Introduction
Listeria spp. is gram-positive, rod-shaped, non-spore-forming and facultative anaerobe (Nyarko and Donnelly, 2015; Wieczorek et al., 2012). Listeria monocytogenes is the major pathogenic species in the genus and causes listeriosis, which can be a serious or fatal illness in immunocompromised people including pregnant women, newborns, and elderly people (Indrawattana et al., 2011; Leong et al., 2014; Maertens de Noordhout et al., 2014). Listeria spp. is found in natural environments such as soil, water, vegetation, and animals (Heredia and García, 2018; Vijayakumar and Muriana, 2017). People who eat listeria-contaminated ready-to-eat foods and unpasteurized foods including fruits, vegetables, milk-based dairy products, and meats can be infected (Chan and Wiedmann, 2008; Farber and Peterkin, 1991; Lecuit, 2007). In particular, L. monocytogenes can grow at refrigeration temperatures (4°C), which is an obstacle to efficiently control the pathogenic bacteria in uncooked foods, including cheese (Kale et al., 2017; Lee et al., 2020).
To control L. monocytogenes in dairy and meat products, various methods have been evaluated. Lactic acid bacteria including Leuconostoc mesenteroides and Lactobacillus curvatus isolated from kimchi could control the growth of L. monocytogenes when the bacteria were applied for making soft cheese as starter cultures. The combination of the Leu. mesenteroides starter culture and a package of polybutylene adipate-co-terephthalate with grapefruit seed extract significantly inhibited L. monocytogenes in the cheese (Lim et al., 2020). Nisin, which is a bacteriocin produced from Lactococcus lactis strains has been used for controlling L. monocytogenes in dairy products including cheese (Ibarra-Sánchez, 2020). In a laboratory cheese model, the sensitivity of L. monocytogenes to nisin was dependent upon conditions such as pH and temperature and its efficacy was stronger when the cheese was stored at lower temperatures and prepared at higher pH (Henderson et al., 2020). Nisin also showed antibacterial activity against L. monocytogenes, which was artificially inoculated into raw pork loin. In the study, a mixed natural preservative including nisin, grapefruit seed extract, and cinnamaldehyde showed synergistic effects to control the pathogenic bacteria in the meat (Yu et al., 2019). Pediocin PA-1/AcH, a class IIa bacteriocin, has also been used for controlling L. monocytogenes in food systems (Cui et al., 2012). Pediocin PA-1 showed a synergistic effect on the control of L. monocytogenes in milk when treated with high pressure and bacteriophage (Komora et al., 2020). Pediocin AcH which bound to heat-killed producer cells presented an anti-listerial activity in sterilized raw chicken breast meat at low temperature (5°C).
Bacteriocins from lactic acid bacteria have been attracted by relevant researchers for safety reason and many bacteriocins have been revealed and considered as biopreservatives for controlling pathogenic bacteria including L. monocytogenes in food systems (O’Connor et al., 2020). Nevertheless researchers are still looking for novel bacteriocins which have good applicability in industrial fields. To meet the trend, a lactic acid bacterium producing a bacteriocin was isolated for controlling L. monocytogenes and the bacteriocin was partially characterized in this study.
Materials and Methods
Enterococcus faecium and L. monocytogenes strains were cultured in De Man, Rogosa, and Sharpe (MRS) broth (Difco, Sparks, MD, USA) at 37°C without shaking. The E. faecium CJNU 2524 strain was previously isolated from Makgeolli and selected as a bacteriocinogenic lactic acid bacterium by screening of 100 isolates in our laboratory.
E. faecium CJNU 2524 strain was cultivated in different media [MRS or brain heart infusion (BHI) broth] and temperature (25°C, 30°C, or 37°C) conditions to determine the optimal culture conditions for the production of anti-listerial bacteriocin from the strain. At times (0, 3, 6, 9, 12, and 24 h), cell growth (OD600) and bacteriocin activities were measured in the culture samples. The bacteriocin activity was expressed as AU (arbitrary units)/mL, where AU indicated the reciprocal of the highest two-fold dilution presenting an inhibition zone (Daeschel, 1992).
A crude bacteriocin preparation from E. faecium CJNU 2524 strain was obtained by a previously published method using acetone as the extraction solvent (Chung et al., 2011). To investigate the physicochemical stability, the crude bacteriocin was treated at various pH (2–11) and temperatures conditions (60°C–100°C); with solvents such as methanol (Honeywell B & J, Ulsan, Korea), ethanol (Honeywell B & J), acetone (Honeywell B & J), acetonitrile (Honeywell B & J), and chloroform (Avantor Performance Materials, Center Valley, PA, USA); and enzymes such as protease (Tokyo Chemical, Tokyo, Japan), lipase (Tokyo Chemical), and α-amylase (Sigma-Aldrich, St. Louis, MO, USA).
L. monocytogenes KCTC 3569 strain was cultured in MRS broth overnight and the culture was inoculated into 5 mL of peptone water (0.1%, w/v) at a 1% final concentration. The crude bacteriocin was added into the inoculum with 10 and 100 AU/mL as final concentrations and the viable L. monocytogenes KCTC 3569 cell counts (CFU/mL) were measured at times (0, 1, 2, 3, 4, 5, and 6 h) to determine the mode of action, i.e., bacteriostatic or bactericidal.
Results and Discussion
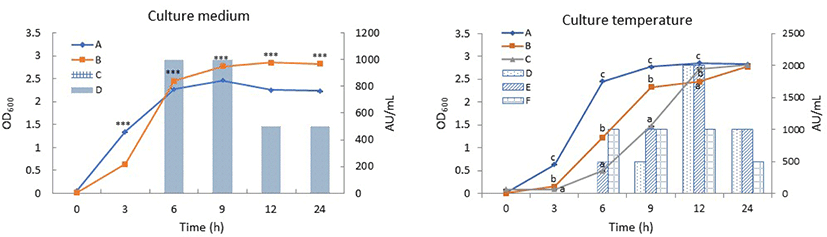
The E. faecium CJNU 2524 strain was previously isolated from makgeolli and confirmed to produce a bacteriocin exerting anti-listerial activity. In this study, the bacteriocin was partially characterized and the optimal culture conditions for the production of bacteriocin by E. faecium CJNU 2524 strain were also established. BHI broth was optimal for the cell growth of E. faecium CJNU 2524 during exponential phase compared to MRS broth but bacteriocin activity in the medium was not seen until 24 h of culture, whereas 1,000 AU/mL of bacteriocin activity was measured at 6 and 9 h of culture in MRS broth (Fig. 1, culture medium). For temperature, 37°C was optimal for the cell growth of the strain compared to 25°C and 30°C but the highest bacteriocin activity was seen at 25°C and 30°C, where 2,000 AU/mL of bacteriocin activity was measured at 12 h in MRS broth (Fig. 1, culture temperature). These results indicate that the optimal conditions for the cell growth of E. faecium CJNU 2524 were not consistent with those for the production of bacteriocin from the strain. Similarly, enterocins from E. faecium CTC492 were optimally produced in MRS broth at temperatures between 25°C and 35°C (Aymerich et al., 2000), but a bacteriocin from E. faecium GM-1 was optimally produced in the broth between 35°C and 40°C (Kang and Lee, 2005).
The crude bacteriocin from the E. faecium CJNU 2524 strain was treated by exposure to various pH and temperature conditions, and different organic solvents and enzymes to evaluate physicochemical stability (Table 1). The bacteriocin was stable at a range of pH values from 2 to 10 where the activities were maintained at the level of the control (4,000 AU/mL), whereas the activity decreased to 2,000 AU/mL at pH 11. It also showed tolerance against heat treatments including 100°C for 60 min where the bacteriocin activity was maintained at 4,000 AU/mL. The bacteriocin was resistant to organic solvents such as methanol, ethanol, acetone, acetonitrile, and chloroform (Table 1). Finally, the bacteriocin was treated with enzymes to confirm its proteinaceous structure. As expected, it was resistant to α-amylase and lipase but not to protease, indicating the proteinaceous nature of the bacteriocin (Fig. 2A). Enterocin TJUQ1 produced from E. faecium TJUQ1 presented very similar stability with different enzymes, pH, temperatures, and solvents (Qiao et al., 2020). Four crude bacteriocins from E. faecium strains, I/Dz, Bri, IK25, and P10, showed strong resistance to heat (45°C–121°C) but their stability in different pH conditions varied. Namely, the bacteriocin of the I/Dz strain was stable at pH 6–7 but the other bacteriocins were stable at pH 3–7 (Bri strain) or pH 3–6 (IK25 and P10 strains) (Kubašová et al., 2020). Circular bacteriocins such as garvicin ML, carnocyclin A, uberolysin A, gassericin A, and butyrivibriocin AR10 which show anti-listerial activity also have heat and pH stability due to their compact structures (Gabrielsen et al., 2014).
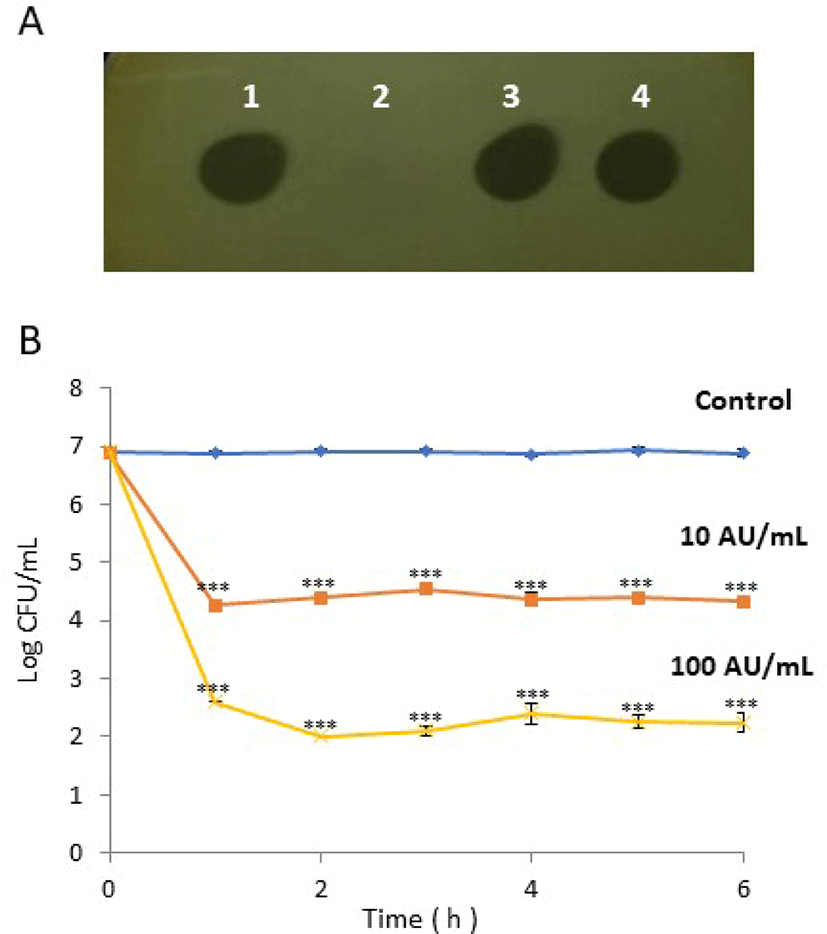
The crude bacteriocin from the E. faecium CJNU 2524 strain was added to 5 mL of peptone water where L. monocytogenes KCTC 3569 had already been inoculated. At the beginning of the culture, viable cell counts of L. monocytogenes were dramatically decreased and maintained afterward (Fig. 2B). The viable cell count started at 6.91 log CFU/mL and was maintained at 6.89 log CFU/mL until 6 h in the controls, whereas the counts decreased to 4.26 and 2.60 log CFU/ml after 1 h when 10 and 100 AU/mL of the bacteriocin was added, respectively. Based on the results, the bacteriocin of E. faecium CJNU 2524 showed a bactericidal mode of action.
Bacteriocins from lactic acid bacteria have been attracted by relevant research scientists for safety reasons. Particularly, class I (nisin, etc.) and II (pediocin, etc.) bacteriocins could be easily applied to the food industry since they have physicochemical stability and strong antimicrobial activity against food spoilage and pathogenic bacteria such as L. monocytogenes and Staphylococcus aureus (Todorov et al., 2020). Nisin has been broadly used as a biopreservative to control pathogenic bacteria in food products including cheese but the bacteriocin is only active in acidic conditions, which might limit its application to food products with neutral or alkaline pH (Khelissa et al., 2020; Pandey et al., 2020). The bacteriocin produced from E. faecium CJNU 2524 showed strong anti-listerial activity and was resistant to heat (60°C–100 °C) and a broad range of pH conditions (2–10). The physicochemical characteristics of the bacteriocin from E. faecium CJNU 2524 might compensate for the drawbacks of nisin. Therefore, it can be a biopreservative candidate to control L. monocytogenes in dairy and meat products. The efficacy of the bacteriocin would be confirmed in food systems in near future.













