported foods of animal origin in the Republic of Korea. Food Addit Contam Part B. 8:106-112



Lindsay DS, Dubey JP, Kennedy TJ. 2000; Determination of the activity of ponazuril against
Sarcocystis neurona in cell cultures. Vet Parasitol. 92:165-169



Pawar RP, Durgbanshi A, Bose D, Peris-Vicente J, Albiol-Chiva J, Esteve-Romero J, Carda-Broch S. 2021; Determination of albendazole and ivermectin residues in cattle and poultry-derived samples from India by micellar liquid chromatography. J Food Compost Anal. 103:104111


Rejczak T, Tuzimski T. 2015; A review of recent developments and trends in the QuEChERS sample preparation approach. Open Chem. 13:980-1010


Wilkowska A, Biziuk M. 2011; Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 125:803-812


Yoo KH, Park DH, Abd El-Aty AM, Kim SK, Jung HN, Jeong DH, Cho HJ, Hacimüftüoğlu A, Shim JH, Jeong JH, Shin HC. 2021; Development of an analytical method for multi-residue quantification of 18 anthelmintics in various animal-based food products using liquid chromatography-tandem mass spectrometry. J Pharm Anal. 11:68-76




Zeleny R, Ulberth F, Gowik P, Polzer J, van Ginkel LA, Emons H. 2006; Developing new reference materials for effective veterinary drug-residue testing in food-producing animals. TrAC Trends Analyt Chem. 25:927-936