Introduction
The food industry has developed along with globalization, resulting in an increased risk of foodstuffs being contaminated with pathogens, chemical residues, and toxins. The proliferation of pathogenic and spoilage bacteria should be controlled to guarantee food safety. Conventional preservatives are a group of synthetic chemical substances including nitrates/nitrites, sulfites, sodium benzoate, propyl gallate, and potassium sorbate. The use of these conventional preservatives in food has known side effects (Sharma, 2015). Nitrites and nitrate have been linked to leukemia, colon, bladder, and stomach cancer. Sorbate and sorbic acid are rare; however, they are related to urticaria and contact dermatitis. Benozates have been suspected to relating to allergies, asthma, and skin rashes.
During recent decades, investigation on food preservation have focused on more natural and healthier food (Caminiti et al., 2011; Fangio and Fritz, 2014). Biopreservation has dealt with extending food shelf life and enhancing food safety using plants, animals, microorganisms, and their metabolites (Settanni and Corsetti, 2008). Particularly, meat and meat products are perishable materials, and are controlled by the Hazard Analysis Critical Control Point (HACCP) approach. The risk of contracting foodborne illnesses is reduced by various food preservation methods; thermal processing, drying, freezing, refrigeration, irradiation, modified atmosphere packaging, and the addition of antimicrobial agents, salts, or other chemical preservatives. Unfortunately, these techniques cannot be applied to all food products because of undesired effects (texture, color, etc.) depending on food type, such as ready-to-eat foods and fresh foods. Especially, preserving meat products is more complex, with higher pH and mild pasteurization temperatures required.
Natural preservative are the chemical agents derived from plants, animals, and microorganisms, and are usually related to the host defense system (Singh et al., 2010; Tiwari et al., 2009). As the demand for biopreservation in food systems has increased, new natural antimicrobial compounds of various origin are being developed, including animal-derived systems (lysozyme, lactoferrin, and magainins), plant-derived products (phytoalexins, herbs, and spices), and microbial metabolites (bacteriocins, hydrogen peroxide, and organic acids) (Lavermicocca et al., 2003). The requirements of natural preservatives are: safety, stability during food processing (pH, heat, pressure, etc.), and antimicrobial efficacy. The representative food pathogens are Escherichia coli, Salmonella spp., Listeria monocytogenes, Staphylococcus aureus, Bacillus cereus, Yersinia enterocolytica, Clostridium perfringens, Clostridium botulinum, and Campylobacter jejuni. The pathogenic fungi often related to food-borne diseases are toxin-producing Aspergillus flavus and Aspergillus paraciticus (Prange et al., 2005).
This review summarizes the current knowledge about natural preservatives regarding their antimicrobial effects, antimicrobial mechanism, application, and regulation in food systems.
Natural Preservatives of Plant Origin
Plant preservatives are composed to polyphenols and phenolics, essential oils, and plant antimicrobial peptides (pAMPs). These substances have evolved to possess antibacterial and antioxidant effect (Dua et al., 2013). Phenolics and polyphenols have various antimicrobial structures: simple phenols (caffeic acid, catechol, eugenol, and epicatechin) and phenolic acids (caffeic acid and cinnamic acid), quinones (hypericin), flavones, flavonols, flavonoids (epigallocatechin-3-gallate, catechin, and chrysin), tannins (pentagalloylglucose, procyanidine B-2), coumarins (coumarin, warfarin, and 7-hydroxycourmarin), terpenoids (menthol, artemisin, and capsaicin), and alkaloids (berberine and harmane) (Table 1) (Cowan, 1999; Hintz et al., 2015). The pAMPs are represented by thionin, plant defensins, lipid transfer proteins (LTPs), myrosinase-binding proteins (MBPs), hevein- and knottin-like peptides, snakins, cyclotides, and peptides from hydrolysates (Hintz et al., 2015).
| Class | Subclass | Examples | References |
|---|---|---|---|
| Phenolics | Simple phenols | Catechol | Hintz et al., 2015 |
| Epicatechin | Mason and Wasserman, 1987 | ||
| Phenolic acids | Cinnamic acid | Cowan, 1999 | |
| Hintz et al., 2015 | |||
| Quinones | Hypericin | Cowan, 1999 | |
| Hintz et al., 2015 | |||
| Flavonoids | Chrysin | Dua et al., 2013 | |
| Quercetin | Lee et al., 2011 | ||
| Flavones | Abyssinone | Cowan, 1999 | |
| Flavonols | Totarol | Cowan, 1999 | |
| Tannins | Ellagitannin | Dua et al., 2013 | |
| Lee et al., 2016 | |||
| Coumarins | Coumarin | Cowan, 1999 | |
| Warfarin | Hintz et al., 2015 | ||
| 7-Hydroxycoumarin | |||
| Terpenoids, essential oils | Capsaicin | Bajpai et al., 2008 | |
| Eugenol | Gutierrez et al., 2008 | ||
| Thymol | Helander et al., 1998 | ||
| Carvacrol | Tiwari et al., 1998 | ||
| Alkaloids | Berberine | Cowan, 1999 | |
| Harmane | Garba and Okeniyi, 2012 | ||
| Piperine | |||
| Lectins and polypeptides | Mannose-specific agglutinin | Cowan, 1999 | |
| Fabatin | Cowan, 1999 | ||
| Polyacetylenes | 8S-Heptadeca-2(Z),9(Z)-diene-4,6-diyne-1,8-diol | Cowan, 1999 | |
| Antimicrobial peptide (pAMP) | Potato defensin, hevein, thionines, | Hintz et al., 2015 | |
| snakins, lipid transfer protein etc. | Jessen et al., 2006 |
Plant polyphenol extracts have been used as natural meat preservatives, including extracts from oregano, cranberry, sage, rosemary, grape seed, and others. Polyphenols can act as reducing agents and metal ion chelators in the presence of various hydroxyl radicals.
Oregano and cranberry extracts were evaluated for antimicrobial activity against L. monocytogenes in laboratory media, beef, and fish (Lin et al., 2004). These phenolicbased plant extracts are widely used in food preparation and are classified as Generally Regarded as Safe (GRAS). The effects of neem oil on the meat pathogens Carnobacterium maltaromaticum, Brochothrix thermosphacta, E. coli, and Pseudomonas fluorescens, were investigated as a preservative for fresh retail meat (Del Serrone et al., 2015a, Del Serrone et al., 2015b). Citrus species extracts were investigated as antifungal agents against spoilage fungi including Mucor sp. and Rhizophus sp. (Mohanka and Priyanka, 2014). Ethanol extract of Citrus species showed a higher antifungal effect than water extract did, and the minimum inhibitory concentration of the extract ranged from 6.25 to 25 mg/mL. Inula britannica ethanol extract showed an antimicrobial effect against five B. cereus strains in low fat milk, and the antimicrobial effect depended on terpene and polyphenol compounds (Lee et al., 2012). Brassica juncea extract showed an antiviral effect against influenza virus A/H1N1 in nonfat milk (Lee et al., 2014). Chestnut inner shell extract containing gallic acid and quercetin was shown an antimicrobial effect against C. jejuni in chicken meat at 1 and 2 mg/mL (Lee et al., 2016). Eight different flavonoids [quercetin, kaempferol, apigenin, luteolin, 5,4-dihydroxy-7-methnozyflavone (genkwanin), narigenin, hesperetin and hesperidin] were tested for antimicrobial effects against B. cereus strains (P14 and KCCM 40935) (Lee et al., 2011). Among these flavonoids, only kaempferol and apigenin were inhibitory, and kaempferol showed the greatest antimicrobial effect at 100 μM.
Essential oil or terpenes are secondary metabolites that provide flavor and aroma. The addition of adding essential oils from marjoram and rosemary was investigated in beef patties (Mohamed and Mansour, 2012). These essential oils were beneficial for antioxidant activity and sensory evaluation.
Plant antimicrobial peptides (pAMPs) were discovered in 1942 as natural defense compounds against pathogens (Hintz et al., 2015). The pAMPs were named as thionins, plant defensins, lipid transfer proteins (LTPs), myrosinase-binding proteins (MBPs), hevein- and knottin-like peptides, snakins, cyclotides, and peptides from hydrolysates. These substances have been isolated from Triticum aestivum (wheat), Impatients balsamina, Hordeum vulgare (barley), Arabidopsis thaliana, Hevea brasiliensis, Solanum tuverosum (potato), Oldenlandia offinis, etc.
The antimicrobial mechanisms of phenol compounds depend on their concentration. Phenols affect enzyme activity related to energy production at low concentrations; however, they cause protein denaturation at high concentrations (Fig. 1). These abilities affect microbial cell permeability, thereby interfering with membrane function (material transport, nucleic acid synthesis, and enzyme activity) (Bajpai et al., 2008; Fung et al., 1977; Rico-Munoz et al., 1987). The high antibacterial activity of phenolic compounds can be due to alkyl substitution into the phenol nucleus, forming phenoxy radicals, which does not occur in more stable molecules such as the ethers myristicin or anethole (Dorman and Deans, 2000; Gutierrez et al., 2008). Catechol and pyrogallol possess phenolic toxicity to microorganisms through enzyme inhibition by oxidized compounds, possibly by reacting with sulfhydryl groups or through more nonspecific interactions with proteins (Mason and Wasserman, 1987). The antimicrobial targets of quinones may include surface-exposed adhesins, cell wall polypeptide, and membrane-bound enzymes (Cowan, 1999). The antimicrobial activities of isothiocynates derived from Brassicaceae vegetables, such as cauliflower, broccoli, mustard, and cabbage are related to 1) loss of cell membranes integrity, 2) inhibiting enzyme or regulatory activity by quorum sensing (in Helicobacter pylori, Pseudomonas aeruginosa, Chromobacterium violaceum, etc.), 3) inhibition of respiratory enzymes, 4) induction of heat-shock and oxidative stress, and 5) induction of a stringent response (Dufort et al., 2015). Carvacrol, (þ)-carvone, thymol, and trans-cinnamaldehyde decrease the intracellular ATP (adenosine triphosphate) content of E. coli O157:H7 cells (Helander et al., 1998).
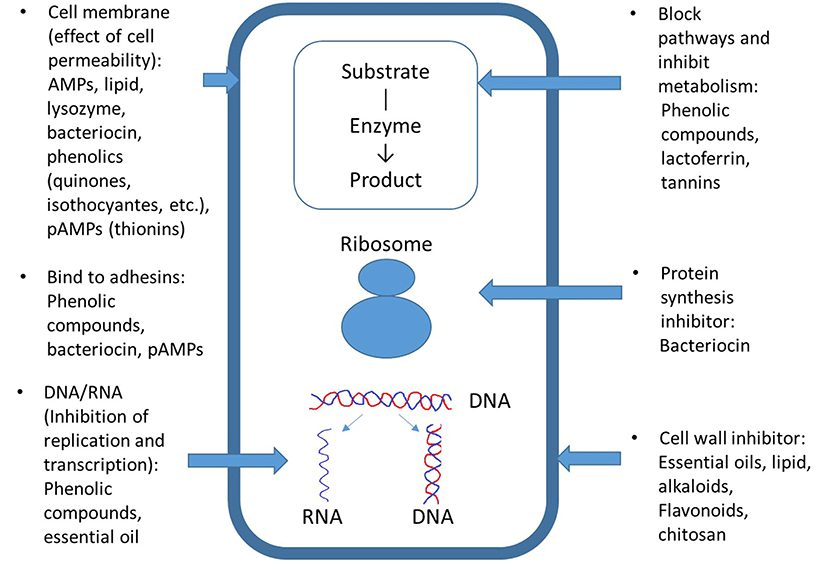
Essential oils have multiple cellular targets. Their hydrophobicity results in reactions with lipids on bacterial and fungal cell membranes, increasing membrane permeability and disturbing the original cell structure (Hintz et al., 2015; Pinto et al., 2009). In addition, antiviral effects are achieved by inhibiting viral protein synthesis at multiple stages of viral infection and replication (Wu et al., 2010).
The antimicrobial mechanism of most pAMPs involves cell membranes of targeted organisms and is driven by net positive charge, flexibility, and hydrophobicity to enable interaction with bacterial membranes (Jessen et al., 2006). Their antifungal mechanisms are involved in cell lysis, interference with fungal cell wall synthesis, permeabilization, binding to ergosterol/cholesterol in the membrane, depolymerization of the actin cytoskeleton, and targeting intracellular organelles such as mitochondria. Antiviral activity is also related to viral adsorption and entry processes.
Natural Preservatives of Animal Origin
There are numerous antimicrobial systems of animal origin related to host defense mechanisms. Preservatives of animal origin include lysozymes, lactoperoxidase, lactoferrin, ovotransferrin, antimicrobial peptide (AMP), chitosan, and others (Table 2).
| Examples | Sources | Bacterial target | References |
|---|---|---|---|
| Chitosan | Crustaceans and arthropods | Antifungal and antimicrobial activity | Ben-Shalom et al., 2003; Je and Kim, 2006; Liu et al., 2006 |
| Defensin | Vertebrates and invertebrates | Bacteria, fungi, and virus | Ganz, 2003 |
| Dermaseptin S4 | Frog skin | Bacteria, fungi, and virus | Mor and Nicolas, 1994 |
| Lactoperxoidase | Milk | Gram-negative and -positive bacteria | Russell, 1991; de Wit and van Hooydonk, 1996 |
| Lactoferrin | Milk | Gram-negative and -positive bacteria, fungi, and parasites | Al-Nabulsi and Holley, 2005 |
| Lipids | Milk, animal | Gram-negative and -positive bacteria | Isaacs et al., 1990; Lampe et al., 1998; Wang and Johnson, 1997 |
| Lysozyme | Chicken egg whites | Gram-negative and -positive bacteria | Tiwari et al., 2009 |
| Magainin | African clawed frog | Gram-negative and -positive bacteria | Zasloff et al., 1988 |
| Ovotranferrin | Egg | S. aureus and E. coli | Ibrahim et al., 2000 |
| Pleurocidin | Skin of winter flounder | Bacteria, fungi, and virus | Cole et al., 1997 |
| PR-39 | Porcine | Gram-negative and -positive bacteria | Shi et al., 1996 |
Lysozyme is obtained from chicken egg whites, and is known as a bacteriolytic enzyme. Lysozyme has been used commercially under the name Inovapure, and can be used against a wide range of food spoilage organisms for extending the shelf life of various food products including raw and processed meats, cheese, and other dairy products (Tiwari et al., 2009).
The lactoperxoidase is a naturally active enzyme in milk with strong antimicrobial effects against both Gramnegative and -positive bacteria (de Wit and van Hooydonk, 1996; Russell, 1991) and fungi. Lactoperoxidase catalyzes the hydrogen peroxide (H2O2) oxidation of several acceptors; it has been utilized in industries such as dairy, oral care, cosmetics, cancer, and viral infection.
Lactoferrin and ovotranferrin are glycoproteins derived from bovine milk and hen egg respectively, that can bind iron, thereby restricting or preventing bacterial growth. Lactoferrin shows strong antimicrobial effects against various Gram-negative and -positive bacteria, fungi, and parasites in neutral pH and refrigeration temperature (Al-Nabulsi and Holley, 2005). Ovotranferrin peptide fragment OTAP-92 has strong bactericidal activity against both S. aureus and E. coli strains through membrane damage (Ibrahim et al., 2000). However, these transferrin peptides cannot be utilized in food systems because of their high cost.
AMPs are widely distributed in nature and are essential components of nonspecific host defense systems (Park et al., 1997; Tossi et al., 2000). The AMPs produced by animal cells include magainin (Zasloff et al., 1988), MSI-78 (Ge and Yan, 2002), PR-39 (Shi et al., 1996), pleurocidin (Cole et al., 1997), and dermaseptin S4 (Mor and Nicolas, 1994). AMPs are considered a promising solution for antibiotic resistance because of their non-specific molecular targets and fast membrane destruction. Pleurocidin is isolated from the winter flounder (Pleuronectes americanus) is active against Gram-negative and -positive bacteria (Cole et al., 2000). It is stable in heat and salt; however, it is unstable in supraphysiological concentrations to magnesium and calcium. An antimicrobial effect of pleurocidin was reported in foodborne organisms including Vibrio parahemolyticus, L. monocytogenes, E. coli O157: H7, Saccharomyces cerevisiae, and Penicillium expansum (Burrowes et al., 2004). Defensins are widely found in mammalian epithelial cells from chicken, turkey, and others (Brockus et al., 1998). Their antimicrobial spectrum included Gram-negative and -positive bacteria, fungi, and enveloped viruses (Ganz, 2003; Murdock et al., 2007).
Chitosan is a natural biopolymer obtained from the exoskeletons of crustaceans and arthropods, and has been used as an antifungal and antimicrobial agent (Ben-Shalom et al., 2003; Je and Kim, 2006; Liu et al., 2006). Chitooligosaccharides have a bacteriostatic effect on Gramnegative bacteria, E. coli, Vibrio cholera, Shigella dysenteriae, and Bacteriodes fragilis (Benhabiles et al., 2012). Chitosan (0.25, 0.5, and 1%) was studied as an antimicrobial ingredient in fresh pork sausage (Bostan and ’Isin Mahan, 2011).
Lipids of animal origin have antimicrobial activity against a wide range of microorganisms. Free fatty acids at mucosal surfaces have been shown to inactivate S. aureus (Bibel et al., 1989). Milk lipids are active against Gram-positive bacteria including S. aureus, C. botulinum, B. subtilis, B. cereus, and L. monocytogenes, and Gramnegative bacteria such as P. aeruginosa, E. coli, and Salmonella enteritidis (Isaacs et al., 1990; Lampe et al., 1998; Wang and Johnson, 1997).
AMPs, transferrins, and lipids can influence cell membranes and peptide synthesis (Fig. 1) (Brogden, 2005; Zasloff, 2002). AMPs can interact directly with the microbial cell membrane and result in the leaching out of vital cell components (Cole et al., 2000; Hancock, 1997). Lipids mainly inhibit bacterial cell walls or membranes, intracellular replication, or intracellular targets. Lysozymes inhibit bacterial cell membranes by hydrolyzing β-1,4-glycosidic linkages between N-acetylmuramic acid and N-acetylglucosamine in bacterial peptidoglycan.
Natural Preservatives from Microorganisms
The preservative of microbial origin include nisin, natamycin, pullulan, ε-polylysine, organic acid, and others (Singh et al., 2010) (Table 3).
| Examples | Sources | Bacterial target | References |
|---|---|---|---|
| Bacteriocins | |||
| Nisin, diplococcin, acidophilin, bulgaricin, helveticin, lactacin, pediocin, and plantarin | Lactococcus lactis, Lactobacillus acidophilus, Lactobacillus bulgaricus, etc. | Gram positive bacteria | Lee et al., 2013; Anastasiadou et al., 2008; Bhunia et al., 1988 |
| Natamycin | Streptomyces natalensis | Molds and yeasts | EFSA, 2009 |
| Reuterin | Lacotobacillus reuteri | Gram-negative and -positive bacteria, yeasts, and filamentous fungi | Axelsson et al., 1989 |
Lactic acid bacteria produce antimicrobial compounds like organic acids, diacetyl, hydrogen peroxide, and proteinaceous bacteriocins (Lee et al., 2013). Bacteriocins are antimicrobial peptides or proteins produced by mainly lactic acid bacteria; these compounds are small and ribosomally synthesized. Most bacteriocins have potential as food preservatives because of their antimicrobial effect against food pathogens. The representative bacteriocins are nisin, diplococcin, acidophilin, bulgaricin, helveticin, lactacin, pediocin, and plantarin. Of these bacteriocins, nisin and pediocin have been used as commercial natural preservatives.
Nisin is a representative bacteriocin produced by various Lactococcus lactis strains, and has activity against food pathogens including Alicyclobacillus spp., L. monocytogenes, Bacillus spp., Micrococcus spp., Clostridium spp., Pediococcus spp., Desulfotomaculus spp., S. aureus, Enterococcus spp., Streptococcus haemolyticus, Lactobacillus spp., Sporolactobacillus spp., and Leuconostoc spp. Nisin is proteinaceous polypeptide that is most stable in acidic conditions. Nisin is soluble in aqueous conditions and is unstable in alkali solutions and heat. It has been used in various food products alone or in combination with other compounds. Nisin is the most widely used bacteriocin approved by the FDA as a food preservative. Dairy and meat products are applied with doses of 50-200 mg/kg. In the USA, nisin is used to inhibit outgrowth of C. botulinum spores and toxin formation in pasteurized processed cheese spread with fruits, vegetables or meats with a limited dose of about 250 ppm in finished products.
Pediocin is GRAS bacteriocin produced by strains of Pediococcus acidilactici (AcH, PA-1, JD, and 5) and P. pentosaceus (A, N5p, ST18, and PD1) (Anastasiadou et al., 2008). Most pediocins are stable in heat and a wide range of pH vlues. Pediocin AcH is effective against both spoilage and pathogenic organisms, including L. monocytogenes, Enterococcus faecalis, S. aureus, and Clostridium perfringens (Bhunia et al., 1988).
Natamycin is an antifungal substance produced by Streptomyces natalensis that is effective against almost all molds and yeasts; however, it has little or no effect on bacteria (EFSA, 2009). Natamycin has been used in dairy, meats, and other foods for antifungal effects, and its use as a surface preservative is regulated (E 235).
Reuterin (β-hydroxypropionaldehyde), an antimicrobial compound produced by Lacotobacillus reuteri, is a watersoluble nonproteinaceous metabolite of glycerol (Axelsson et al., 1989). Its broad antimicrobial spectrum includes Gram-negative and -positive bacteria, yeasts, and filamentous fungi (Nom and Rombouts, 1992).
The antimicrobial mechanism of bacteriocin involves pore formation in the cytoplasmic membrane of target microorganisms (Fig. 1). This leads to cell death by loss of intracellular molecules and a collapse of the proton motive force (Driessen et al., 1995). Bacteriocin originating from Gram-positive bacteria is only effective for Grampositive bacteria, and is less effective on Gram-negative bacteria due to their selective membrane permeability (Lee et al., 2003). These disadvantages could be compensated for by using other preservatives and preservative methods.
Natamycin has an antimicrobial effect by binding to ergosterol, a cell membrane sterol, in fungal membranes (EFSA, 2009). The structure of natamycin contains a large lactone ring with a rigid lipophilc chain containing conjugated double bonds, and a flexible hydrophilic portion bearing several hydroxyl groups. The hydrophobic groups form a polar pore with ergosterol in the membrane, and this complex affects material passage (K+, H+, amino acids, and other metabolites) (Deacon, 1997).
Application of Natural Preservatives toward Livestock Food Systems
Raw meat, meat products, milk, and milk products are major sources of foodborne pathogens, and a variety of methods have been considered to reduce bacterial contaminants. These methods include (a) chemical decontamination with organic acids (Gill and Badoni, 2004; Goncalves et al., 2005; Nissen et al., 2001) and trisodium phosphate (Bashor et al., 2004; Okolocha and Ellerbroek, 2005); (b) physical processes such as irradiation (Badr, 2005; Kim et al., 2004), high pressure processing (Oliveira et al., 2015), steam (Kang et al., 2001a; Kang et al., 2001b; Logue et al., 2005; Stivarius et al., 2002), and UV; (c) natural antimicrobials such as bacteriocins (de Martinez et al., 2002; Gogus et al., 2004) and iron chelating compounds; and (d) combination treatment (Bashor et al., 2004; Koohmaraie et al., 2005).
Challenge studies using meat samples mainly reported efficacy against L. monocytogenes, B. cereus, C. jejuni, and S. aureus (Barman et al., 2014). The efficacy of natural preservatives was tested against commercial formulation (Microgard 100, Microgard 300, nisin, Altak 2002, Perlack 1902) (Lemay et al., 2002). These natural preservatives were investigated in an acidified chicken meat model (pH 5.0). E. coli ATCC 25922 and Brochothrix thermosphacta CRDAV452 were inhibited, however Lactobacillus alimentarius BJ33 (FloraCarn L-2) was not inhibited.
The use of fruit byproducts, including rinds of grapefruit, orange, and mandarin with or without γ-irradiation, was applied in raw ground beef (Abd El-khalek and Zahran, 2013). These substances demonstrated antioxidant and antimicrobial properties on microbial growth, lipid oxidation, and color change of raw ground beef meat. The antimicrobial effects on the survival of Salmonella typhimurium, E. coli and B. cereus were demonstrated.
A combination of plant extracts and MAPs was applied in meat products. Thymol and thymol-MAP were applied in sausage to inhibit Pseudomonas spp.; however, the performance is unacceptable respect to sensory acceptability (Mastromatteo et al., 2011). Bay essential oil with MAP (20% CO2 and 80% N2) was applied in ground chicken meat, and extend the shelf life without L. monocytogenes and E. coli (Irkin and Esmer, 2010). Oregano oil was added to fresh chicken breast meat under MAP (Chouliara et al., 2007). At 1%, oregano oil had a very strong taste in the sensory evaluation; however 0.1% oregano oil and MAP extended the shelf life by 5-6 d without strong taste.
Plant preservatives like clove oil showed a synergistic effect with lactic acid and vitamin C for antioxidant and antimicrobial effects (Naveena et al., 2006). Ntzimani et al. (2010) used a mixture of EDTA, lysozymes, rosemary, and oregano oil, and the shelf life of semi-cooked coated chicken fillets was extended under vacuum packaging at 4℃ to more than 2 wk.
Nisin was applied with lactoferrin in Turkish-style meatballs. Counts of total aerobic bacteria, coliform, E. coli, and other species were decreased by lactoferrin alone and by the mixture of lactoferrin and nisin (Colak et al., 2008). A mixture of lysozyme, nisin, and EDTA effectively inhibited L. monocytogenes and meat-borne spoilage bacteria in ostrich patties packaged in air and vacuum (Kim et al., 2002; Mastromatteo et al., 2010).
Regulation of Natural Preservatives in Livestock Foods
Preservatives permitted in livestock foods are sodium acetate, natamycin, pimamycin, nisin, nitrites (potassium nitrite and sodium nitrite), nitrates (potassium nitrate and sodium nitrate), sorbates (sorbic acid, sodium sorbate, potassium sorbate, and calcium sorbate), and sulphites (sulfur dioxide, sodium sulfite, sodium bisulfite, sodium metabisulfite, potassium metabisulfite, potassium sulfite, and potassium bisulfite) (Food and Drug Administration, 2016).
Natural food preservatives are regulated by maximum permitted levels for food safety and health (Table 4). The only natural preservatives regulated by legislation are natamycin and nisin. Natamycin (E235) is permitted for use in over 150 countries in the surface treatment of hard, semi-hard and semi soft cheeses and dried, cured sausages with a maximum permitted level of 6-40 mg/kg. Nisin (E234) is permitted for use in over 80 countries worldwide, including the United States and European Union, and has been in use as a food preservative for over 50 years (Adams, 2003; EFSA, 2006). The maximum permitted levels in meat, poultry, game products are 5.5-7 mg/kg.
ESFA (2006, 2009); GSFA (1995); KFDA (2016).
Natural preservatives are considered safer than synthetic preservatives because of their existence in nature and long history of use. However, the use of natural preservatives in food is not powerful enough when considering added amounts in food system. Therefore, effective use levels of conventional and plant extracts/oils against microorganisms are less than 0.1% and 10-20%, respectively (Browne et al., 2012). Therefore, the regulation of these natural preservatives as food additives is necessary regarding their safety, toxicity, and effectiveness.
Conclusion
Chemical preservative have side effects related to the emergence of drug-resistant strains and chronic toxicity. Traditional methods of preservation including refrigeration, pasteurization, and low pH are not completely effective in controlling food pathogens. Therefore, the efficacy of combining natural preservatives with traditional methods has been tested. Combination with other substances or different food preservation systems, coatings, or microand nano-capsulation should be tested to assure safety and nontoxicity of natural preservatives. In addition, the use of natural preservatives must be regulated by law for safety, toxicity, and effectiveness.













