Introduction
Traditional dairy products are the natural habitats of microbes, especially lactic acid bacteria. The Mongolian race is well-known for their production and consumption of dairy products; thus, a large number of natural LAB strains presented in these dairy products have passed from generation to generation during the households manufacturing process. Baotou and Bayannur are located in the midwest of Inner Mongolia with rich natural pastoral areas, which could contributed to the dairy production. The local inhabitants use traditional methods to produce unique and diverse fermented foods and the numerous households have developed and handed down their own characteristic dairy products. These traditional fermented dairy products include fermented cow milk, urum, huruud, kumiss, tarag (fermented milk of cows, yaks, goats or camels), airag (an alcoholic fermented horse milk), yak milk, goat milk, kurut and so on, what’s more, these products were made by natural fermentation without adding any commercial starter cultures. Rhee et al. (2011) suggested that lactic acid bacteria are widely distributed in natural fermented foods as indigenous microflora. Also previous studies have been performed to analyze the diversity of the microbial species existing in traditional fermented dairy products in various locations, such as Turkey (Gurses and Erdogan, 2006), Africa (Mathara et al., 2004), Italy (Losio et al., 2014), Mongolia (Takeda et al., 2013), Iran (Azadnia and Khan Nazer, 2009), Morocco (Ouadghiri et al., 2009) etc.
Our research team has integrally and systematically analyzed the biodiversity of LAB in various conventional dairy foods in different minority regions of China, for instance, Yunnan (Liu et al., 2009), Sichuan (Bao et al., 2012b), Gansu (Bao et al., 2012a), Qinghai (Sun et al., 2010), eastern Inner Mongolia (Liu et al., 2012; Yu et al., 2011), Tibetan (Chen et al., 2010) and Xinjiang (Sun et al., 2009). In addition, we have already screened out numerous novel strains for their functional properties and desirable beneficial effects, including Lactobacillus (Lb.) casei Zhang (Wu et al., 2009), Lb. plantarum P-8 (Wang et al., 2013) and Lb. helveticus H9 (Chen et al., 2014). Our previous studies have demonstrated that traditional dairy products are rich sources for isolating precious LAB resources.
To our knowledge, there were only limited studies that described LAB composition present in urum, huruud in midwestern Inner Mongolia. Here, we isolated and characterized the LAB communities in sixty-six samples of urum, huruud and fermented cow milk that were collected from two regions named Baotou and Bayannur in midwestern Inner Mongolia by traditional culture method and 16S rRNA gene analysis. To precisely depict the dominant LAB populations, quantitative polymerase chain reaction method was applied.
Materials and Methods
Three types of samples including fermented cow milk (fermented at room temperature with the household’s traditional starter), huruud (dry curd of cheese produced by boiling the spontaneously fermented cow milk and squeezing the sediment) and urum (cow milk spontaneously fermented over a day at room temperature in the household’s traditional wooden cask and then on the surface of the dairy would form the thin cream called urum) were produced by nomadic families. Sixty-six samples were collected from thirteen sampling sites located in two cities called Baotou and Bayannur, the midwest of Inner Mongolia, in June 2015. About 50 mL of each sample was aseptically collected and stored in sterile polyethylene bottle. The collected samples were then transported to our laboratory in a vehicle-mounted refrigerator kept at 4℃, followed by longer term storage at 80℃ in the laboratory until further microbiological analysis and LAB isolation. The information of samples is listed in Table 1.
DM: samples from Baotou; BM: samples from Bayannur.
One milliliter of a sample was mixed with 9 mL sterile physiological saline (0.85% w/v, NaCl) to make an initial dilution. Serial dilutions were made for each sample and then 1 mL of the appropriate dilution was mixed with the melted MRS agar to enumerate the total LAB with the pour plate method. Cycloheximide at a concentration of 0.01% (v/v) was added to the MRS plates in order to prevent the growth of fungi. Meanwhile, the appropriate dilution were evenly spread onto the MRS (Difco Laboratories, USA) and M17 (Oxoid Ltd., UK) plates before being incubated under anaerobic condition at 30℃ for 48-72 h. Colonies with distinct morphological differences (based on color, shape, size, rough or smooth surface) were selected and then purified using another agar plate of the same culture medium. The catalase activity and Gram reaction of all the isolates were assessed. Gram-positive, catalase-negative and non-motile microorganisms were preserved in 10% (w/v) skim milk containing 0.1% (w/v) sodium glutamate and stored at 80℃.
DNA was extracted from strains that grew in MRS and M17 culture broth at 37℃ by a revised cetyltrimethylammonium bromide (CTAB) method. Purified DNA template was diluted to 100 ng/μL for 16S rRNA gene amplification. The 27F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 1495R (5’-CTACGGCTACCTTGTTACGA-3’) primers were used for amplification of the partial 16S rRNA gene (Liu et al., 2009).
The PCR mix (50 μL) contained 2 μL DNA templates (100 ng/μL), 5 μL 10 × buffer (Mg2+), 4 μL dNTP (10 mmol/L), 1.5 μL primer FA-27F (10 pmol/μL), 1.5 μL primer RA-1495R (10 pmol/μL) , 0.5 μL Taq DNA polymerase (5 U/μL) and 35.5 μL tri-distilled water. The thermal cycling program consisted of an initial denaturation step at 94℃ for 5 min and 30 cycles of 94℃ for 1 min, 58℃ for 1 min and 72℃ for 2 min with a final extension at 72℃ for 10 min and 4℃ for heat preservation. PCR amplification was carried out on an automatic thermal cycler (PTC-200, MJ Research, USA). The sequencing of purified products was performed by Shanghai Sangni Biosciences Corporation of China. Subsequently, the 16S rRNA gene sequences of all isolates were submitted to the National Center for Biotechnology Information (NCBI, http://www.blast.ncbi.nlm.nih.gov) for BLAST search. MEGA version 6.0 software (http://www.mega software.net) was used to create phylogenetic trees by the neighbor-joining (NJ) method.
Total DNA of each sample was extracted as described previously (Lick et al. 1996; Xu et al. 2014). The DNA quality was checked by the spectrophotometry and agarose gel electrophoresis. All extracted DNA were stored at 20℃ until further processing. The predominant LAB in traditional dairy products in Baotou and Bayannur of midwestern Inner Mongolia were enumerated by q-PCR, as listed in Table 2.
| Target bacteria | Primer pairs (Forward/Reverse) | Oligonucleotide sequences (5’-3’) | Product size/bp | Tm (℃) | Reference |
|---|---|---|---|---|---|
| Lb. plantarum | Lp-F | CAGAATTGAGCTGGTGGTGG | 210 | 55 | (Marco and Kleere-bezem, 2008) |
| Lp-R | TGTTACTTTCGCAACCAGAT | ||||
| Lac. lactis subsp. lactis | Lac-F | ATGCGTAAACTTGCAGGAC | 262 | 57 | (Passerini et al., 2010) |
| Lac-R | CAACCTTGAATGGTGGAG | ||||
| Leu. mesenteroides | Leu-F | ATACAGGCGAACAGGGGATTA | 269 | 45 | (Olsen et al., 2007) |
| Leu-R | GGGTGTAGTTTCTGGGTTTC |
Q-PCR was carried out on an ABI Step-One detection system (Applied Biosystems, USA). The PCR mix (20 μL) contained 2 μL DNA templates (100 ng/μL), 10 μL SYBR Premix Ex Taq, 0.4 μL 50 × ROX, 0.4 μL primer F, 0.4 μL primer R and 6.8 μL tri-distilled water. The thermal cycling program consisted of an initial denaturation step at 95℃ for 20 s and 40 cycles of 95℃ for 20 s, 60℃ for 40 s and 72℃ for 50 s. Melting curve analysis was performed at 95℃ for 15 s, 75℃ for 1 min and 95℃ for 15 s to assess the specificities of the amplifications.
Results
The average count of total LAB of the three types of dairy food samples collected from Baotou and Bayannur are presented in Table 1. The LAB viable counts of these sixty-six samples ranged from 6.74 to 9.15 Log CFU/mL. As can be seen from Table 1, the average count of LAB from urum in Bayannur was 8.7 Log CFU/mL, which was slightly higher than that of Baotou (8.01 Log CFU/mL). No significant difference was shown between the LAB counts of fermented cow milk and huruud collected from the two sampling cities. Urum and huruud collected from Baotou showed a lower average LAB count than fermented cow milk. In contrast, urum from Bayannur had the highest detectable LAB compared to huruud and fermented cow milk.
After isolation and purification, we obtained two hundred and thirty-seven Gram-positive and catalase-negative isolates, which were presumptively identified as LAB. Subsequently, 60% of the isolates were rod-shaped (67 and 75 isolates cultivated from MRS and M17, respectively), while the remaining ones were cocci (70 and 25 isolates cultivated from MRS and M17, respectively).
To precisely confirm the identity of these isolates at species level, the sequence of the 16S rRNA gene (around 1,400 bp) was determined and searched with the NCBI BLAST program (http://www.ncbi.nlm.nih.gov) for their closest relatives/reference strains with an homology of over or equal to 99%. Phylogenetic tree analysis (Fig. 1) was performed to reveal the relationship between the representative isolates and the known reference strains.
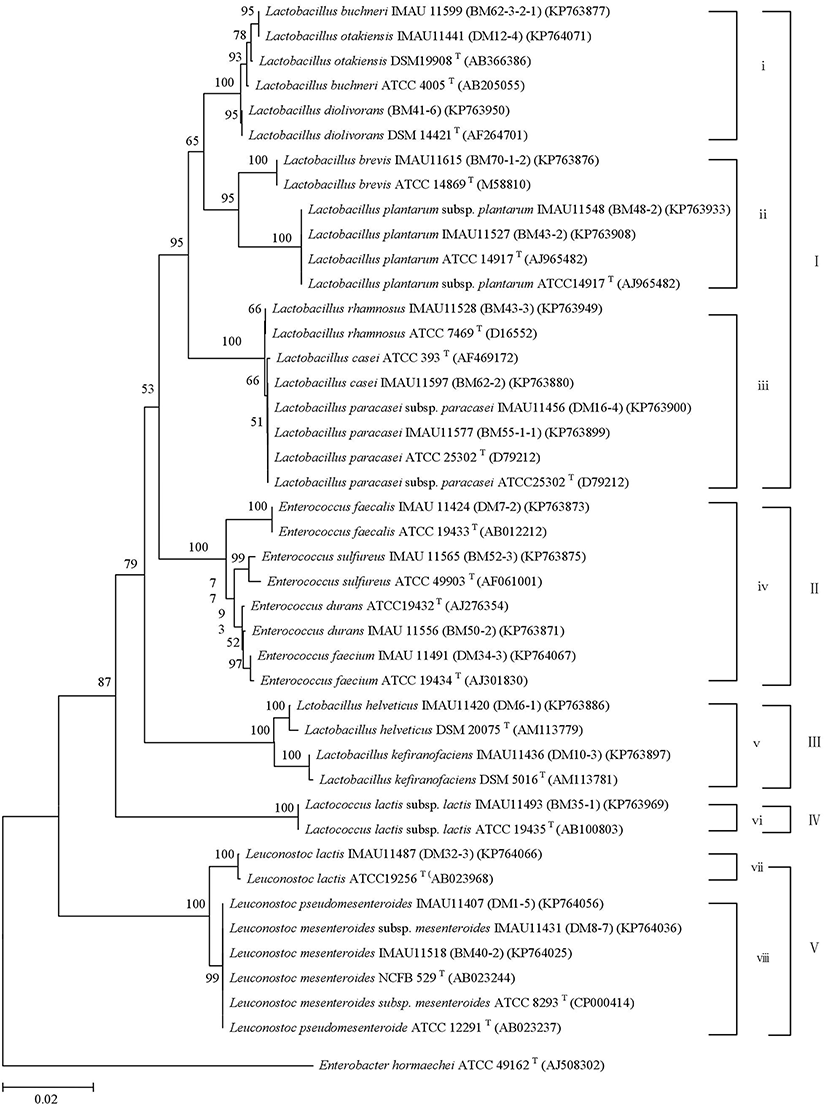
The phylogenetic analysis categorized all isolates into five clusters (−) and eight sub-clusters (i-viii), including four genera and twenty-one species and subspecies (Fig. 1). Cluster and cluster were the Lactobacillus (Lb.) group, containing the sub-cluster i, ii, iii, v (Lb. buchneri, Lb. otakiensis, Lb. diolivorans, Lb. brevis, Lb. plantarum subsp. plantarum, Lb. plantarum, Lb. rhamnosus, Lb. casei, Lb. paracasei subsp. paracasei, Lb. paracasei, Lb. helveticus, Lb. kefiranofaciens). Cluster was the Enterococcus (E.) group including E. faecalis, E. sulfureus, E. durans, E. faecium. Cluster was the Lactococcus (Lac.) lactis subsp. lactis group. Cluster was the Leuconostoc (Leu.) containing sub-cluster vii, viii (Leu. lactis, Leu. pseudomesenteroides, Leu. mesenteroides subsp. mesenteroides, Leu. mesenteroides).
These 202 LAB isolates belonged to 21 species and subspecies (Table 3). The obtained nucleotide sequences were deposited in GenBank and assigned the following accession numbers: KP63871 to KP64073.
E., Enterococcus; Lb., Lactobacillus; Lac., Lactococcus; Leu., Leuconostoc. n: the number of samples. -: not detected.
Lac. lactis subsp. lactis was the largest taxonomic group, consisting 65 strains (32% of the total isolates). The second most frequent species was Lb. plantarum, comprising 12.3% of all isolates, followed by Leu. mesenteroides accounting for 11.33% of the total isolates. The amounts of LAB in fermented cow milk from Baotou and Bayannur accounted for 28.7% and 44% of all isolates, respectively (Table 3). And the number of LAB in urum from Baotou and Bayannur accounted for 5.45% and 11.39% of all isolates, respectively, which showed that the same type of fermented dairy food produced in different regions had variable microbial diversity and composition. Nevertheless, the amount of LAB in huruud from these two regions showed no significant difference.
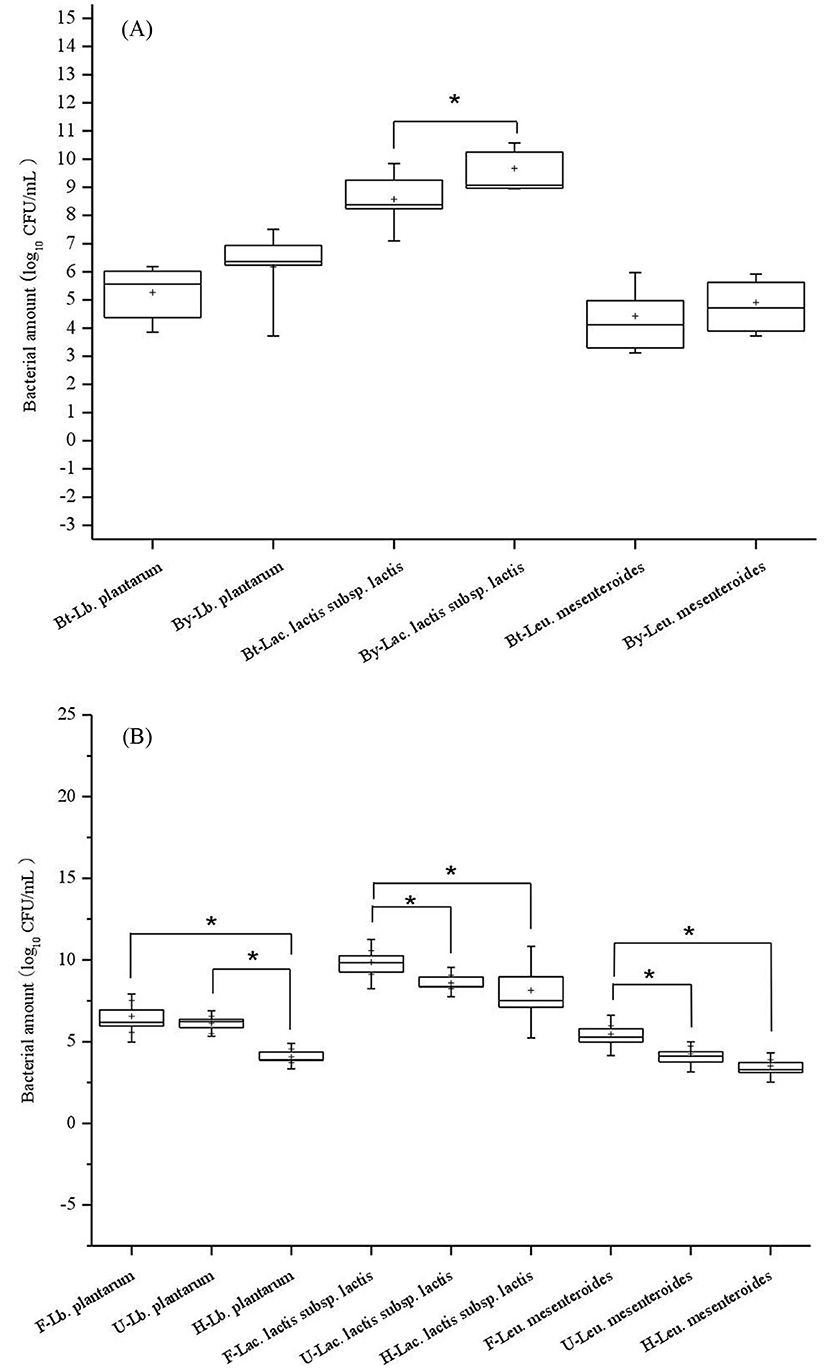
The average quantities of Lb. plantarum, Lac. lactis subsp. lactis, Leu. mesenteroides of samples from Baotou were 5.26±0.9, 8.58±0.9, 4.43±1.01 (Log CFU/mL; mean±SD), respectively, whereas the quantities of samples from Bayannur were 6.17±1.32, 9.67±0.73, 4.92±0.86 (Log CFU/mL; mean±SD), accordingly. The bacterial amount of Lac. lactis subsp. lactis in Baotou samples was significantly lower (p<0.05) than that in Bayannur samples (Fig. 2A). The bacterial amount of Lb. plantarum in huruud was significantly lower (p<0.05) than that in fermented cow milk and urum (Fig. 2B). The quantities of Lac. lactis subsp. lactis was not significantly different between huruud and urum, however, that was significantly lower (p<0.05) than the quantities in fermented cow milk samples. The numbers of Leu. mesenteroides in fermented cow milk reached 5.47±0.41 (Log CFU/mL; mean±SD), which was significantly higher (p<0.05) than that in huruud and urum, representing 4.25±0.41and 3.51±0.36 (Log CFU/mL; mean±SD), respectively.
Discussion
The Mongolian ethnic group has developed and maintained their unique style fermented dairy products from one generation to the next. During the process of natural selection, good quality LAB strains have been reserved and handed down in these traditional fermented products. In recent years, more and more studies concerning the microbial composition and microorganism resources in traditional dairy products has been conducted. The conventional home-made dairy products such as fermented cow milk, huruud and urum play important roles in the Mongolian diet because of their nutritive value and economic value. However, the quality of these products is not homogenous and lack of quality standards. Thus, in order to preserve the dairy quality as well as to further expand the probiotic potential of these Mongolian style fermented products, it is of interest to study the microbial diversity of these products and to analyze isolated strains’ desirable properties.
The studied samples had a generally high LAB count (ranging from 6.74 to 9.15 CFU/mL) compared with some previous reports on other types of natural dairy foods (Liu et al. 2012; Watanabe et al. 2008). It may suggest that a high viability of LAB in the Mongolian styled products, which is an important requirement for further functional food development. Therefore, it may useful to further characterize the microbial diversity and composition of these conventional dairy products. A previous study (Watanabe et al., 2008) on the LAB diversity in 22 samples of airag and 31 samples of tarag of Mongolia showed that the identification of 367 isolates of LAB classified into 6 genera (including Enterococcus, Lactobacillus, Lactococcus, Leuconostoc, Pediococcus and Streptococcus) and 19 species and subspecies by phylogenetic analysis based on the 16S rRNA gene sequences. Dewan and Tamang (2007) conducted a research on 58 samples of Himalayan ethnic fermented milk products and a total of 128 isolates of LAB were isolated and classified into 3 genera and 10 species and subspecies.
In this study, we revealed the LAB composition of conventional Mongolian dairy products by traditional culture method as well as 16S rRNA gene sequence-phylogenetic analysis and the results indicate the obvious difference in species diversity compared with previous studies (Dewan and Tamang 2007; Watanabe et al. 2008). These compositional differences are likely due to the types of dairy source, the process of dairy food production, sampling sites, environmental factors etc. A previous study (Zamfir et al., 2006) on the LAB diversity in sour cream of Romanian found that the predominant species were Lac. lactis subsp. lactis, Leu. mesenteroides, E. durans, E. faecium. Torres-Llanez et al. (2006) conducted a research on artisanal Mexican Fresco cheese, they concluded that the predominant LAB were Lac. lactis subsp. lactis, E. faecium and Lb. casei. Bulut et al. (2005) carried out a research on the traditional Comek peynr cheese from the Cappadocia region and reported E. faecium, Lb. paracasei subsp. paracasei as the prevalent bacteria.
In our study, the predominant LAB cultivated with MRS and M17 culture medium of fermented cow milk, huruud, and urum from midwestern Inner Mongolia were Lac. lactis subsp. lactis (65 isolates), Lb. plantarum (25 isolates), Leu. mesenteroides (23 isolates). All these results suggest that the genera of LAB species was finite, however, the species distribution and the quantity was various in dairy production. Also, the microbial composition is related to the fermentation time. Sakai et al. (2014) conducted a research on detecting the microbial community composition during production of Takanazuke and they found that the species, amount and the proportion of microflora changed during the fermentation process.
To obtain more accurate quantitative information of the dominant LAB, we utilized q-PCR method that is economical, easy to perform and provides more accurate quantitative data. The results indicate that the quantity of the same species varied greatly between sample types. This may suggest that the microbial difference was related to the unique household methods as well as the variation of the intrinsic starter composition. Even though no considerable difference was observed in the quantity of Lb. plantarum and Leu. mesenteroides in samples collected from both regions, significant variation was observed in the quantity of Lac. lactis subsp. lactis, with a much higher quantity in the samples from Bayannur compared to Baotou (Fig. 2A). Similarly, Yu et al. (2015) conducted a research on fermented dairy products of Russia by q-PCR analysis and found that the amounts of Lb. plantarum, Lb. helveticus, Lb. acidophilus were not significantly different, while the amounts of other species, namely Lb. delbrueckiii subsp. bulgaricus, Lb. fermentum, Lb. paracasei, were significantly different among three Russian cities. Although the sampling sites chosen in this study might geographically close, it already contribute to the difference in the microbial composition between similar sample types.
Conclusion
In this study, traditional culture method and 16S rRNA gene analysis as well as q-PCR were applied to analyze the diversity and composition of traditional dairy products (including fermented cow milk, huruud and urum) from Baotou and Bayannur, midwest of Inner Mongolia. A total of two hundred and two LAB isolates were identified and classified into twenty-one species and subspecies. Lactobacillus plantarum, Lactococcus lactis subsp. lactis, Leuconostoc mesenteroides were considered as the predominated LAB species among these sixty-six samples under the condition of cultivating in MRS and M17 culture medium. Q-PCR were performed to quantify the dominant LAB and the result revealed that the number of predominant species varied from samples to samples and from region to region. This research contributes to an understanding of the composition and diversity of LAB in traditional dairy products of midwestern Inner Mongolia, which could provide some raw data and strain resources for further study involved in probiotics strain selection and starter culture design.













