Introduction
Several interventions are being used in food processing and production industry to control food contamination by pathogens. Food pathogens pose a serious problem to the health of the human kind. World Health Organization (WHO) has estimated that 420,000 people die every year due to contamination food (WHO fact sheet 2017). Bacteria contribute to 90% of the deaths due to food poisoning under which Listeria monocytogenes and Escherichia coli are responsible for 31% of the cases. United States Department of Agriculture (USDA) has reported 21 recalls due to L. monocytogenes and 9 recalls for Shiga-toxin producing E. coli (STEC) in 2018 in the US which has led to 4, 415, 454 pounds of product being recalled (USDA recall summary, 2018). There has been an increase in the number of contamination and prevalence of these pathogens in fresh produce including spinach. Recall of organic spinach and spring mix in 2012 due to STEC contamination in the US (CDC report, 2012) and recall of baby spinach in Canada due to L. monocytogenes in 2018 (Canadian Food Inspection Agency report, 2018) has caused significant loss and health issues to people.
Current measures to eliminate contamination of food by pathogens including heating, refrigeration, freezing, modified packaging, antimicrobial washes and addition of antimicrobials in the finished product. Most of the antimicrobials being used to control food contamination are synthetic and have limitations on efficacy. Also, as consumers are becoming aware of the ingredients, they seek for safe products with clean label antimicrobials.
Several strategies and approaches are being followed to develop clean label antimicrobial for food application including use of plant extracts (Hintz et al., 2015), essential oils for plants and plant products (Pandey et al., 2017) and use of beneficial microbes (Cleveland et al., 2001; Yang et al., 2014). Use of normal commensals and beneficial microbes from the environment and food sources have been studied and evaluated extensively. Food has been a good source of these beneficial microbes with activity against food pathogens (Zaid, 2018). A major portion of the beneficial microbes isolated from food belong to the class lactic acid bacteria which produce multiple antimicrobial compounds including organic acid and bacteriocins. Lactic acid bacteria isolated from food are reported to possess antimicrobial activity against E. coli and L. monocytogenes (Arques et al., 2015). Leuconostoc spp. which falls under the lactic acid bacteria groups are also known to be effective against E. coli, L. monocytogenes, Salmonella enterica serovar Typhi and S. enterica serovar Typhimurium (Giles-Golmez et al., 2016; Benmechernene et al., 2013). Leuconostoc spp. have been isolated from several foods including such as chill-stored and fermented meats, vegetables, and dairy products (Liu et al., 2016). They have also been isolated from meat processing plants (Goto et al., 2003; Nissen et al., 1994).
Previously, Leuconostoc spp. have been isolated from chicken and pork samples from Indian (Thangavel et al., 2019) which showed promising results against E. coli, L. monocytogenes, Staphylococcus aureus and S. Typhimurium. The objective of the present study was to evaluate the efficacy of the Leuconostoc isolates in controlling E. coli and L. monocytogenes in spinach stored at 4°C for a period of 5 d.
Materials and Methods
Two challenge studies were conducted in spinach leaves with E. coli and L. monocytogenes. Spinach leaves were treated with cell free supernatants (CFSs) of the Leuconostoc isolates, then inoculated with the pathogen (E. coli and L. monocytogenes) and enumerated at regular intervals while stored at 4°C for 5 d. Each of the challenge study was conducted in triplicates with 2 sampling at each time point/replicate. The groups for the study included inoculated control with no antimicrobial treatment and 5 groups inoculated with pathogen and treated with CFS of the isolated. Detailed description of each of the step of the study, materials and methods are provided below.
The food-borne pathogens that were selected for the study were E. coli MTCC 443, and L. monocytogenes MTCC 657. The strains were procured from MTCC, India. The samples were received as freeze-dried cultured in glass vials. Upon receiving the samples, the cultures were sub-cultured by inoculating the samples into 10 mL of sterile Tryptone Soya Broth (TSB, HiMedia M641, Chennai, India) and incubating for 24 h at 37°C. After the incubation period, sterile 50% glycerol on deionized water was added with the cultures at 1:1 ratio to make a final concentration of 25% glycerol (Sigma Aldrich, G9012) in the glycerol culture stocks. The samples were transferred to cryovials and were frozen at −80°C until further use.
The glycerol stock of the cultures (E. coli and L. monocytogenes) were thawed to room temperatures and 100 μL of strain from the stock were aseptically transferred to 10 mL of TSB broth and incubated at 37°C for 18–20 h. One hundred μL of the overnight culture was transferred again to 10 mL of TSB broth and incubated at 25°C for 18–20 h. After the incubation period, the cells were harvested by centrifugation (2,500×g, 20 min) and suspended in 10 mL of phosphate buffered saline pH 7.2 (PBS, HiMedia, M1452, Chennai, India). The working inoculum of the pathogens was prepared by diluting the cultures to achieve ~6 to 7 Log10 CFU/mL respectively. The counts of the working inoculum were verified by plating on to their selective media.
The Leuconostoc isolates designated as CM17, CM19, PM30, PM32, and PM36 were identified earlier as Lc. mesenteroides subsp. mesenteroides J18; CP003101, Lc. mesenteroides LM2, Lc. mesenteroides (T); ATCC 8293; CP000414, Lc. gelidum subsp. gasicomitatum LMG 18811; type strain: LMG 18811; FN822744 and Lc. mesenteroides; LM2; AY675249 respectively. Glycerol stocks of these isolates were passaged twice in De Man, Rogosa and Sharpe (MRS) broth (HiMedia, M369) and incubated for 24°C for 48 h. The samples were centrifuged at 2,500×g for 20 min to remove all cells and the CFSs were used for the antimicrobial treatment. The CFSs were stored in the refrigerator until use within the same day.
Spinach leave samples (Semi-savoy type) were procured from the local vegetable market, they were cleaned with water to remove dirt, air drier to remove excess moisture. The leaves were packed in 25 g into Ziploc pouches and stored in refrigerator until use. The samples were inoculated with the pathogen on the same day.
Twenty-five gram of the spinach leaves in the Ziploc pouches were inoculated with 250 μL of the working inoculum of either E. coli or Listeria. The pouches were hand massage gently to help with distribution of the inoculum over the leaves. The samples were allowed to stand for 60 min at room temperature to enable the attachment of the pathogens to the leaves. After 60 min, each of the groups was treated with 1 mL of one of Leuconostoc CFS. Control samples treated with only MRS broth was maintained to compare with the treatment groups.
Ziploc pouches containing the spinach leaves were stored at 4°C for 5 d and enumerated for the counts of pathogens at regular intervals. The samples were spread out in the refrigerator to enable uniformity in the temperature. This storage condition and duration was followed to mimic the typical consumers practice.
The samples were enumerated for the counts of pathogens on 0, 1, 2, and 5 d of storage. Twenty-five milliliters of PBS was added to each of the pouch, hand massaged to facilitate the detachment of the cells and aliquots were serially diluted and plated using selective media for the pathogens. In the case of Listeria, Listeria Identification Agar Base (PALCAM, HiMedia M1064, Chennai, India) supplemented with Listeria Selective Supplement (PALCAM) (FD061) was used as the plating media whereas in the case of E. coli, Levine Eosin-Methylene Blue Agar Medium (EMB agar, Himedia MU022). Both PALCAM and EMB were incubated at 37°C for 24 to 48 h. After the incubation period, the colonies were counted and represented as Log10 colony forming unit CFU/mL of the rinse. Control samples of the leaves with no microbial inoculation were enumerated on 0 d to rule out any background contamination from the leaves. Two samples per group were tested per time period and the study was conducted in three replicates.
The data from the enumeration studies are reported as average Log10 CFU/mL of rinse±SD for three separate studies (n=3) conducted for each of challenge studies with E. coli and L. monocytogenes. Differences between the treatments and the untreated control were analyzed by one-way analysis of variance (ANOVA) using the STATGRAPHICS© Centurion XV. All statistically significant differences in the study were reported at p<0.05 level.
Results and Discussion
Uninoculated control spinach leaves were plated on 0 d onto PALCAM agar to rule out any background Listeria contamination in the samples and the leaves were found to be free of Listeria. The study showed that L. monocytogenes did not grow significantly (p=0.4406) in the inoculated control samples when stored at 4°C for 5 d (Table 1). Despite the fact that Listeria can survive and thrive at refrigerated condition, there was no growth observed within the 5 d of testing period. This is in alignment with the previous observations (Carrasco et al., 2008) wherein a 5.6 d lag phase was observed with L.monocytogenes in ready-to-eat iceberg lettuce stored at 5°C. The longer time period would have witnessed growth of Listeria in the leaves, however, 5 d was chosen as it is the traditional shelf life of spinach. In the case of the leaves treated with the CFS of the Leuconostoc isolates, they did not show any difference (p=0.8621) in the counts of Listeria at 0 d as compared to the control. However, at 1, 2, and 5 d all the groups treated with the CFS showed significant reduction (p<0.05) in the Log10 CFU/mL of the rinse as compared to the control (Fig. 1, Table 1). The % of reduction in the counts ranged from 30.75% to 73.28% on 1 d reaching up to 64.16% to 89.95% on 5 d (Table 1). CFS of Leuconostoc isolate CM19 (Lc. mesenteroides LM2) showed the least reduction by the end of 5 d (64.19%) whereas isolates PM 30 (Lc. mesenteroides (T); ATCC 8293; CP000414) and PM32 (Lc. mesenteroides; LM2; AY675249) showed the highest reduction throughput the study period, with 85.75% and 89.95% respectively on 5 d. Earlier study by Nakamura et al. (2012) showed the inhibitory effect of Leuconostoc isolated from a fermented fish dish on L. monocytogenes infection in A/J mice and in Caco-2 cells. Similarly, Leuconostoc isolated from fresh fruits and vegetables were found to efficient in controlling Listeria in wounds of Golden Delicious apples and iceberg lettuce leaf cuts (Trias et al., 2008). One of the strain CM 160 was most effective resulting in a ten-fold reduction of the viable pathogen concentration (ED90) which is close to the efficacy observed with PM 36 (90% reduction).
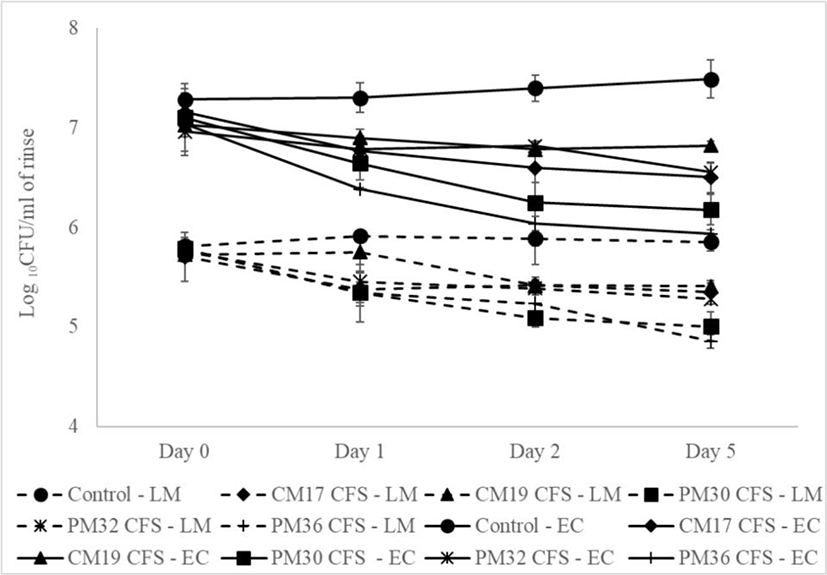
Statistical analysis of the counts of Listeria within the group over the testing period showed significant reduction from 1 d in all the groups treated with CFS except for CM 17 which showed reduction from 2 d. Within the groups that showed the efficacy from 1 d, no statistically significant difference was observed beyond 1 d expect from PM 30 which showed a gradual reduction over the entire testing period to reach 85.75% reduction by the end (Table 1).
Plating of the uninoculated control spinach leaves on 0 d for E. coli showed that the sample was free of the E. coli that could picked in the EMB agar. No statistically significant difference (p=0.2857) were observed in the count of E. coli in the inoculated control samples when stored at 4°C, over the 5 d testing period (Table 2). Leaves treated with the Leuconostoc CFSs, they did not show any difference (p=0.4037) in the counts of E. coli at 0 d as compared to the control. However, from 1 d all the antimicrobial treated groups showed significant reduction (p<0.05) in the Log10 CFU/mL of the rinse as compared to the control (Fig. 1, Table 2). The percentage of reduction in the counts ranged from 61.70% to 88.37% on 1 d reaching up to 79.69% to 97.35% on 5 d (Table 2). As observed with Listeria, CFS of Leuconostoc isolate CM19 showed the least reduction by the end of 5 d (79.69%) and isolates PM 30 and PM32 showed the highest reduction throughput the study period, with 95.21% and 97.35% respectively on day 5. Leuconostoc isolated from ground beef was found to inhibit E. coli O157 H7, S. aureus, Salmonella spp., L. monocytogenes, and spoilage bacteria Brochothrix thermosphacta in beef (Koo et al., 2015). The activity of the CFS of the isolate was attributed to organic acids produced by the isolates. Similarly, Lc. mesenteroides KCCM35046 fermented aged garlic extract were found to be effective in controlling E. coli in the excreta of chicken (Hossain et al., 2016).
The counts of Listeria within the group over the testing period showed significant reduction from 1 d in all the groups treated with CFS except for the control. No significant difference was observed in the counts in any of the groups beyond 1 d indicating that the inhibition was achieved with 24 h and no further statistical reduction was observed. However, numerical difference was observed after 1 d in all groups except for CM 19 (Table 2).
Over all, the study showed that the CFSs of the Leuconostoc isolates from Indian meat were able to reduce the counts of L. monocytogenes and E. coli in spinach leaves. PM 36 isolate (Lc. mesenteroides; LM2; AY675249) was found to be the most potent of all the isolates with 90 and 95% reduction in the pathogens at the end of 5 d. PM 32 (Lc. gelidum subsp. gasicomitatum LMG 18811) also showed high reduction in the pathogens.
Leuconostoc spp. isolated from different sources has been reported to possess antimicrobial activity by several researchers (Borges et al., 2019; Bellil et al., 2014). The antimicrobial activity of Leuconostoc have been attributed to production of organic acid (Koo et al., 2015) and bacteriocins (Hechard et al., 1992; Martinez et al., 2006). Several bacteriocins are being produced by Lc. mesenteroides, including Leucocin A-VAL 187, Leucocin A, Mesentericin YlOS, Bacteriocin ST33LD, Leucocyclicin Q (Masuda et al., 2011; Stiles et al.; 1994, Todorov et al., 2005). Use of Leuconostoc in food applications has been studied by Shi et al. (2016), where in the Leucocin K7 produced by Leuconostoc mesenteroides K7 isolated from fermented pickle was found to be effective in controlling L monocytogenes for a period of 7 d in milk. Similarly, novel bacteriocins isolated from Lc. mesenteroides spp. mesenteroides IMAU:10231 were found to be effective in Listeria control in Serbian Sremska sausages (Moracanin et al., 2013). Harding et al. (1990) demonstrated the efficacy of Leuconostoc gelidum against closely related species and L. monocytogenes. However, no efficacy was found against spore forming and other gram-positive bacteria like S. aureus. This is contrary to the promising results that we have obtained with our strain.
Leuconostoc spp. has been isolated from multiple and different habitats and they play an integral role in the fermentation of food. Leuconostocs are generally considered as GRAS (generally regarded as safe) organisms (Bjorkroth et al., 2014) as they are associated with food fermentation. However, some species of Leuconostoc have been associated with opportunistic infection (Kumudhan et al., 2004) in immune compromised patients and this microorganism can cause spoilage in some types of food matrices (de Paula et al., 2015).
Although these microbial strains may have a negative impact in food application due to their ability to cause food spoilage by slime, rope formation and imparting sourness, the CFSs can be a potential biopreservative to control food pathogens in food. This would be a potential clean label antimicrobial to replace existing synthetic antimicrobials in the food.













