Introduction
The genus Prunus L. belongs to the subfamily Amygdaloideae of the family Rosaceae and consists of shrubs or trees distributed mainly in temperate regions of the Northern Hemisphere, as well as tropical regions of Asia and the Americas. Globally, approximately 200 species have been identified (Kalkman, 2004). Among the 22 species of cherry trees in Korea, 4, including Prunus yedoensis Matsumura, Prunus sargentii Rehder, Prunus spachiana f. ascendens (Makino) Kitam, and Prunus verecunda (Koidz.) Koehne, have been historically recognized as valuable ornamental trees, with the Japanese flowering cherry tree being the most cultivated species (Jang, 2008; Kim et al., 1998). Among these, P. yedoensis, commonly known as the Yoshina cherry or Japanese flowering cherry, is the most commonly planted tree along streets and is easily seen in everyday life (Kim et al., 2019b). The Japanese flowering cherry is known for its beautiful flowers and can reach a height of 15 meters with a trunk diameter of 50 centimeters. It thrives in well-drained, fertile soil and typically flowers in April with white or light pink blossoms before leaving out. The berry of the cherry tree, known as “beotji”, is approximately 1 cm in diameter, black in color, and matures between May and June (Kim et al., 2019a). Beotchi is used as an herbal medicine for various purposes in both traditional and modern medicine in Korea. It is known to be effective for pain relief, cough, heart diseases, mammary tumors, edema, toothache, leukorrhea, wind disorders, regulating menstruation, promoting bowel movements and fatigue recovery (Kim et al., 1998). It is rich in sugars, organic acids, and pigments, primarily anthocyanins, with cyanidin-5 being the main component of the pigments. Nutritional analysis has shown that beotchi contains water, protein, fat, carbohydrates, minerals (especially potassium), amino acids (such as aspartic acid and glutamic acid), organic acids, sugars, and bioactive compounds like anthocyanins and polyphenols (Lee et al., 2009).
Lactic acid bacteria (LAB) are members of a heterogeneous group of bacteria that play an important role in various fermentation processes, notably playing a central role in the creation of yogurt (Bintsis, 2018). LAB is characterized as gram-positive, non-spore-forming cocci or rods, catalase-negative, and fastidious organisms with resilience to low pH (van Geel-Schutten et al., 1998). Further, LAB is Generally Recognized as Safe (GRAS) by the U.S. Food and Drug Administration (FDA) and have received the “Qualified Presumption of Safety” status by the European Food Safety Authority (EFSA; Plavec and Berlec, 2020; Rajoka et al., 2017). Lacticaseibacillus (previous name, Lactobacillus), is a major LAB in the Lactobacillaceae family and includes various facultative anaerobic species of rod-shaped gram-positive bacteria with a width of 2.0 to 4.0 μm and length of 0.8 to 1.0 μm; members of this versatile genus are widely utilized in probiotic food production and are primarily found in fermented dairy products like milk, yogurt, bio-yogurt, kefir, and cheese (Collins et al., 1989; Fijan, 2014; Pinto et al., 2020; Tannock, 2004). Now, the applications of these bacteria have been expanded to include non-dairy fermented probiotic products, such as soy milk, fruits, vegetables, vegetable juices, meat, and bread (Nguyen et al., 2019; Pinto et al., 2020; Swain et al., 2014).
Studying the characteristics of LAB is essential for developing new and industrial cultures. Kılıç et al. (2022) highlighted the notable lack of research on isolating and identifying LAB from specific natural environments, emphasizing the need to address this gap for future advancements. While P. yedoensis has been studied before, its associated bacteria have not been explored. Accordingly, the present study aimed to isolate and identify novel LAB strains from the natural habitat of P. yedoensis, and to evaluate their bioprotective properties, including antibacterial activity and probiotic potential, thereby contributing to the discovery and potential application of beneficial microorganisms from natural sources.
Materials and Methods
Fresh P. yedeonsis cherries were collected from street trees in Anseong city, south Korea. After anaerobic fermentation for 6 wk in an incubator at 37°C, these cherries were homogenized by adding sterilized distilled water and subjected to LAB isolation by plating the homogeneous cherry liquid on De Man Rogosa-Sharpe (MRS) agar (Difco, Franklin Lakes, NJ, USA) supplemented with 0.01% sodium azide (Sigma-Aldrich, St. Louis, MO, USA). The plated MRS agar was incubated anaerobically or aerobically at 37°C until colony formation. Colonies thus formed were selected for the morphological identification of LAB based on shape and size via Gram staining and microscopic observation. The selected LAB was examined for sugar utilization using the API 50CHL kit (BioMérieux, Marcy-l'Étoile, France). Specifically, a CHL medium inoculated with a single colony was dispensed into each capsule at 100 μL per well and incubated at 37°C for 48 h to observe color changes. The fermentation patterns of each sugar were then entered into the API web software (https://apiweb.biomerieux.com/) to confirm the identification results. Furthermore, the selected LAB was identified by a consignment company (Solgent, Daejeon, Korea).
The isolated strain of Lacticaseibacillus paracasei was cultured in MRS broth (Difco) under aerobic conditions at 37°C for 24 h. Following incubation, the culture suspension was centrifuged at 4°C at 1,690×g for 20 min to separate the bacterial cells from the supernatant. The supernatant was carefully collected, and its pH was measured. To obtain a sterile cell-free supernatant (CFS), the collected supernatant was filtered through a 0.22 μm syringe filter (Advantec, Tokyo, Japan). The CFS was then stored at –20°C for use in subsequent experiments. The bacterial cells were resuspended in 30% glycerol (v/v) and stored at −80°C for future experiments. The probiotic strains, Streptococcus thermophilus KCCM 40430 and Lactobacillus bulgaricus KCCM 35463 were obtained from the Korean Culture Center of Microorganisms and used in this study. Pathogenic strains Escherichia coli KCCM 11569, E. coli KCCM 11587, E. coli KCCM 11591, E. coli KCCM 11596, and E. coli KCCM 11600 were purchased from the Korea Microbial Conservation Center (KCCM). Salmonella Enteritidis NCCP 11702, Salmonella Thompson NCCP 11704, and Salmonella Typhimurium NCCP 11732 were purchased from the National Pathogen Resource Bank (NPRB) and used in the experiment. Prior to use in experiments Escherichia and Salmonella were cultured in Luria-Bertani (LB) broth (Difco) and incubated overnight at 37°C.
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay was used to measure the antioxidant activity. To calculate the DPPH radical scavenging ability, freshly prepared 0.2 mM 2,2-diphenyl-1-carboxylic acid in ethanol was mixed with CFS at a 1:1 ratio and allowed to react for 30 min in a dark room and the absorbance of this mixture was measured at 490 nm using the Epoch Microplate Spectrophotometer (Biotech, USA). The mixture of MRS broth and ethanol (1:1 ratio) was used as control and the mixture of CFS and ethanol (1:1 ratio) was used as blank. Percentage inhibition (%) of DPPH scavenging activity was calculated according to the following formula:
Hemolytic activity of the isolated L. paracasei was assessed in accordance with the method proposed by Lkhagvasuren et al. (2023). Toward this, L. paracasei pre-cultured in MRS broth for 24 h were plated on Blood Agar Base (Kisan Biotech, Seoul, Korea) containing 5% (w/v) defibrinated whole sheep blood (Kisan Biotech) and incubated at 37°C for 48 h.
The acid resistance of the isolates was evaluated using the method proposed by Oh and Jung (2015) with slight modifications. After culturing L. paracasei for 12 h, the pH was adjusted to 2.0 using 1 N HCl. After a 2 h incubation at 37°C, viable cells that survived the low pH were counted on MRS agar using the plate counting method. The survival rate (%) was calculated according to the following formula:
The antibacterial activity of P. yedoensis-derived L. paracasei CFS against eight pathogenic bacteria was confirmed using the diffusion method involving the use of paper discs. First, 1% of each pathogenic bacterium, including Escherichia coli (E. coli KCCM 11569, E. coli KCCM 11587, E. coli KCCM 11591, E. coli KCCM 11596, and E. coli KCCM 11600), and Salmonella (S. Enteritidis NCCP 11702, S. Thompson NCCP 11704, and S. Typhimurium NCCP 11732) was inoculated into individual 20 mL aliquots of Luria Bertani (LB) agar. For each pathogenic bacterium, the concentration of CFS obtained from P. yedoensis-derived L. paracasei was adjusted to 100%, 50%, 25%, and 12.5% (v/v), following which, it was absorbed onto sterile paper discs (Advantec), placed on the bacteria-inoculated LB agar plates, and incubated at 37°C for 3 h to measure the diameter of the inhibition zone, which is a readout of antibacterial activity.
The antibacterial activity of P. yedoensis-derived L. paracasei CFS against eight pathogenic bacteria at various concentrations was measured using the 96-well plate method. The prepared supernatant was added at concentrations of 20%, 10%, 5%, and 2.5% (v/v). After pre-culturing, 1% of each pathogenic bacterium (Escherichia coli and Salmonella) was inoculated. The growth of each pathogenic bacterium was monitored by measuring the optical density of the resultant culture at 655 nm using a microplate reader (Bio-Rad, Hercules, CA, USA) at 3 h intervals.
In the pH stability test, the antibacterial activity of the CFS obtained from P. yedoensis-derived LAB against pathogenic strains in the face of pH changes was measured by adjusting its pH using 1 N NaOH and then incubating 5% of the pH-adjusted CFS with the pathogenic bacteria. In the thermal stability test, individual pathogenic bacteria were independently exposed to various heat-treated CFS (65°C for 30 min; 75°C for 15 min; 85°C for 10 min; 100°C for 5 min) and the antibacterial activity of these CFS was measured. The growth of each pathogenic bacterium was monitored by measuring the absorbance of the cultures at 655 nm at 6 h intervals during culture at 37°C for 24 h.
Results and Discussion
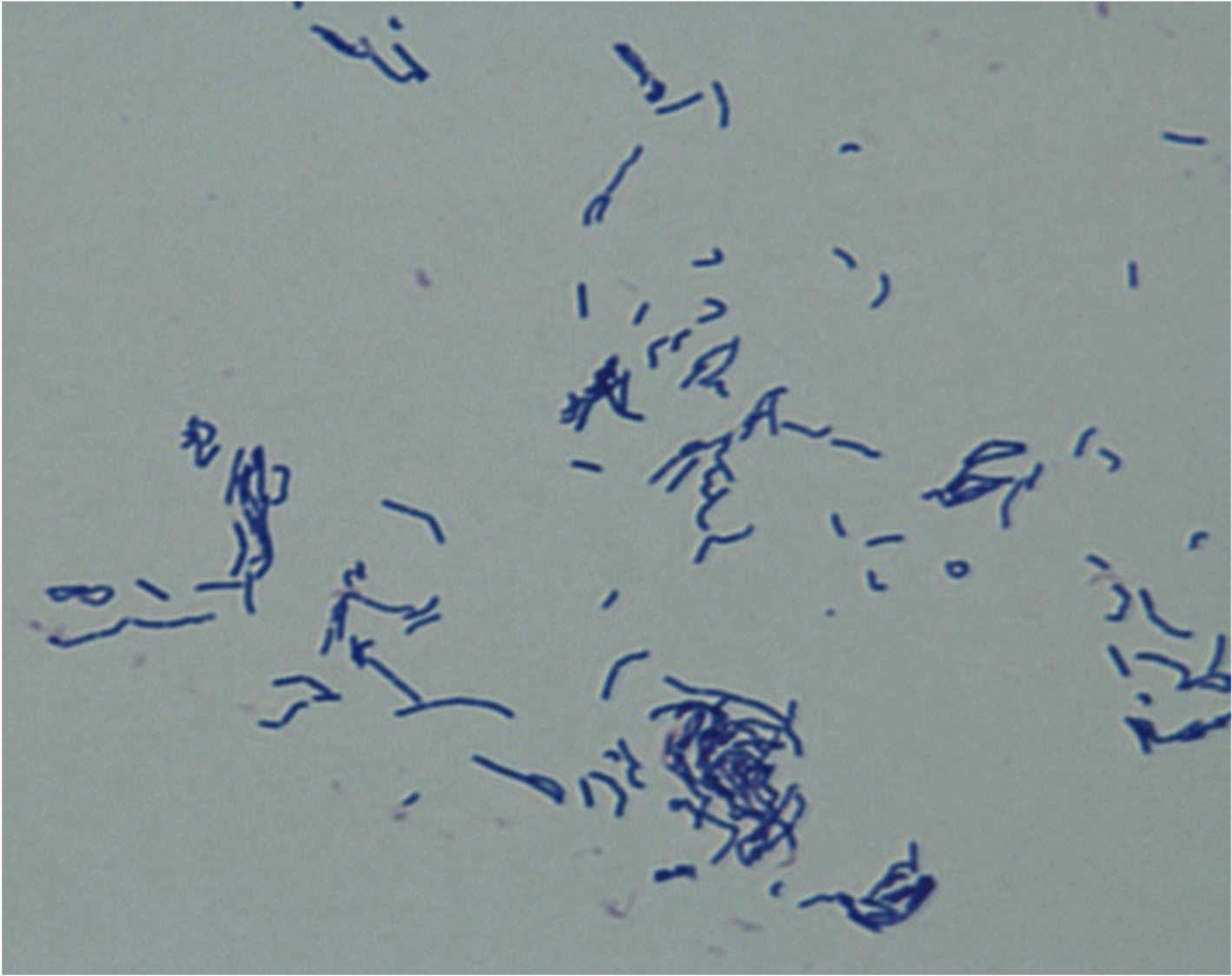
After 24–48 h of culturing, one strain was selected based on the formation of wide and white colonies on MRS agar. This strain was identified as Lactobacillus spp. The colony morphology characterization results confirmed that the bacteria were gram-positive and rod-shaped (Fig. 1). The analysis of sugar utilization of the selected LAB using the API 50CHL kit showed 99.5% homology with L. paracasei (Table 1). Furthermore, the analysis of the 16S rDNA nucleotide sequence and homology search confirmed 99.9% homology with L. paracasei R094 as shown in Fig. 2.
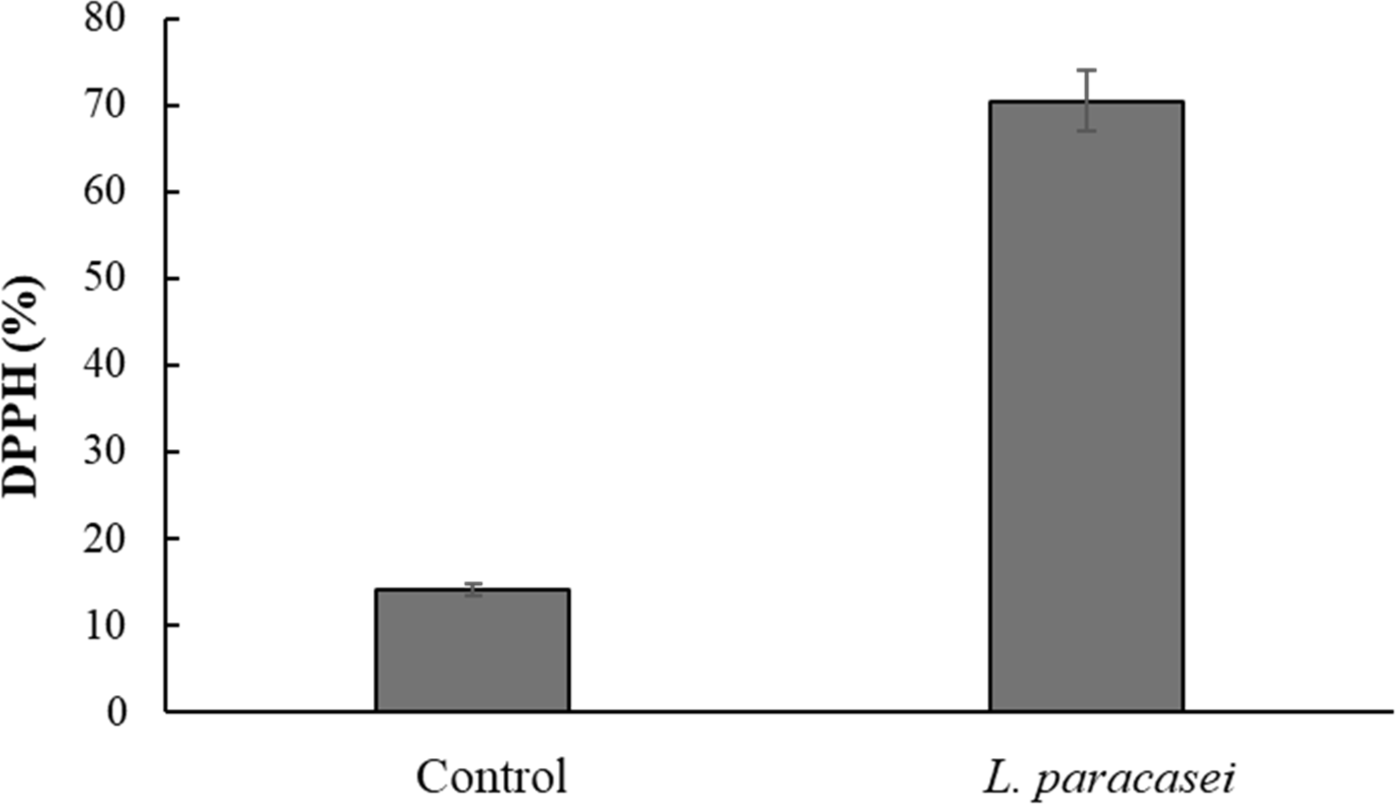
In the DPPH free radical scavenging assay, the CFS of the isolated L. paracasei demonstrated a strong DPPH scavenging ability of 70.40%, which was significantly (p<0.05) higher than that of the control MRS broth (Fig. 3). This value is comparable to the antioxidant activity observed in Bifidobacterium animalis 01 (73.11%) reported by Shen et al. (2011) and Lactobacillus plantarum CCFM-JU 8661 (75.94%) reported by Xing et al. (2015). These findings highlight the robust antioxidant potential of L. paracasei in scavenging free radicals, consistent with the findings of previous studies on probiotic strains.
Hemolytic activity was measured to evaluate the safety of the isolated L. paracasei, which was then compared with that of S.thermophilus KCCM 40430 and L. bulgaricus KCCM 35463 (control strains). All of the control LAB strains and isolated L. paracasei were determined to be γ-hemolytic, showing no hemolysis in agar medium containing sheep blood (Fig. 4). The hemolytic activity of probiotics is classified into the following three types: α-hemolysis, which involves incomplete lysis of red blood cells and produces a green ring around the colony; β-hemolysis, which involves the complete lysis of red blood cells and produces a white or yellow circle; and γ-hemolysis, in which there is an absence of hemolytic activity and ring.

These results are consistent with the studies of Riebel and Washington (1990), indicating that L. paracasei is non-hemolytic and safe for food fermentation.
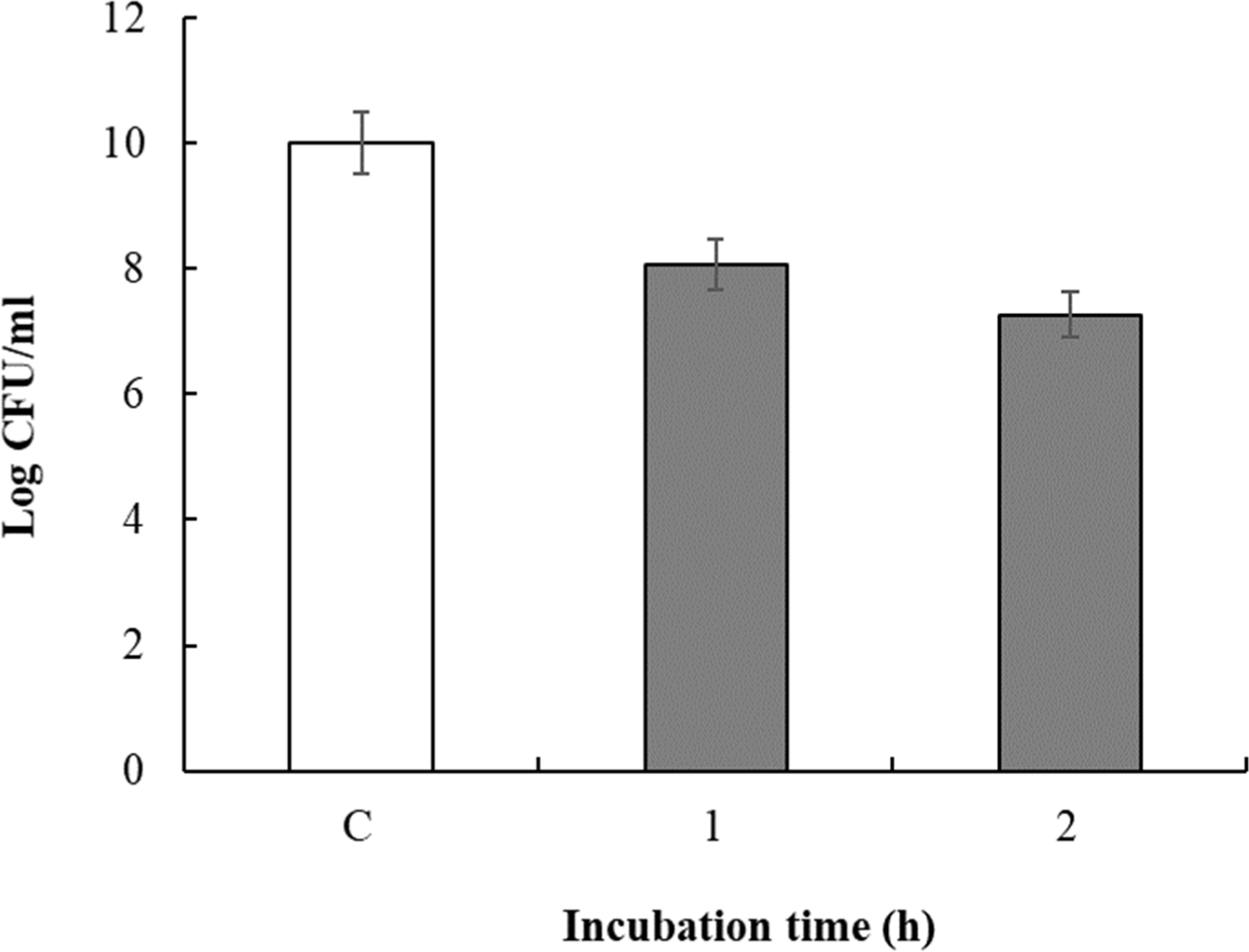
To measure survival under acidic conditions, the pH of the culture fluid was adjusted to 2.0 with 1 N HCl and the culture was incubated for 1 or 2 h (acid exposure). Viable cell counts in the culture fluid over various intervals of pH exposure are shown in Fig. 5. The viable cell count immediately after incubation of the isolated L. paracasei was 10.0 Log CFU/mL, which changed to 8.07 Log CFU/mL and 7.26 Log CFU/mL after 1 h and 2 h exposure to pH 2.0, respectively. Therefore, isolated L. paracasei showed 72.63% survival under acidic conditions. In this study, intrinsic resistance to low pH was rare among the commercial probiotic cultures examined. All commercial strains lost viability after a 2 h to pH 2.0 (data not shown), consistent with the findings of Pereira and Gibson (2002). However, our isolated L. paracasei demonstrated resilience under similar pH conditions (which were comparable to the pH conditions prevalent in the gastric juice, i.e., pH 1.5–3.5; Freeman and Kim, 1978), suggesting good survival in the stomach. Additionally, as the stomach pH increases beyond 3.0 after food intake (Martini et al., 1987), our isolated L. paracasei may have enhanced survival, supporting its potential as an effective probiotic, especially as LAB must withstand acidic conditions and bile to be effective (McDonald et al., 1990; Ouwehand et al., 1999).
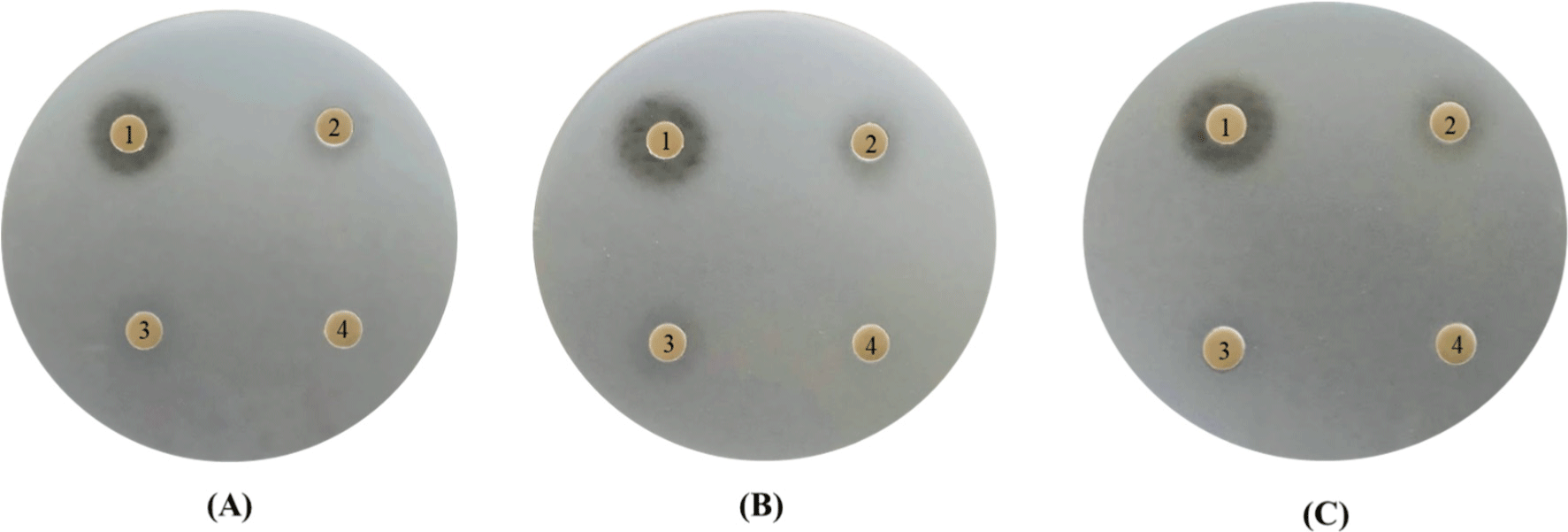
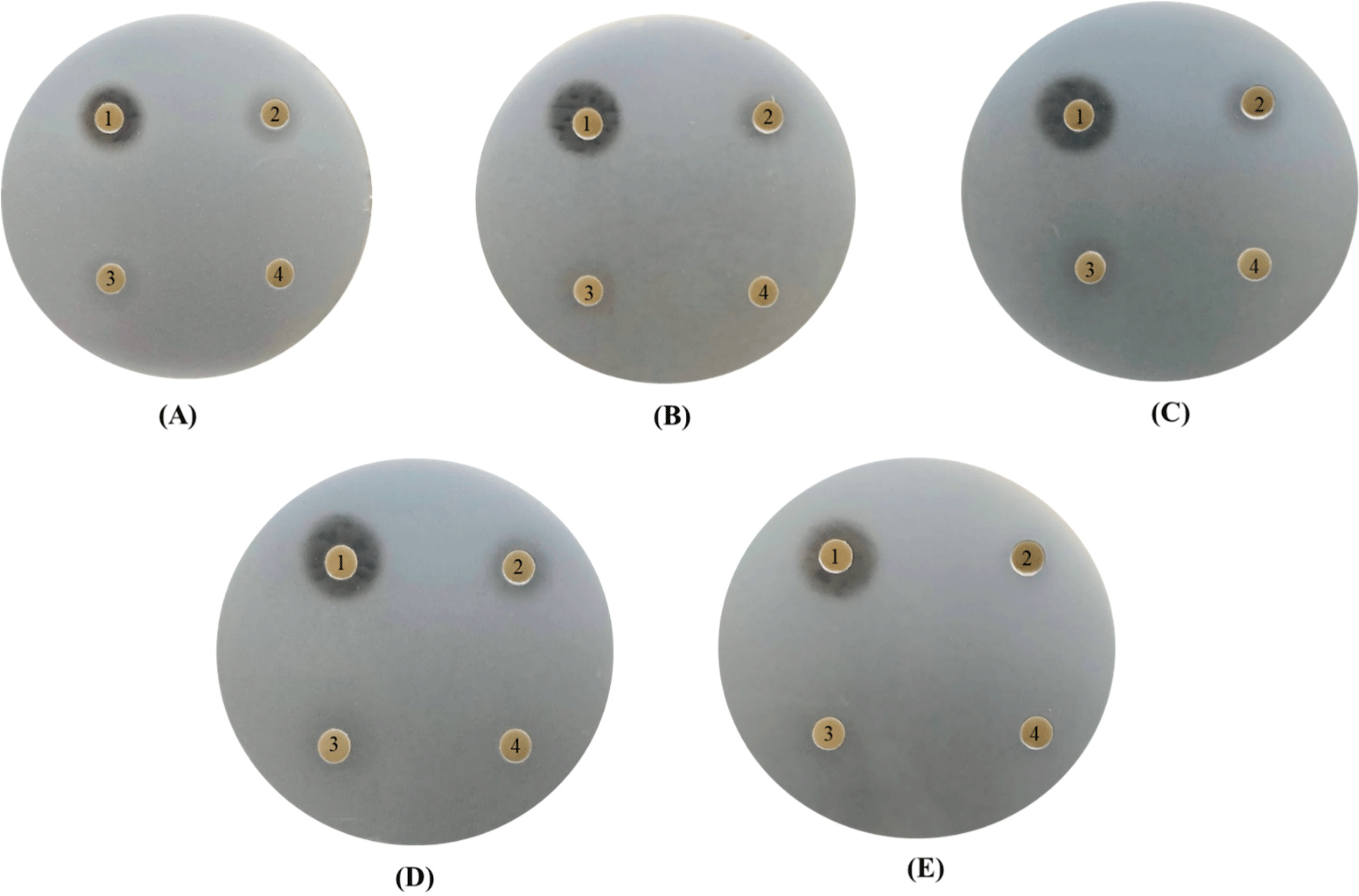
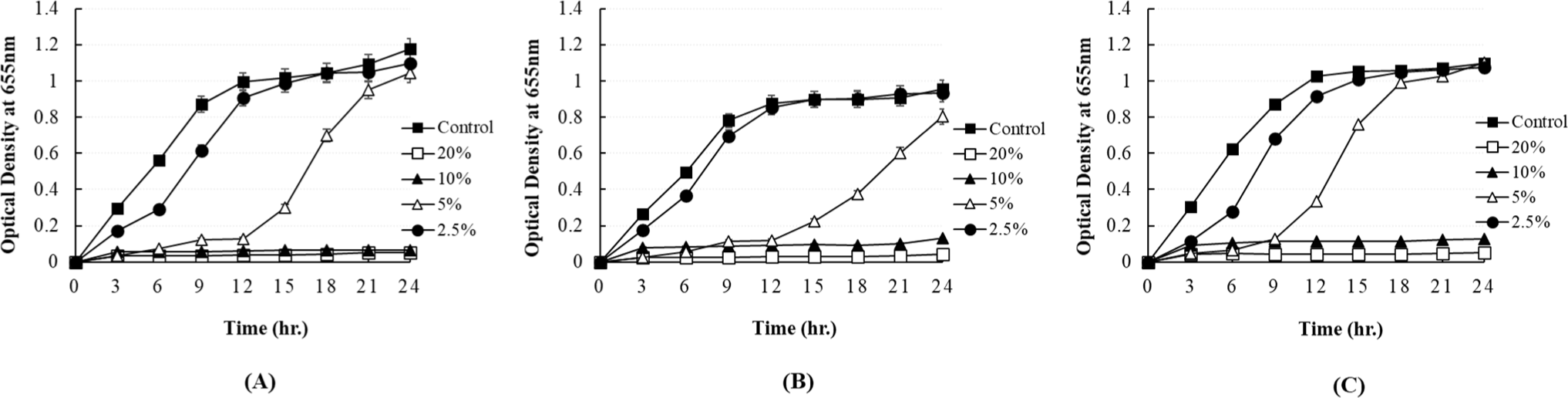
In our study, the antibacterial activity of the P. yedoensis-derived L. paracasei CFS was assessed using multiple methods. The paper disc diffusion method revealed significant antibacterial activity against both Salmonella strains (Fig. 6) and E. coli strains (Fig. 7), with clear inhibition zones observed at higher concentrations (100% and 50% v/v). These findings indicate that the CFS of L. paracasei isolated from P. yedoensis effectively inhibits the growth of both Salmonella and E. coli. Further validation using the 96-well plate method confirmed these findings, as shown in Figs. 8 and 9. These figures demonstrate the antibacterial activity of the CFS against various strains of Salmonella and E. coli under different concentrations. The CFS from P. yedoensis-derived L. paracasei significantly inhibited the growth of S. Enteritidis NCCP 11702, S. Typhimurium NCCP 11732, and S. Thompson NCCP 11704 (20% and 10% CFS) from the beginning to the end of culture (Fig. 8). At 5% CFS, the growth of S. Enteritidis NCCP 11702 and S. Typhimurium NCCP 11732 was inhibited for up to 12 h of culture (Figs. 8A and B), whereas that of S. Thompson NCCP 11704 was inhibited for up to 9 h (Figs. 8A, B, and C). Moreover, at 2.5% CFS, slight growth inhibition was observed for S. Enteritidis NCCP 11702, S. Typhimurium NCCP 11732, and S. Thompson NCCP 11704 (Fig. 8). The P. yedoensis-derived L. paracasei CFS (20% and 10%) exhibited significant antibacterial activity against all five E. coli strains investigated, i.e., E. coli KCCM 11569, E. coli KCCM 11587, E. coli KCCM 11591, E. coli KCCM 11596, and E. coli 11600, from the beginning of the culture to 24 h (Fig. 9). At 5% CFS, the growth of E. coli KCCM 11587 was inhibited for up to 9 h and the antibacterial activity gradually decreased after 12 h of culture, whereas that of E. coli KCCM 11591, E. coli 11596, and E. coli 11600 was inhibited for up to 6 h; at 5% CFS, the growth of E. coli KCCM 11569 was inhibited for only 3 h (Fig. 9). Meanwhile, at 2.5% CFS, slight growth inhibition was observed for all E. coli strains (Fig. 9).
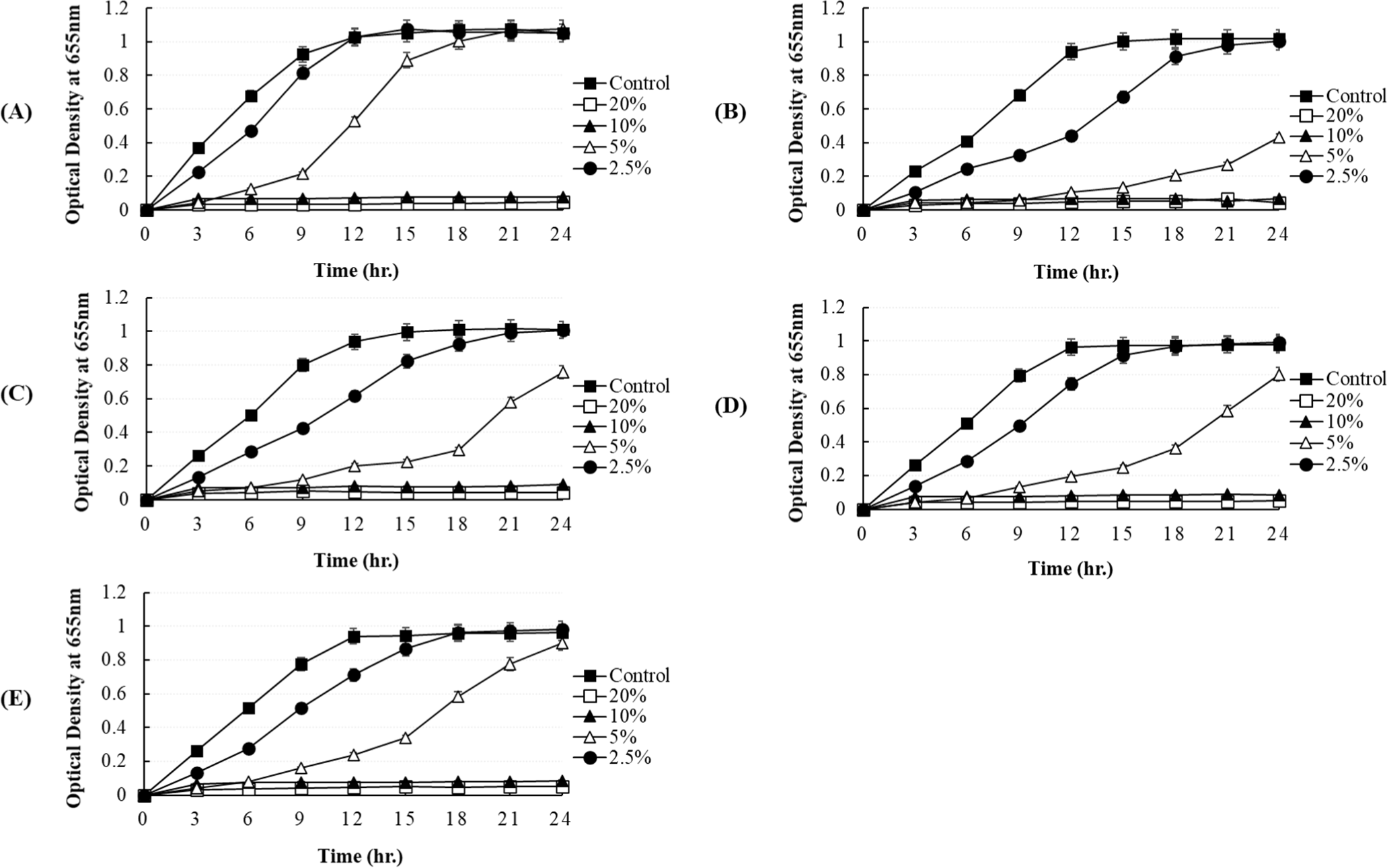
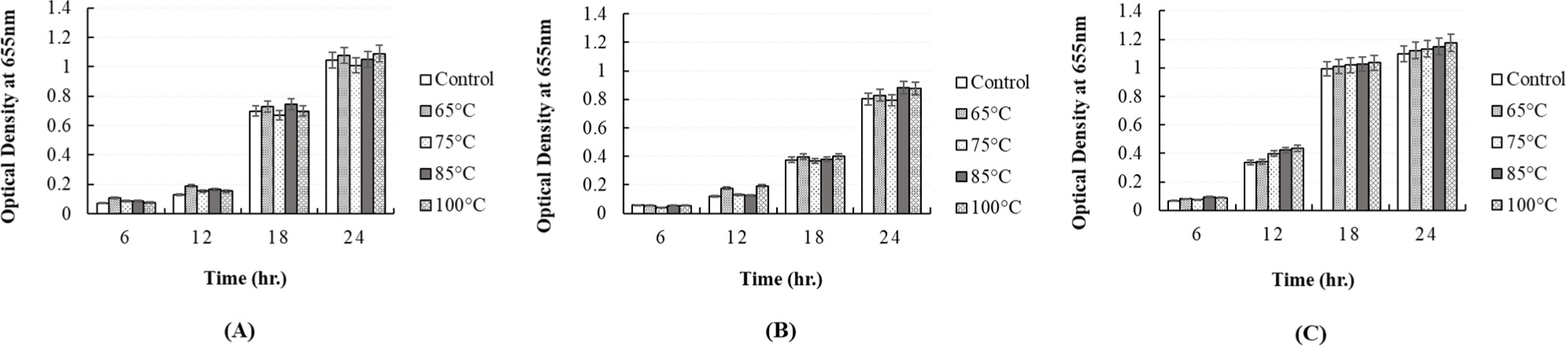
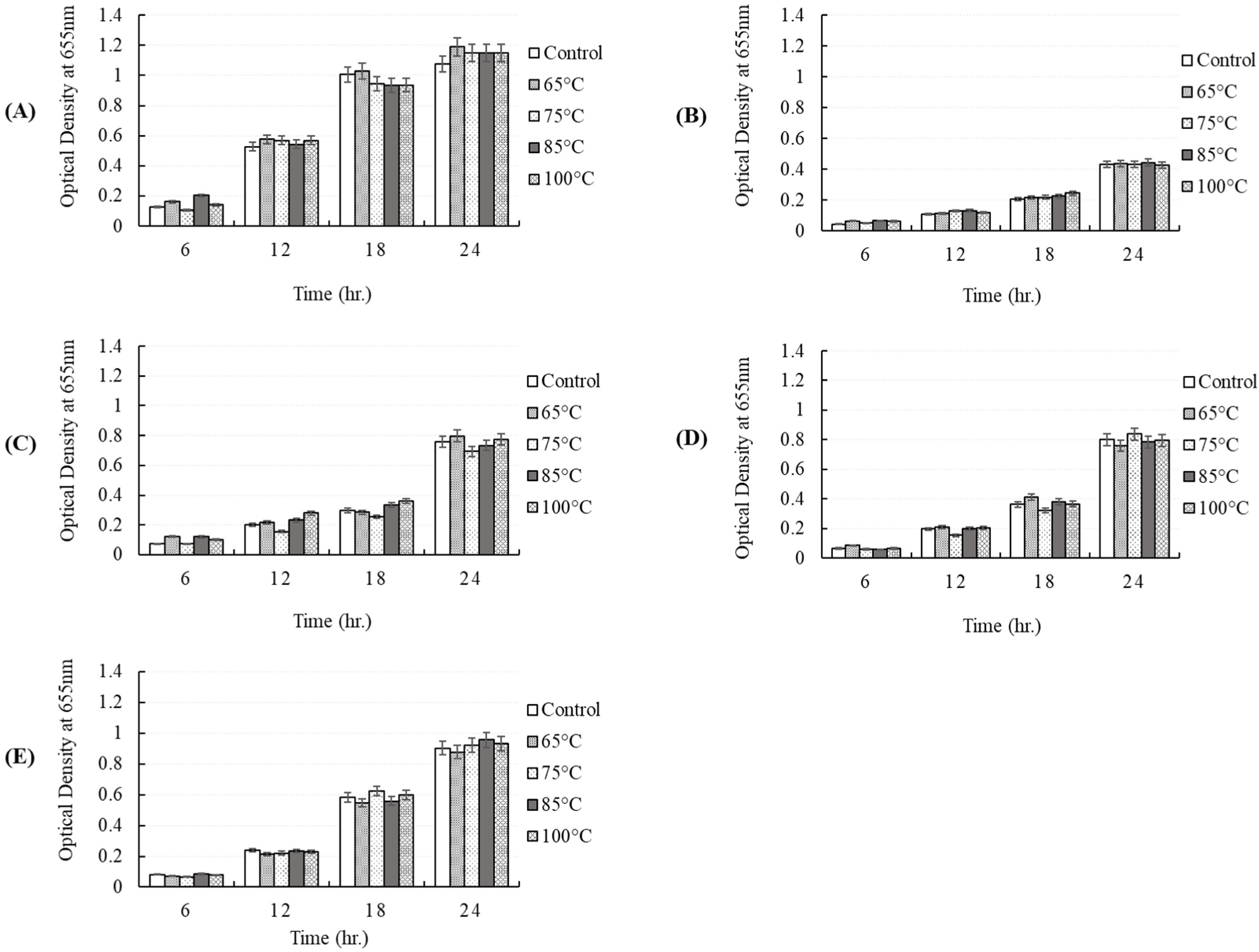
To determine whether the antibacterial activity of the CFS obtained from P. yedoensis-derived L. paracasei is thermostable, the CFS was independently incubated at 65°C for 30 min, 75°C for 15 min, 85°C for 10 min, and 100°C for 5 min. After heat treatment, the antibacterial activity of the CFS against pathogenic strains was measured by adding 5% CFS to individual bacterial cultures. When 5% heat-treated CFS individually was added to S. Enteritidis NCCP 11702, S. Thompson NCCP 11704, and S. Typhimurium NCCP 11732, the growth of these cultures was found to be similar that observed in the control group (CFS without heat treatment), i.e., no reduction in antibacterial activity (Fig. 10). A similar result was obtained when 5% heat-treated CFS was individually added to E. coli KCCM 11587, E. coli KCCM 11591, E. coli KCCM 11596, and E.coli KCCM 11600 and cultured for 24 h (Fig. 11). Thus, the CFS remained heat-stable, maintaining its antibacterial properties after heat treatment. Therefore, the antibacterial components present in L. paracasei-derived CFS were shown to be heat-stable.
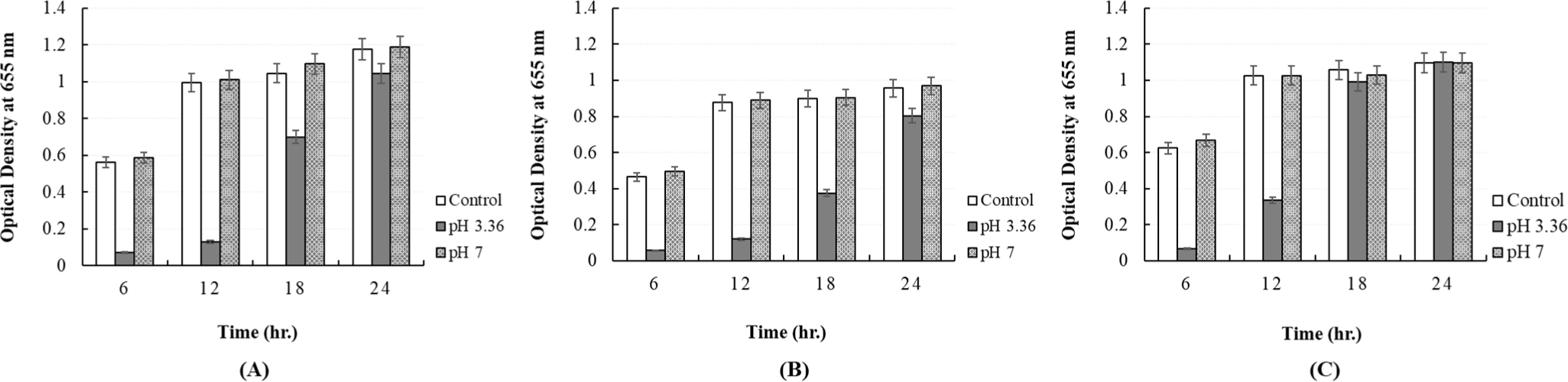
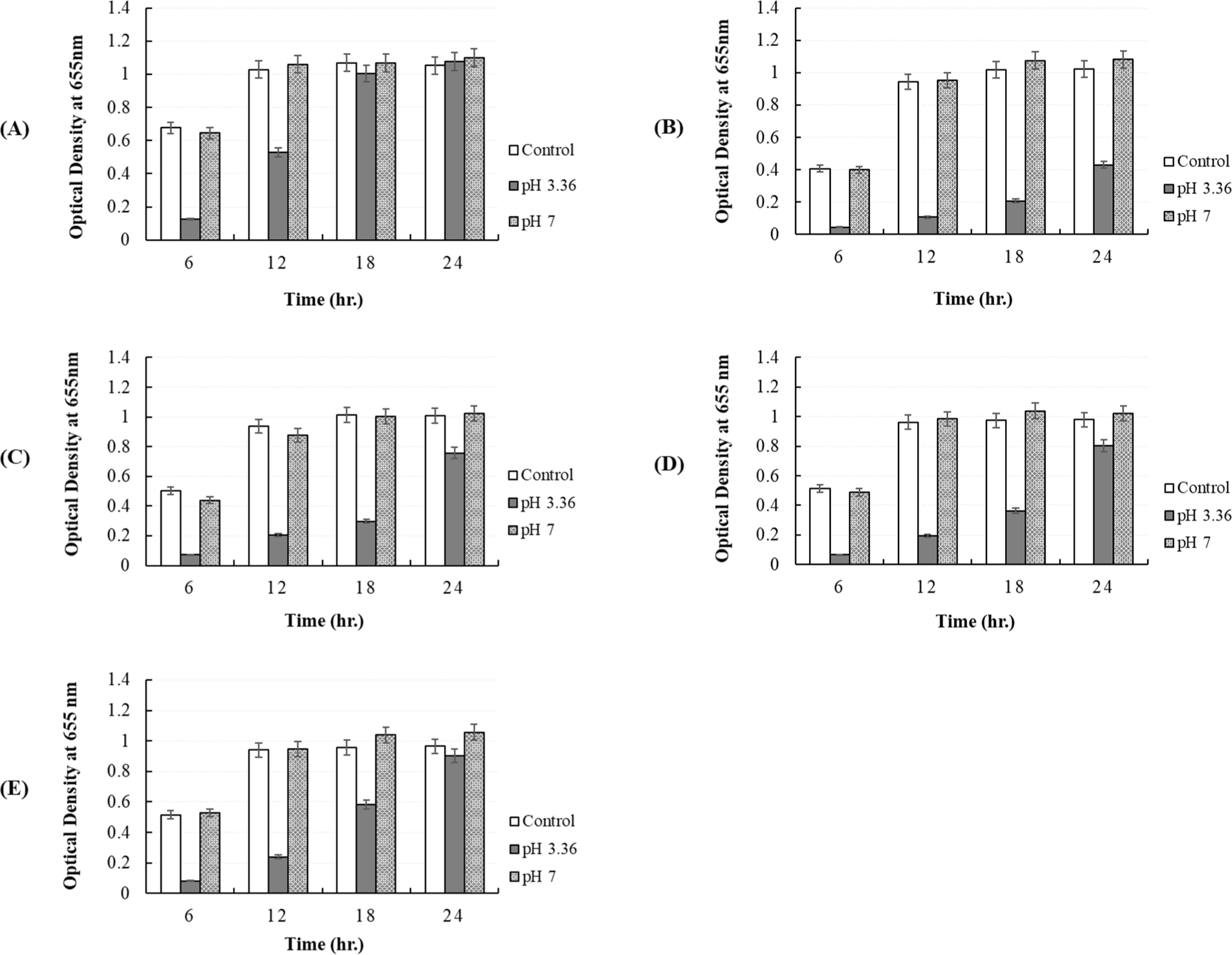
The pH of the CFS obtained from P. yedoensis-derived L. paracasei was found to be 3.36. To determine whether the antibacterial activity of the CFS was due to organic acids or bacteriocins, its pH was adjusted to 7.0 using 1 N NaOH. Exposure of S. Enteritidis NCCP 11702, S. Thompson NCCP 11704, and S. Typhimurium NCCP 11732 to pH-adjusted CFS (pH 7) did not result in growth inhibition, i.e., no antibacterial activity (Fig. 12). In contrast, exposure of these Salmonella spp. to regular CFS (pH unadjusted) resulted in substantial growth inhibition, i.e., significant antibacterial activity, suggesting that organic acids were the primary inhibitory factor. As in case of Salmonella spp., exposure of E. coli KCCM 11569, E. coli KCCM 11587, E. coli KCCM 11591, E. coli KCCM 11596, and E. coli KCCM 11600 to pH-adjusted CFS (pH 7) did not result in growth inhibition, i.e., no antibacterial activity (Fig. 13).
Overall, our findings demonstrate the significant antibacterial activity of the P. yedoensis-derived L. paracasei CFS against pathogenic bacteria, particularly Salmonella and Escherichia spp. This observation aligns with the latest research highlighting the antibacterial properties of L. paracasei (Sornsenee et al., 2022; Xin et al., 2024) and other LAB against various pathogens (De Vuyst and Leroy, 2007; Hill et al., 2018). The concentration-dependent antibacterial effects observed in our study suggest the involvement of bioactive compounds present in the CFS. Interestingly, adjusting the pH of the CFS to neutral levels resulted in the loss of antibacterial activity against Salmonella and Escherichia strains, indicating the contribution of organic acids to this inhibitory effect. This finding corroborates existing literature on the antimicrobial activity of organic acids produced by LAB, which play a crucial role in food preservation and pathogen inhibition (Bintsis, 2018). According to Dunne et al. (2001) and Gupta et al. (2018), for a probiotic to be effective, it must be safe for human use and capable of withstanding acidic conditions. In this study, the L. paracasei isolate demonstrated significant antibacterial activity against pathogens such as Salmonella and E. coli spp., meeting key probiotic criteria. These findings suggest its potential use in yogurt production, emphasizing both its safety and efficacy as a candidate probiotic.
Conclusion
To the best of our knowledge, this is the first study to report the isolation of L. paracasei with probiotic abilities from P. yedoensis and evaluate its potential as a probiotic strain with notable antimicrobial and antioxidant properties and as a postbiotic and starter culture for fermented milk products. The strain exhibited significant dose-dependent antibacterial activity against Salmonella and E. coli spp.; this was primarily attributed to its ability to synthesize organic acids. Furthermore, the CFS showed remarkable thermal stability; however, its antibacterial activity was reduced after pH neutralization. The strain also demonstrated potent antioxidant activity and was confirmed to be non-hemolytic, ensuring its safety for potential food applications. These findings underscore the probiotic potential of L. paracasei, particularly for use in yogurt production and other functional food products, owing to its safety, acid tolerance, and effective antibacterial activity. Further research is warranted to explore its application in food preservation and human health.













