Introduction
Antibiotic-resistant bacteria are serious threat to global public health. The overuse of antibiotics accelerates the appearance of antibiotic-resistant strains (Zhang et al., 2015). Lactobacillales are generally recognized as safe (GRAS) (Yang et al., 2012). Because lactic acid is the main metabolite produced by these bacteria, they are called lactic acid bacteria (LAB) (De Vuyst and Leroy, 2007). LAB produces antibacterial peptides including bacteriocin and bacteriocin-like inhibitory substance (BLIS) (Bowdish et al., 2005; Cintas et al., 2001; Shokri et al., 2014). Bacteriocins produced by LAB are ribosomally synthesized antibacterial proteins or peptides that are effective against narrow or broad spectrum bacteria (Bowdish et al., 2005; Cotter et al., 2005; Yang et al., 2012). Among the LAB, enterococci are important due to high production of bacteriocin. Enterococci are gram positive, non-spore forming, catalase and oxidase negative, and aerotolerant bacteria (Rodriguez et al., 2012; Sun et al., 2014). Most of the bacteriocins are only active against Gram positive bacteria; this is a limitation for application of the bacteriocin as a biopreservative in the food industry and as substitute for chemical antibiotics in pharmaceutical industry (Abee et al., 1995; Acuna et al., 2012). Nisin A, nisin Z and pediocin PA-1 have broad antibacterial effect against Gram positive bacteria such as food-borne pathogens, but these bacteriocins are inactive or have a narrow spectrum antibacterial effect against Gram negative bacteria (Acuna et al., 2012; Settanni et al., 2005). This study reports isolation, screening and molecular identification of bacteriocin-producing enterococci from native dairy products of Kerman province with a broad antibacterial effect against Gram positive and Gram negative pathogens.
Materials and Methods
Milk, yogurt, and cheese samples were collected in sterile bottles from different regions of Kerman province in Iran and were immediately transported to the laboratory in an insulated ice box.
Five grams of each sample was homogenized and serially diluted six-fold in 10 ml of sterile saline phosphate buffer and were plated on de Man, Rogosa and Sharp (MRS) medium (Biolife, Italy). Plates were incubated for 48-72 h under anaerobic conditions at 37°C. Colonies were evaluated by phenotypic methods. Enterococci suspicious isolates were isolated according to growth on kanamycin aesculin azide agar (KAA) and 6.5% NaCl media. The culture media were centrifuged at 10,000 × g for 10 min at 4°C and then filtered through 0.22 μm filters to separate cell-free supernatants (CFS) (Saranya and Hemashnpagam, 2011). The supernatants were used for purification of bacteriocins. The supernatants were adjusted to pH 7.0 by adding 12 N NaOH. Ammonium sulphate (Fluka, Netherlands) was added to the supernatants (at 4°C) to obtain 60% saturation and stirred overnight. Following centrifugation at 20,000 × g for 1 h at 4°C, the pellets were dissolved in 20 mM potassium phosphate buffer (Aran et al., 2015).
The antibacterial effects of bacteriocins from the isolated strains was investigated on indicator strains bacteria (Gram positive: Listeriamonocytogenes ATCC 7644, Staphylococcusaureus PTCC 1431 and Bacilluscereus PTCC 1015 and Gram negative: Escherichiacoli PTCC 1270, Salmonellaenteritidis PTCC 1709 and Pseudomonasaeruginosa CZO), using agar disk diffusion assay. Muller-Hinton agar (Biolife, Italy) was seeded by 106 CFU/ml of each indicator strain and 25, 50 and 75μl of bacteriocins that were dissolved in 20 mM potassium phosphate buffer placed on 6.4 mm diameter blank paper discs (Padtan Teb Co., Iran) in agar plates seeded with the indicator strains. Plates were left for 1 h at room temperature prior to incubation at the optimum temperature for each indicator strains. Appearance of clear zones of inhibitions was investigated after 24 h. The best bacteriocin producers were selected based on their antibacterial effect against indicator strains (Olasupo et al., 1999; Settanni et al., 2005).
The Effect of the bacteriocin-containing CFS on the growth of L. monocytogenes and P. aeruginosa was investigated. CFS from the best bacteriocin producers prepared as mentioned above. Ten millilitres of bacteriocin- containing CFS was added to 100 ml (3 h-old) culture of indicator strains and incubated at 37°C. The optical density (OD) at 600 nm was scrutinized at every 2 h-interval during 24 h (Ahmadova et al., 2013; Todorov et al., 2010).
The CFS was prepared as mentioned above. To test the effect of pH on antibacterial activity of the bacteriocins, pH was adjusted between 2 and 12 using HCl or NaOH, and then incubated at 37°C for 2 h. Prior to antibacterial activity assay, pH was adjusted to 7. The antibacterial effect was measured against L. monocytogenes ATTC 7644 by agar disk diffusion assay. To determine the effect of temperature on bacteriocin stability, the CFS was incubated at 60°C for 30 min; 100°C for 10, 20 and 45 min; and 121°C for 15 min. The antibacterial effect of CFS was assessed against L. monocytogenes ATTC 7644 using agar disk diffusion assay (Aran et al., 2015; Sonsa-Ard et al., 2015). The influence of trypsin (pH=7) on antibacterial activity of bacteriocins was evaluated. 20 μl of trypsin solution was added to 200 μl of each bacteriocin-containing CFS. After 2 h incubation at 37°C, trypsin activity was stopped by heating at 80°C for 5 min. The antibacterial effect of trypsinized CFS was tested against L. monocytogenes ATTC 7644 by agar disk diffusion assay compared to untreated supernatants (Ahmadova et al., 2013; Aran et al., 2015; Shokri et al., 2014; Sonsa-Ard et al., 2015).
The best bacteriocin-producing enterococci were characterized using phenotypic (Gram staining, motility, catalase, oxidase and hemolysis tests) and molecular methods. Polymerase chain reaction (PCR) was used for molecular identification. Genomic DNA was extracted by DNeasy purification kit (Bioneer, Republic of Korea). The 16S rRNA gene was amplified using the universal primers 27F (5'-AGAGTTTGATCCTGGCTCAG-3') and 1492 R (5'-TACGGTTACCTTGTTACGACTT-3' ). PCR was carried out in final reaction volume of 25 μl containing 10 ng of extracted DNA, 0.4 μmol/L of each primer, 0.2 μmol/L dNTP, 2 μmol/L MgCl2, 2.5 μl PCR reaction buffer and 1 U of Taq DNA polymerase. The thermal reaction condition was as following: initial denaturation at 94°C for 5 min, 35 cycles of denaturation at 94°C for 1 min, annealing at 45°C for 30 sec, extension at 72°C for 90 sec and a final extension step at 72°C for 5 min. Amplification products were resolved on a 1% agarose gel by electrophoresis for 1 h at 80 V. The PCR products were sequenced by Bioneer Company (Republic of Korea).
Phylogenetic analysis was carried out as follows: the sequences were edited using Bioedit V.5.0.9 (Hall, 1999). For identification of the nearest neighbors, a BLAST search at the NCBI genome database server (http://blast.ncbi.nlm.nih.gov/Blast.cgi) was carried (Altschul et al. 1990). Alignment, phylogenetic and molecular evolutionary analysis were conducted using MEGA version 5 (Tamura et al. 2011). To confirm the reliability of the phylogenetic tree, bootstrap test and reconstruction was performed 1,000 times (Felsenstein,. 1985). The nucleotide sequences of 16S rRNA gene of studied bacterial strains (E. faecium C-2 and E. faecium Y-1) have been deposited in GenBank under Accession No: KU200452 and KU200451, respectively.
Results
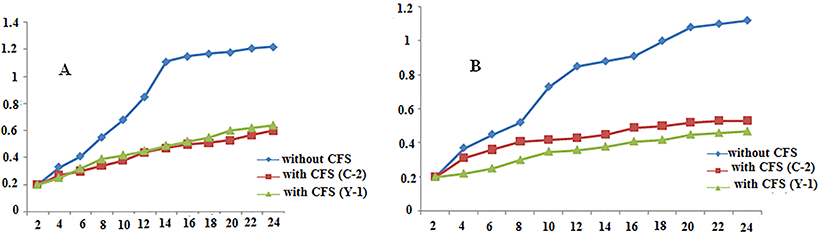
In this research, 15 Enterococcus isolates have been obtained from different native dairies in Kerman province. Ten isolates have antibacterial effects only against Gram positive indicator strains. Five isolates have antibacterial effects against both Gram positive and Gram negative indicator strains. Bacteriocin produced by C-2 (isolated from Zarand cheese) and Y-1 (isolated from Ghanateghestan yogurt) isolates have the highest antibacterial effects against all indicator strains (Table 1). The maximum antibacterial effect against the Gram positive indicator strains was observed for L. monocytogenes and among the Gram negative indicator strains was observed against P. aeruginosa. The growth of L. monocytogenes and P. aeruginosa was significantly reduced after adding bacteriocin-containing CFS from overnight culture of C-2 and Y-1 isolates compared with controls (Fig. 1).
The antibacterial activity of the CFS of C-2 and Y-1 isolates were stable over a wide range of pH from 2 to 12. The CFS of Y-1 isolate was a little more resistance than C-2 isolate. The antibacterial activity of CFS of Y-1 isolates was stable at 100°C for 45 min but the bacteriocin activity of C-2 isolate was reduced by up to 25% in this condition. However, heating at 121°C for 15 min led to 25% and 50% activity loss of the bacteriocins from Y-1 and C-2 isolates, respectively. The bacteriocin activity of the both isolates was lost after treatment with trypsin (Table 2).
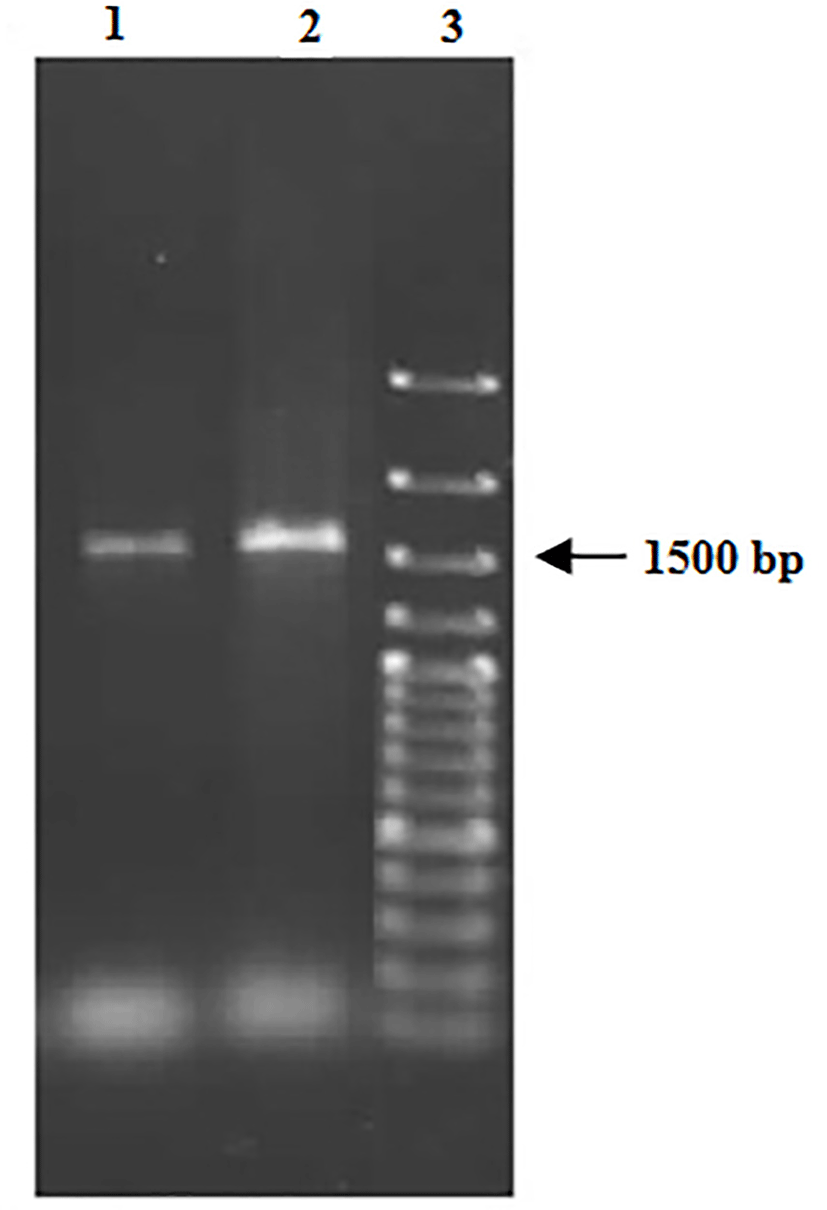
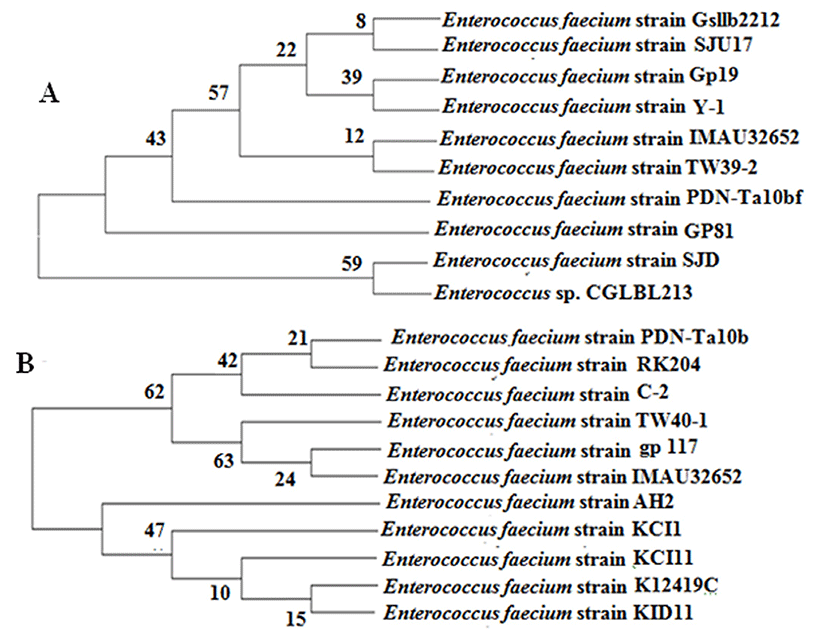
C-2 and Y-1 isolates were Gram positive, no motion, catalyse and oxidase negative, and are alpha-hemolytic. These isolates were characterized by 16S rRNA gene analysis. Amplification of 16S rRNA gene was performed using universal primers as mentioned above. PCR amplification generated a 1500 bp fragment (Fig. 2). Fig. 3 demonstrates the infer phylogenetic relationships derived from neighbor-joining analysis of 16S rRNA gene sequences of the E. faecium C-2 and E. faecium Y-1 with highest validated described species of the genus Enterococcus.
Discussion
From 15 enterococci isolates, bacteriocins produced by the Y-1 isolate from Ghanateghestan yogurt and the C-2 isolate from Zarand cheese, have inhibited all indicator strains. Phylogenetic trees revealed that both isolates located in E. faecium species. Enterococci produce a wide group of bacteriocins (enterocins) that most of them purified and characterized from E. faecium and E. faecalis (Aran et al., 2015; Nascimento et al., 2010). For example, bacteriocin-producing strains such as E. faecalis LMG2333 (Nilsen et al., 2003), E. faecalis KT2W2G (Aran et al., 2015), E. faecium CN-25 (Sonsa-Ard et al., 2015) and E. faecium AQ71 (Ahmadova et al., 2013) have been isolated. Bacteriocins produced by enterococci are generally recognized as safe products.(Javed et al., 2011; Khan et al., 2010). They inhibit the growth of many food borne pathogens such as L. monocytogenes, B. cereus and S. aureus (Barbosa et al., 2014; Chen et al., 2007; Javed et al., 2011; Nilsen et al., 2003). In accordance with our results, Ahmadova et al have reported that the CFS of E. faecium AQ71 inhibited the growth of L. monocytogenes (Ahmadova et al., 2013). Bactericidal activity of bacteriocin against Gram negative bacteria is very low probably for their lipopolysaccharide (LPS) in the outer membrane (Ahmadova et al., 2013; Hammami et al., 2009; Todorov et al., 2010). However, here we reported that Bacteriocins produced by C-2 and Y-1 strains, in addition to Gram positive indicator strains, could also inhibit the growth of Gram negative indicator strains such as P. aeruginosa, S. enterica and E. coli. Similar reports have stated that class II bacteriocin , can inhibit a limited number of Gram negative bacteria, also bacteriocins produced by E. faecium GM-1 isolated from the faces of a newborn human infant inhibited the growth of E. coli and S. typhimurium (Kang and Lee., 2005). Enterococcal bacteriocins belong to class II bacteriocin. These bacteriocins can interact with the receptor in Gram negative bacteria, changing it to an open conformation or the receptor might act as an anchor allowing subsequent bacteriocin insertion and outer membrane disruption (Barraza et al., 2017). The antibacterial effect of the bacteriocin activity of C-2 and Y-1 isolates was lost after treatment with trypsin, indicating proteinaceous nature of antibacterial compound in the CFS. Previous studies revealed that bactericidal activity of the CFS of E. faecium AQ71, E. faecium CN-25 and E. faecalis KT2W2G strains was total lost following the treatment with proteolytic enzymes (Ahmadova et al., 2013; Aran et al., 2015; Sonsa-Ard et al., 2015). Bacteriocins produced by E. faecium C-2 and Y-1 were stable up to 121°C and remained active over a wide pH range from 2 to 12. However, some enterocins lost their antibacterial effect at 100°C and 121°C (Park et al., 2003). The pH stability of enterococci was reported variable. The thermostability and pH stability are useful characteristics for application of the bacteriocins in food and drug processing (Ahmadova et al., 2013; Hadji-sfaxi et al., 2011; Rehaiem et al., 2010).
Conclusion
Here we reported that bacteriocins produced by C-2 and Y-1 isolates have a broad antibacterial activity spectrum against both Gram positive and Gram negative bacteria, in particular pathogenic bacteria. Also, they were resistant against heat and pH ranges. As a result, use of these bacteriocins as substitutes for chemical antibiotics is recommended.













