Introduction
Due to being tasty and cheap, hen eggs are one of the most favourite food on our planet and consumed either as a main dish or used as an ingredient both in processed and home-made food products. Eggs are a natural source of many nutrients, including high-quality protein, vitamins (riboflavin, cobalamin, pantothenic acid, vitamin D, vitamin A etc.), certain essential minerals and trace elements, including phosphorus (Guyonnet, 2015). The FAO has stated that the global production of hen’s eggs (in shells) in 2018 was about 77 million metric tonnes. While China, the USA and India are the top three countries for eggs production, Turkey is the ninth in world hen’s eggs production at about 0.96 million tonnes of unprocessed, in-shell hen’s eggs produced annually (FAO, 2018). Turkey is also the leading hen’s egg producer in Europe and the third exporter of eggs in the world, after the Netherlands and the USA.
Eggs and egg dishes can be a source of food-borne diseases caused mainly by Salmonella Enteritidis if improperly handled (EFSA, 2012). Egg consumers may also be exposed to chemical hazards, including mycotoxins (aflatoxin M1, ochratoxin A), dioxins and dioxin-like polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAH), veterinary drugs and pesticides. While carry-over of mycotoxins, PCBs, PAH and heavy metals from feed to eggs seems negligible, dioxins, as well as pesticides, may be transferred from feed to eggs (Kan, 2007).
Pesticides are man-made chemicals and used for the treatment of many crops against several diseases and pests, including fungi, insects, nematodes etc. International organisations and many local authorities have set maximum residue levels (MRLs) for pesticides in foods, and their use is carefully controlled. Nevertheless, there is still a risk of pesticide residues occurring in foods and animal feeds. Pesticides and environmental hazards can enter laying hens with the inhalation and contact with the skin, but the most important way of transmission of residues to laying hens is via the feed, and they can accumulate in the muscle, liver and fatty tissue of animals as well as in eggs (Song et al., 2019).
On 30 May 2002, The Netherlands transmitted a Rapid Alert System for Food and Feed (RASFF) information notification concerning nitrofen in organic eggs from Germany. Shortly after, Germany notified an increase of cases related to nitrofen in organic eggs (0.07–0.115 mg/kg) and prohibited substance dichlorodiphenyltrichloroethane (DDT) in eggs (0.379–0.567 mg/kg). More recently, on 20 July 2017, Belgium launched a RASFF alert notification concerning fipronil pesticide in eggs (0.003–1.2 mg/kg). Millions of eggs have been recalled from shops and warehouses in Belgium, The Netherlands and Germany in the following weeks. After this first notification on fipronil, there were 110 notifications on fipronil in eggs (0.008–1.9 mg/kg) originating from various European countries, mainly Italy (58.2%) and Poland (16.4%), by the end of 2019.
Following the fipronil egg contamination incident in Belgium, the incidence of pesticide residues in hen eggs has received great attention. While several studies have been performed over the last decade to detect fipronil in eggs (Anagnostopoulos et al., 2019; Guo et al., 2018; Hatta et al., 2019; Li et al., 2019; Tu et al., 2019; Zhang et al., 2016), there is limited data on multi-class residues of pesticides in hen eggs (Akgün et al., 2020; Lichtmannegger et al., 2015; Song et al., 2019).
The goal of the present study was to develop and validate a multi-residue analytical method using quick, easy, cheap, effective, rugged and safe (QuEChERS) methodology in combination with liquid chromatography-tandem mass spectrometry (LC-MS/MS) and gas chromatography-tandem mass spectrometry (GC-MS/MS) to identify and quantify pesticide residues in hen eggs. In this study, we intended to cover 365 GC-amenable and 142 LC-amenable pesticide residues, including fipronil due to their presence in the EU active substance list, and in the list of banned or EU and Turkey MRLs for eggs. Furthermore, the validated method was applied to monitor 100 real eggs consumed in Turkey.
Materials and Methods
LC-MS grade acetonitrile (MeCN), methanol (MeOH) and formic acid (CH2O2) were acquired from Sigma-Aldrich (Steinheim, Germany). Glacial acetic acid (CH3COOH) and ammonium formate (HCOONH4) were from Merck (Darmstadt, Germany). Ultrapure water (18.2 MΩ) was obtained using a Milli Q purification system (Millipore, Molsheim, France). Anhydrous magnesium sulphate (MgSO4) (Sigma-Aldrich), anhydrous sodium acetate (C2H3NaO2) (Merck) and primary-secondary amine (PSA, 40 μm particle size) (Supelco, Bellefonte, PA, USA) were analytical purity. Bondesil C18 bulk sorbent (50 μm particle size) was from Agilent Technologies (Santa Clara, CA, USA).
Certified standards of 507 pesticides were from Dr. Ehrenstorfer (Augsburg, Germany), ChemService (West Chester, PA, USA), Sigma-Aldrich (Steinheim, Germany) and A2S Analytical Standard Solutions (Saint Jean d’Illac, France). The purity of standards was ≥92%. Four stock solutions for GC-amenable pesticides and five stock solutions for LC-amenable pesticides were prepared at 10 mg/L in acetonitrile [or methanol/acetonitrile (50:50, v/v), depending on the analytes’ solubility] to cover all target analytes. These nine multi-standard mixture solutions were used for the preparation of matrix-matched calibration standards (1–100 μg/kg for LC-amenable and 2–250 μg/kg for LC-amenable analytes) and validation study.
A hundred egg packages [containing six medium (≥53 – <63 g) class A eggs from caged hens] were purchased from retail stores and supermarkets in Corum, Turkey. The shell of each six eggs was crushed, and whole eggs (egg yolk and egg white) were poured into an appropriate container. The whole eggs were homogenised with an ultra-turrax basic homogeniser (WiseTis® HG-15A, Witeg Labortechnik GmbH, Wertheim, Germany) for 1 min at about 3,000×g. An aliquot of about 40 mL homogenate egg solution was then poured into three 50 mL polypropylene centrifuge tubes and stored frozen at –18°C until analysis.
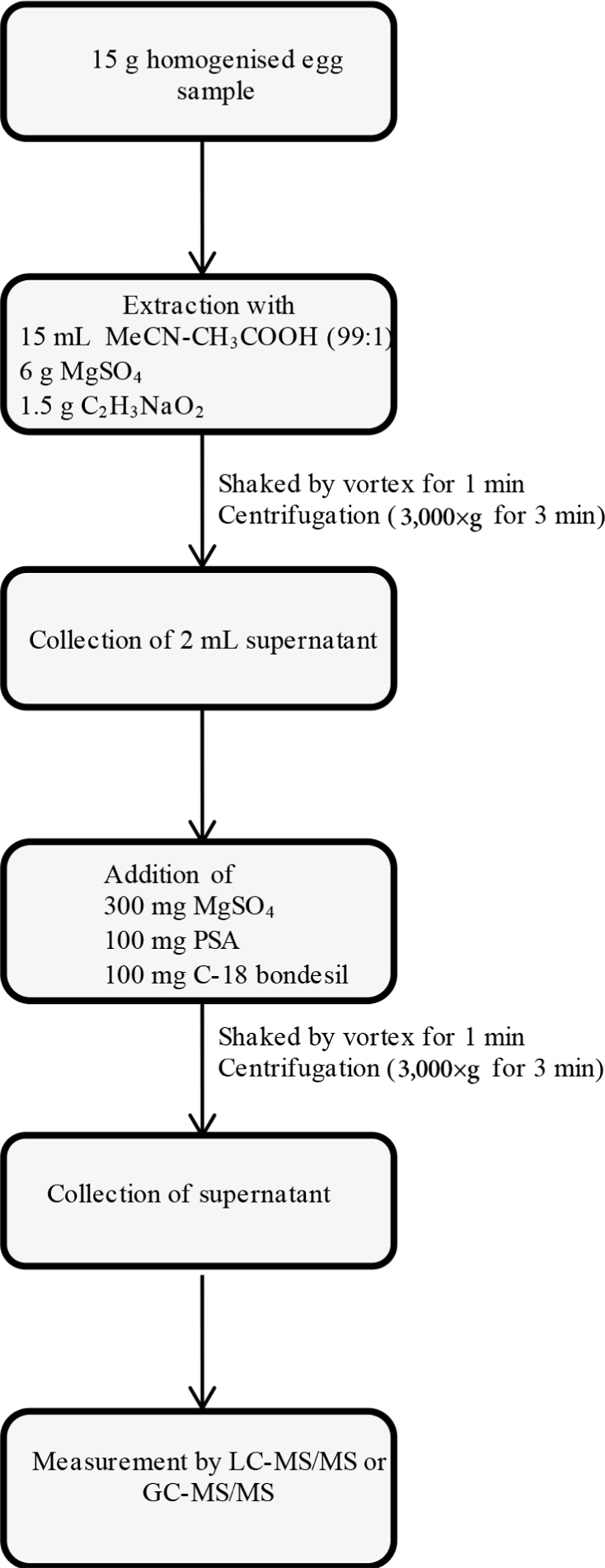
The sample preparation was based on the QuEChERS extraction procedure according to AOAC Official Method (AOAC, 2007), with slight modifications. For the validation experiment, portions of 15 g of the blank egg materials were put into 50 mL polypropylene centrifuge tubes. Then the blank egg materials were fortified with multi-pesticides standard solutions. For recovery, the target mass fractions of pesticides were set to 10 and 50 μg/kg. The QuEChERS extraction and clean-up procedures were summarised in Fig. 1. For the quantification of target analytes in hen eggs, blank samples of homogenised eggs were fortified with multi-standard solutions, resulting in a 7-point set of matrix-matched calibration samples.
Chromatographic separation of pesticide residues was conducted by a Waters Acquity UPLC I-Class system (Waters, Milford, MA, USA), with BEH C18 reversed-phase analytical column (100×2.1 mm, 1.7 μm) (Waters), integrated with a Fusion-RP guard column (4×2.0 mm, 4 μm) from Phenomenex (Torrance, CA, USA), at 50°C, and analyte determination was achieved with a Xevo TQD tandem quadrupole tandem mass spectrometer equipped with electrospray ionisation (ESI) interface source (Waters). The mobile phase was: (A) 95/5 (v/v) water/methanol, (B) 5/95 (v/v) water/methanol, both solutions containing 2 mM ammonium formate and 0.1% formic acid. The elution was started at 25% solvent B and held for 0.5 min, then increased B to 98% in 10 min, and kept at 98% for 1.5 min. Finally, the column was equilibrated at the initial condition for 2 min at a flow rate of 0.45 mL/min. The injection volume was 1 μL. Positive ESI+ mode was used for the analysis of 365 LC-amenable pesticide residues. Instrument control, data acquisition and data processing were performed by Waters MassLynxTM (version 4.1) software. Two specific multiple reaction monitoring (MRM) transitions of the studied residues are shown in Table S1.
GCMS-TQ8050 triple quadrupole GC/MS (Shimadzu, Japan) with split/splitless injector was used for the analysis of 142 pesticide residues. The residues were separated on a Rxi-5 Sil MS (30 m×0.25 mm I.D.×0.25 μm) from Restek. The column was set at a linear velocity of 50 mL min−1 using helium (ultra-high purity) as a carrier gas. The initial column temperature was maintained at 40°C for 1 min, programmed at 40°C/min to 120°C, then programmed at 5°C/min to 240°C, followed by 12°C/min to 280°C, holding for 6 min. The total run time was 36 min. The MS transfer line and ion source temperatures were set at 280°C and 200°C, respectively. In a splitless injection mode, a 2 μL of sample extract was injected with a splitless time of 1.5 min at a constant injection temperature of 250°C. MS analysis was performed using electron ionisation (EI) mode. LabSolutions InsightTM software was used for (quantitative analysis of multi-analyte data) instrument control and data acquisition. For each target analytes, two MRM transitions were analysed, and the collision energy was optimised. The MRM transitions for 142 target analytes are listed in Table S2.
The performance characteristics of the analytical method have been in-house validated according to the European SANTE/11813/2017 Guideline (European Commission, 2017). The following parameters were assessed in the validation study: specificity, linearity, limit of quantification (LOQ), accuracy, precision and measurement uncertainty. For the quantification of target analytes in hen eggs, locally purchased egg samples were used as a matrix. Egg samples were extracted as per the flowchart shown in Fig. 1 to prepare the matrix blank. These blank egg extracts were spiked with multi-standard solutions, resulting in a 7-point set of matrix-matched calibration samples. The matrix-matched calibration standards were prepared at 1, 2, 5, 10, 25, 50, and 100 μg/kg for LC-amenable pesticides, and at 2, 5, 10, 25, 50, 100, and 250 μg/kg for GC-amenable pesticides. After the multi-point calibration, LOQ, accuracy and precision were measured by fortified experiments using blank eggs matrices. LOQ was estimated via the SD of blank egg samples fortified to a suitable low level of targeted pesticides that can be quantified with acceptable accuracy (70%–120%) and precision (relative standard deviations (RSD)% ≤20) as described in SANTE/11813/2017 Guideline. The recovery and precision were performed by the measurement of blank eggs matrices fortified at two concentration levels of 10 and 50 μg/kg. The expanded measurement uncertainty (U’) was measured by multiplying combined uncertainty associated with within-laboratory reproducibility and trueness (bias), with a coverage factor of k=2, as described in detail previously (Golge et al., 2018).
Results and Discussion
This study included 507 pesticide residues, of which 365 were analysed by LC-MS/MS, and 142 were analysed by GC-MS/MS. The instrumental MS/MS conditions are listed in Tables S1 and S2 for LC-amenable and GC-amenable pesticides, respectively.
The specificity of both LC-MS/MS and GC-MS/MS techniques was determined by injecting representative blank samples of hen eggs (n=20) at low spiking concentration (10 μg/kg). For both LC-amenable and GC-amenable analytes, no chromatographic interference was observed even at low spiking level and did not interfere with quantitation.
Matrix matched calibration standards were generated using blank extracted hen eggs. Matrix matched calibration curves were linear within the quantitation limits established for each LC- and GC-amenable analytes, which was compound dependent but ranged from as low as 1 μg/kg to 250 μg/kg, with R2 values greater than 0.99 and residuals were within 20%.
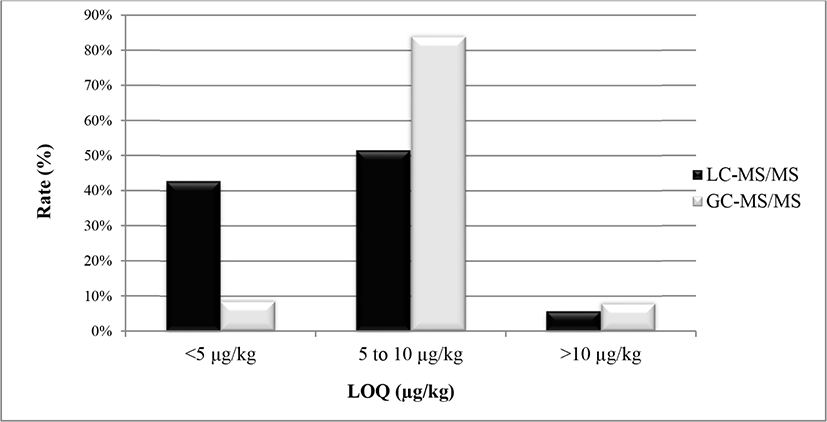
The limit of detection (LODs) and LOQs were determined based on data of the spiking experiment. The LODs and LOQs of target analytes were determined by three and ten times the SD of replicate analyses (n=10) of blank egg samples fortified at 10 μg/kg. The LOQs for all target analytes are listed in Table S3. Overall, as summarised in Fig. 2, 93.7% of residues either LC- or GC-amenable had satisfactory sensitivity in hen eggs with LOQs of 1.4–10 μg/kg, while 6.3% of analytes had LOQ higher than 10 μg/kg. In LC-MS/MS analysis, 42.7% of residues had LOQ lower than 5 μg/kg, 51.5% of residues had LOQ values between 5 and 10 μg/kg, and only 5.8% of residues had LOQ greater than 10 μg/kg. Imazalil showed the highest sensitivity resulting in LOQ=1.4 μg/kg, while disulfoton pesticide had the lowest sensitivity with LOQ of 19.3 μg/kg in hen eggs by LC-MS/MS. In GC-MS/MS analysis, LOQs were below than 5 μg/kg for 8.5% of residues, at between 5 and 10 μg/kg for 83.8% of residues, and higher than 10 μg/kg for 7.7% of pesticides. The highest LOQ obtained was 22.7 μg/kg for o,p’-DDT pesticide, while chlorothalonil observed the lowest LOQ of 3.4 μg/kg in GC-amenable compounds (Table S3). For fipronil, the determined LOQ (4.8 μg/kg) was lower than both EU MRL of 5 μg/kg and national legislation of 15 μg/kg.
Method recovery was assessed by spiking pesticides at two levels of 10 and 50 μg/kg in hen eggs and comparing the response to that observed from matrix-matched standards. Recoveries and precision (repeatability and within-laboratory reproducibility, %RSDs) values for all compounds are presented in Table S3. Results outside of the acceptance criteria are highlighted in bold. The overall recoveries observed for 98.6% of LC-amenable pesticides and 95.1% of GC-amenable pesticides were in the range of 70% to 120%, while 0.5%–2.1% and 0.8%–2.8% of LC- and GC-amenable analytes showed recoveries <70% and >120%, respectively. With GC-MS/MS, three pesticides, namely, chlordane (cis-), chlordane (trans-) and oxyfluorfen had recoveries lower than 70%, whereas four GC-amenable pesticides with average recoveries of >120% were, butylate, cycolate, dinobuton and endosulfan (alpha). In LC-MS/MS, two pesticides (bromoxynil and imazapyr) showed low recoveries (<70%), whereas three GC-amenable pesticides (famoxadone, profoxydim and thiobencarb) had recoveries in excess of 120%.
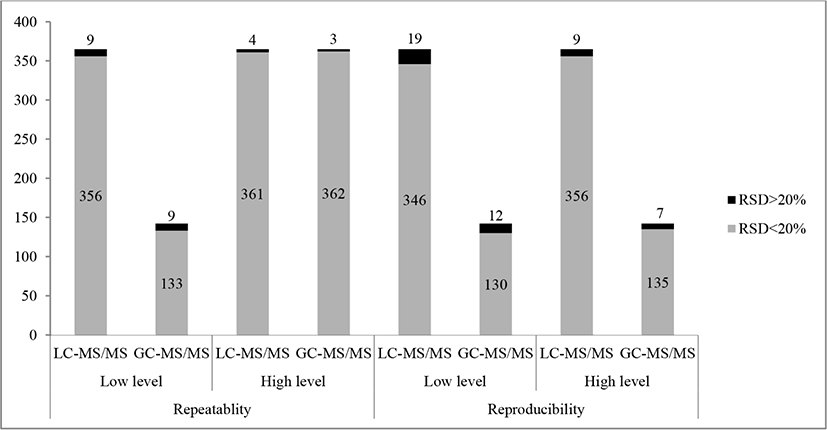
The results show that %RSD under repeatability conditions were less than 20% for 97.5%–98.9% of LC-amenable and 93.7%–97.9% of GC-amenable target analytes, depending on the spiking level. The %RSD under reproducibility conditions <20% were observed for 94.8%–97.5% of LC-amenable and 91.5%–95.1% of GC-amenable pesticides (Fig. 3), depending on the spiking level, which indicates that high analytical precision was achieved when analysing multi-residue pesticides simultaneously. The RSDs were showed more variability at a low spiking level of 10 μg/kg. The maximum RSD value of 29.77% and 28.91% for the low spiking level were obtained for bitertanol and bromoxynil by GC- and LC-MS/MS analysis, respectively.
As can be seen in Table S3, the measurement uncertainty calculated for 99.2% of LC-amenable and 97.9% of GC-amenable analytes showed values below 50%. The measurement uncertainty was higher than 50% for only three LC-amenable pesticides, namely, bromoxynil (54.8%), imazapyr (51.7%) and thiobencarb (50.9%) and three GC-amenable pesticides, namely, captan (52.0%), chlordane (trans-) (53.1%) and cycloate (51.5%). Therefore, a default expanded MU can be used for the interpretation of results based on the SANTE/11813/2017 guidelines.
The method validation data of the present study was also compared with the previous studies for the analysis of pesticide residues in hen eggs and chicken muscle. As can be seen in Table 1, the LOQ, recovery and precision values obtained in the present study are in good agreement with the previously reported studies. Method for analysis of pesticides provides good recovery and detection of high protein foods. Similar findings were also previously reported for chicken muscle (Weng et al., 2020).
| Matrix | Spiking levels (μg kg−1) | Recovery (%) | Precision (RSD%) | LOQ (μg kg−1) | Reference |
|---|---|---|---|---|---|
| Hen eggs | 10–50 | 91.1–94.1 | 2.1–8.9 | 4.8 | Current study |
| Hen eggs | 2.5–100 | 71.6–99.1 | 5.0–14.4 | 2.5 | Anagnostopoulos et al., 2019 |
| Hen eggs | 2.5–50 | 74.2–112.0 | 4.4–12.4 | 2.5 | Charalampous et al., 2019 |
| Hen eggs | 5–100 | 88.8–93.8 | 1.6–4.3 | 2.0 | Song et al., 2019 |
| Hen eggs | 2–40 | 92.8–107.0 | 1.6–2.2 | 1.0 | Guo et al., 2018 |
| Hen eggs | 5–50 | 78.0–88.0 | 4.0–5.0 | 5.0 | Hildmann et al., 2015 |
| Hen eggs | 0.01–1 | 79.7–90.5 | 0.8–6.1 | - | Zhang et al., 2016 |
| Chicken muscle | 0.01–1 | 81.3–95.7 | 1.9–8.8 | - | Zhang et al., 2016 |
To evaluate the applicability of the validated method, 100 egg samples collected from retail stores and supermarkets in Turkey, were analysed for the presence of 507 multi-class pesticide residues. None of the target pesticides was detected above the quantitation limit in all egg samples. These results are inconsistent with a previous Turkish study by Akgün et al. (2020), who did not detect any of the 59 pesticide residues in 35 egg samples. In Greece, only one of the 18 processed egg samples contained fipronil at a level of 0.0067 mg/kg (Anagnostopoulos et al., 2019). In contrast to our results, in Korea, Song et al. (2019) monitored 60 pesticides in hen eggs and determined seven pesticides, namely disulfoton, fipronil sulfone, cyromazine, o,p-DDT, p,p-DDD, p,p-DDT and permethrin in 27.6% (16/58) of egg samples at levels of 5–10 μg/kg. In 2017 in Brazil, Pereira et al. (2020) analysed only 13 samples for a total of 26 pesticide residues. While pirimiphos, mephosfolan and pyraclostrobin were detected in a single different sample, spiroxamine was quantified in 62% of egg samples up to a level of 8.3 μg/kg, but well below the EU MRL of 50 μg/kg. In a recent study by Luo et al. (2020), 325 pesticides were monitored in 40 chicken egg samples from China, and 9 GC-amenable residues (benzenehexachloride (BHC)-beta, BHC-gamma, biphenyl, chloroneb, p,p’-DDD, p,p’-DDE, p,p’-DDT, hexachlorobenzene and phenothrin) and four LC-amenable residues (2,4,5-T, etoxazole, propetamphos and simazine) were observed in egg samples. The maximum level of BHC-gamma in eggs exceeded the national MRL of 100 μg/kg.
Conclusion
The analytical method was successfully validated for the determination of more than 500 pesticide residues in eggs. The limits of quantification achieved for most of the LC- and GC-amenable pesticides analysed are well below the EU MRLs. While there are some recoveries and RSD values slightly lower or higher than the values recommended by the SANTE/11813/2017 Guideline, the results obtained are satisfactory (recoveries 70%–120% and RSDs ≤20%) for 92.9% of LC-amenable and 86.6% of GC-amenable pesticide residues. The validated method was applied to 100 eggs from caged hens, and none of the target pesticide residues was detected above the quantitation limit. It can be concluded that this method can be easily implemented in the analysis of high protein foods for multi-residue pesticides in routine testing laboratories.