Introduction
The demand for functional foods has recently escalated, as food consumption has increasingly focused on gaining additional health benefits than simply managing energy intake. This trend has been intricately linked to technological development, the growing aging population, and an emphasis on immunity (Ali et al., 2022). Among the several functional ingredients, probiotics are recognized for their health benefits, including enhancing nutritional value, managing cholesterol levels, improving immunity, and mitigating the risk of colon cancer (Zendeboodi et al., 2020). As numerous studies have highlighted the benefits of probiotics, multiple researchers have focused on developing functional foods that incorporate these beneficial microorganisms (Misra et al., 2021).
Among the most commonly used probiotic species, Bifidobacterium spp. are well-known to be “Generally Recognized as Safe”. They play a crucial role in the human intestinal microbiota, offering various functional benefits, such as lactose digestibility, anti-carcinogenic activity, cholesterol level reduction, vitamin B synthesis, and calcium absorption (Abdi et al., 2022; Barone et al., 2022; Faghfoori et al., 2021; Mysore Saiprasad et al., 2023; Uebanso et al., 2020). Among the diverse functionalities of Bifidobacterium, research on conjugated linoleic acid (CLA) production has actively been conducted (Gao et al., 2020). CLA is recognized for its numerous health advantages as a functional ingredient, such as its anti-carcinogenic, anti-atherosclerotic, anti-obesity, and anti-inflammatory properties (Basak and Duttaroy, 2020). Because of these health benefits, research into developing probiotic foods with enhanced CLA content using Bifidobacterium spp. is currently underway (Mei et al., 2022).
However, Bifidobacterium spp. encounter several challenges during production because of their anaerobic nature and poor resistance to low pH, rendering it difficult to maintain minimum viable cell counts of 106–107 CFU/mL or g, as required for probiotic foods (Gao et al., 2021; He et al., 2023; Terpou et al., 2019). As a solution, the application of bifidobacteria to dairy products has been proposed. Dairy products are well-known probiotic carriers as they possess a high buffering capacity, which helps protect probiotics during passage through the human gastrointestinal tract (Vivek et al., 2023). Additionally, the incorporation of probiotic bacteria into dairy products offers a natural means of delivering these microorganisms to consumers.
Among dairy products, fermented milk is a common probiotic carrier; however, its low pH (<4.6) would have negatively affected Bifidobacterium survival by the time it reaches consumers. In contrast, cheese provides a more favorable environment with a higher pH and solid content, rendering it a preferred carrier (Rolim et al., 2020). Therefore, this study aimed to evaluate CLA production by Bifidobacterium breve JKL2022 in reconstituted skim milk (RSM). Further, the study assessed the survivability of B. breve JKL2022 in three representative dairy products: whole milk, yogurt, and cream cheese along with its chemical characteristics. Ultimately, this study evaluated the potential of B. breve JKL2022 as a CLA-producing probiotic adjunct culture for dairy products.
Materials and Methods
B. breve JKL2022 used in this paper is registered in the Korean Agricultural Culture Collection (KACC) as B. breve KACC 81214BP. The ability of B. breve JKL2022 to convert linoleic acid (LA) to CLA in RSM broth was evaluated. Briefly, 10% (v/v) skimmed milk powder (SMP) was prepared in distilled water and supplemented with various combinations of glucose and yeast extract, as listed in Table 1. The modified RSM broths were sterilized in an autoclave (121°C, 15 psi, for 15 min) or via heat treatment (90°C–95°C for 10 min), followed by cooling in the ice bath. Thereafter, all media were supplemented with 0.50 mg/mL LA before inoculating a 1.0% (v/v) overnight culture of B. breve JKL2022.
| Treatment | Base media | Supplements1) | |
|---|---|---|---|
| Glucose | Yeast extract | ||
| RSM | 10% RSM | - | - |
| RSG | 10% RSM | 2.00% | - |
| RSY | 10% RSM | - | 0.10% |
| RSGY | 10% RSM | 2.00% | 0.10% |
The cultures were incubated under aerobic and anaerobic conditions at 37°C for 12–24 h. After incubation, pH, viable cell count, and CLA production were measured. The pH of each treatment was measured using a BP3001 Benchtop pH meter (Trans Instruments, Singapore). The viable cell count was determined by plating on de Man, Rogosa, and Sharpe agar (Difco, Franklin Lakes, NJ, USA) with 0.05% L-cysteine hydrochloride. The plates were incubated at 37°C for 24 hours under anaerobic conditions using the GasPakTM system (Becton Dickinson, Franklin Lakes, NJ, USA) and the results were reported as Log CFU/mL. CLA concentration was determined using the isopropanol–hexane extraction protocol, with minor modifications (Jung et al., 2006). Briefly, 400 μL of culture was transferred to a sterile 2.0-mL microfuge tube, followed by the sequential addition of 800 μL of isopropanol (Sigma-Aldrich, St. Louis, MO, USA) and 600 μL of hexane (Sigma-Aldrich). The mixture was vortexed for 5 min, followed by centrifugation (980×g, 5 min, 20°C) to facilitate phase separation. The hexane layer (top layer) containing the conjugated fatty acids was diluted in methanol (Sigma-Aldrich) in a 100:900 ratio (v:v) before measuring absorbance at 233 nm using a UV-transparent 96-well plate (UVMaxTM, SPL, Pocheon, Korea). All optical density (OD) readings were performed using an INNO Spectrophotometer (INNO, LTEK, Seongnam, Korea).
Whole milk heat-treated at ultra-high temperature (130°C, 2–5 s) was obtained from a commercial market in Anseong, Gyeonggi-do, Republic of Korea. Subsequently, B. breve JKL2022 was inoculated into 500 mL of whole milk at 2.01×107 CFU/mL and aseptically distributed into 50-mL sterile glass tubes. The samples were stored at 4°C for 15 d.
For probiotic yogurt production, the total solid–nonfat content in 3.2 L of whole milk was adjusted to 11% using commercial SMP. Thereafter, the milk was divided into four groups: T1 (control), containing no additional carbohydrates; T2, supplemented with 2% (w/v) glucose (Duksan, Ansan, Korea); T3, supplemented with 2% (w/v) inulin (Fibrulose® F90, Cosucra, Pecq, Belgium); and T4, supplemented with trans-galactooligosaccharides (TOS; Oligomate® 55NP, Yakult, Tokyo, Japan). All treatments were subsequently heat-treated at 95°C for 10 min and immediately cooled to 40°C in an ice bath. Thereafter, a thermophilic starter culture (TCC-3, Chr. Hansen, Hørsholm, Denmark) containing Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus as well as B. breve JKL2022 was inoculated at 0.01% (w/v) and 2.58×107 CFU/mL in all treatments, respectively. Fermentation was conducted in a 37°C water bath until the mixture had reached pH 4.60 (approximately 4–5 h). Subsequently, the yogurt samples were cooled in the ice bath and subjected to the same conditions mentioned above.
Probiotic cream cheese was manufactured from 40 L of bovine raw milk obtained from a farm affiliated with Chung-Ang University, Anseong, Gyeonggi-do, Republic of Korea. First, the raw milk was pasteurized at 65°C for 30 min and subsequently cooled to 32°C. Thereafter, the total milk fat content was adjusted to 8% using 6 L of fresh cream (38% fat content). Afterward, a Flora Danica starter culture (Chr. Hansen) comprising Lactococcus lactis subsp. cremoris, L. lactis subsp. lactis, L. lactis subsp. diacetylactis, and Leuconostoc spp. was inoculated at 0.03% (w/v) along with 2.45×107 CFU/mL of B. breve JKL2022. To facilitate curd formation, 0.02% (v/v) rennet Naturen® (92% chymosin, 290 IMCU/mL; Chr. Hansen, Hamilton, New Zealand) was added and incubated for 45 min at 32°C. Subsequently, the curd was cut horizontally and vertically into 1.5-cm cubes and fermented at 32°C until achieving a pH of 5.55. Thereafter, heat treatment was applied at 48°C for 10 min to inactivate the starter culture, and the curds were subsequently placed in cheesecloth, followed by whey drainage at 10°C for 3 h. Finally, the cheese samples were salted at 0.5% (w/w) and subsequently packaged into 220-mL plastic containers. All cheese samples were stored as previously described.
B. breve JKL2022 viability in cream cheese was measured during both the manufacturing and storage processes. Samples were collected after inoculation, fermentation, heating, and drainage. For microbiological and chemical analyses, all samples from the three dairy matrices were analyzed every 3 days (0, 3, 6, 9, 12, and 15 d) throughout storage.
To analyze whole milk and yogurt, pH and titratable acidity (TA) were measured, while for cream cheese analysis, pH was measured. To determine the pH of cream cheese, 5-g cheese samples were each mixed with 5 mL of distilled water, and the pH values of the resulting mixtures were measured. The pH values of all three dairy matrices were determined using a pH meter (Trans®, BP3001, Trans Instruments). To measure TA, 0.1% phenolphthalein indicator solution was employed, and 0.1 N NaOH was used to titrate the samples to neutrality. TA was presented as a percentage of samples and calculated using the following formula:
For the microbiological analysis of B. breve JKL2022 in whole milk and yogurt, samples were diluted 10-fold using 1× phosphate-buffered saline (PBS). For cream cheese, 2-g cheese samples were homogenized with 18 mL of 1× PBS using a homogenizer (SHG-15D-Set-A, Daihan Scientific, Wonju, Korea) and diluted in the same manner as previously described. Viable B. breve JKL2022 cells were enumerated in TOS–propionate agar (Sigma-Aldrich) supplemented with 1% (v/v) mupirocin (Medion, Seoul, Korea) and incubated at 37°C for 48 h under anaerobic conditions. The survival rate (%) of B. breve JKL2022 was calculated using the following formula:
Where Log (CFU/mL)Tn is the viable cell count calculated at each storage time point, and Log (CFU/mL)T0 denotes the viable cell count immediately after inoculation.
All experiments were conducted in triplicate within the same batch. The data shown in the Figures and Tables are expressed as the mean±SD (n=3). Statistical analysis was performed using GraphPad Prism (version 8.0.1, GraphPad Software, San Diego, CA, USA). For each experiment, statistical analysis involved one-way analysis of variance (ANOVA) and two-way ANOVA, followed by Tukey’s test for post-hoc analysis to identify differences between means. Statistical significance was set at p<0.05.
Results
B. breve JKL2022 demonstrated the ability to grow and produce CLA in modified RSM medium (Fig. 1). In terms of fermentation activity measured in terms of pH (Fig. 1A) and cell viability measured in terms of CFU/mL (Fig. 1B), the addition of 0.1% yeast extract (RSY) favored B. breve JKL2022 growth more than 2.0% glucose (RSG) supplementation. Meanwhile, the combination of 0.1% yeast extract and 2.0% glucose (RSGY) did not exhibit higher viability or better fermentation than supplementation with 0.1% yeast extract alone. Notably, media prepared via autoclave displayed significantly lower cell growth (p<0.05) under aerobic (RSY, 8.2 Log CFU/mL; RSGY, 7.92 Log CFU/mL) than under anaerobic (RSY, 9.05 Log CFU/mL; RSGY, 9.10 Log CFU/mL) conditions, while those prepared via heat treatment yielded a similar viable cell count range (p>0.05) for RSY and RSGY (8.96–9.14 Log CFU/mL). Generally, incubation under anaerobic conditions enabled better B. breve JKL2022 proliferation. This observation was consistent regardless of the sterilization method.
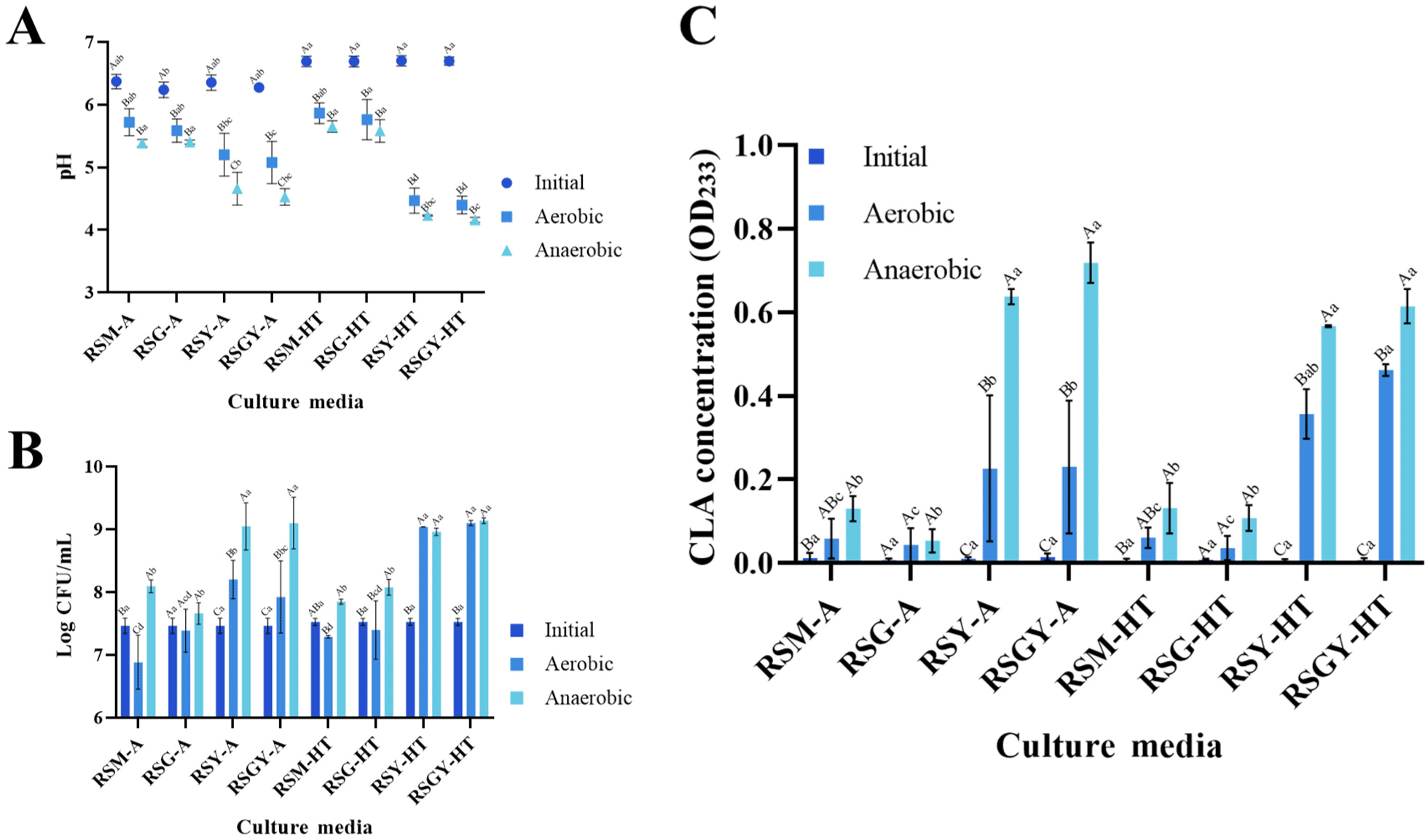
In terms of CLA production (Fig. 1C), B. breve JKL2022 exhibited significant CLA conversion when cultured in RSY or RSGY, independent of the sterilization method. Specifically, autoclaved RSY and RSGY under aerobic conditions reached OD233 values 0.226±0.175 and 0.230±0.159, while heat-treated media yielded 0.357±0.059 and 0.462±0.014, respectively. Moreover, incubation in the same media under anaerobic conditions yielded higher CLA concentrations, reaching OD233 0.638±0.018 and 0.719±0.049 for autoclaved RSY and RSGY, and 0.567±0.003 and 0.615±0.042 for heat-treated media, respectively. The rest of the tested media failed to produce significant CLA during the incubation period. Notably, CLA production was exclusively observed when curd formation occurred during growth, that is, when the pH of the culture medium reached approximately ≤5.0.
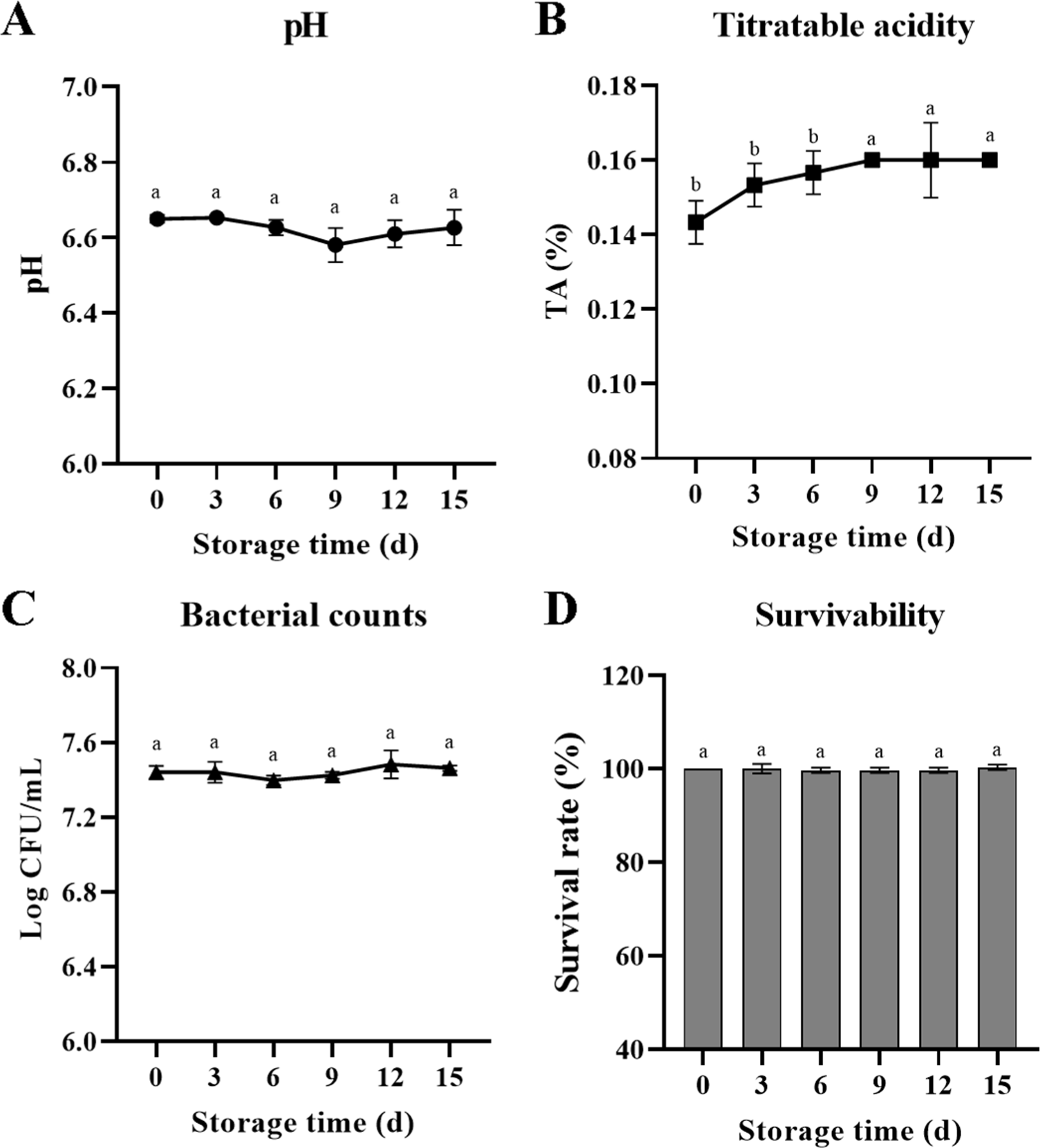
The pH, TA (%), bacterial count, and survivability (%) values of B. breve JKL2022 in whole milk during 15-day refrigerated storage are presented in Fig. 2. The initial pH of whole milk was 6.65, and it gradually decreased to 6.58 after 9 d of refrigerated storage (Fig. 2A). Nevertheless, the changes during the 15-day storage period were not statistically significant (p>0.05). In contrast, TA notably increased to 0.16% at day 9 from an initial value of 0.14% and remained constant until day 15 (Fig. 2B, p<0.05). The initial concentration of B. breve JKL2022 was 7.55 Log CFU/mL and was maintained at 7.46 Log CFU/mL up to 15 d of storage (Fig. 2C, p>0.05). The calculated survival rate (%) of B. breve JKL2022 remained between 99% and 100% throughout the 15-d storage period at 4°C (Fig. 2D, p>0.05).
The pH, TA (%), bacterial count, and survivability (%) values of four different yogurt treatments inoculated with B. breve JKL2022 were compared (Fig. 3). Overall, all treatments displayed significant differences in pH and TA (%) throughout their ripening periods (p<0.05). Notably, a substantial decline in pH was observed until day 9 (p<0.05); thereafter, it remained stable for the remainder of the storage period (Fig. 3A). Among the four yogurt treatment groups, T2 and T3 exhibited the greatest declines in pH from 4.57 and 4.58 after fermentation (day 0) to 4.27 and 4.30 on day 15 of storage, respectively (Fig. 3B). In contrast, T1 and T4 demonstrated relatively smaller declines in pH from 4.59 and 4.56 on day 0 to 4.31 and 4.32 on day 15, respectively. Despite the addition of carbohydrates as an extra energy source, T4 yielded a similar pH value to that of the control. However, during 15-day refrigerated storage, T1 exhibited the highest TA value, which rose from 0.96% on day 0 to 1.04% on day 9 and continued increasing to 1.06% on day 15. A similar trend was observed in T2 wherein TA sharply increased from 0.95% on day 0 to 1.02% on day 9, reaching 1.04% on day 15. This increase correlated with the rapid pH decrease during the initial refrigerated storage period (days 0–9). In contrast, T3 and T4 displayed more gradual increases in TA from 0.95% and 0.94% on day 0 to 0.99% and 0.96% on day 15, respectively.
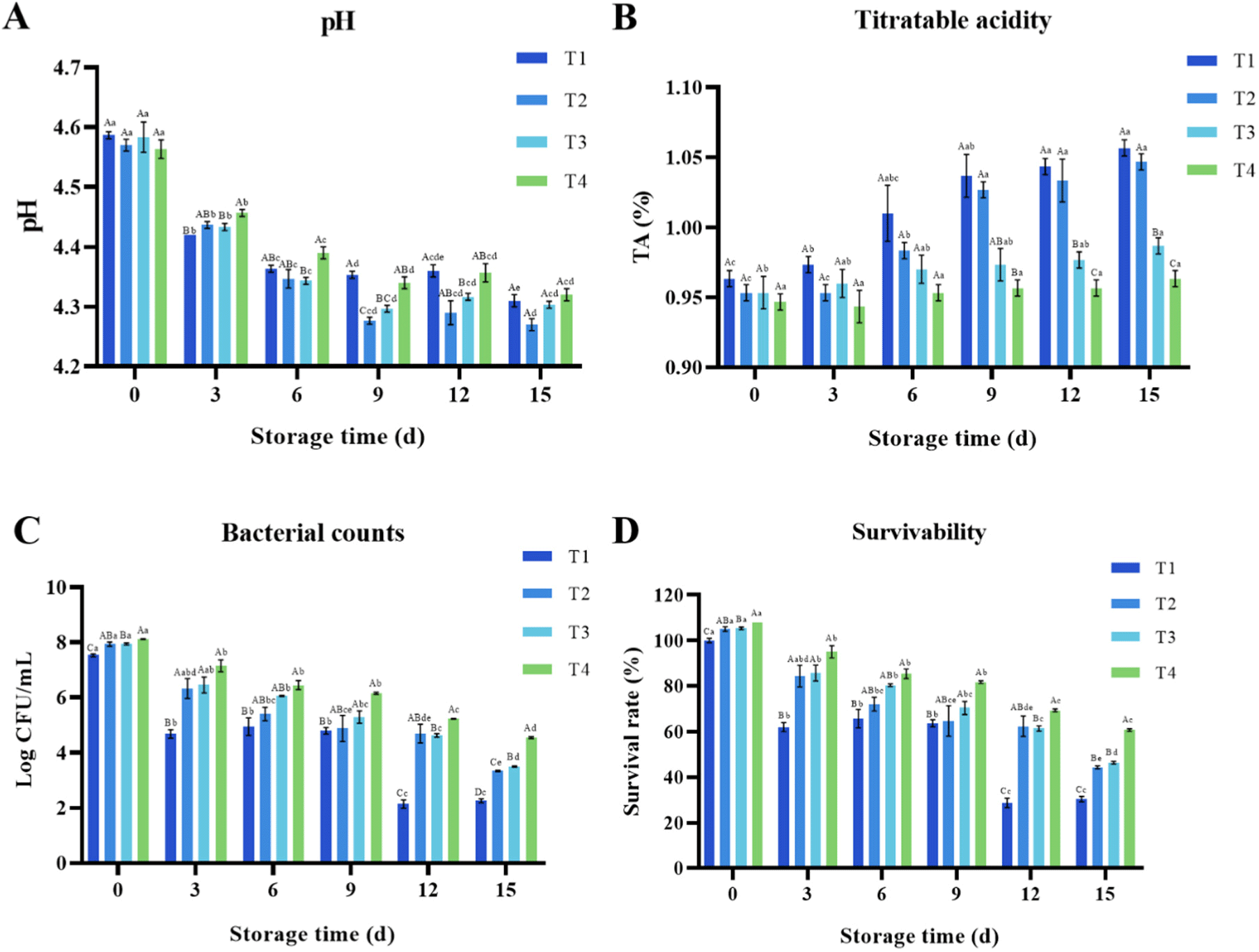
Moreover, viable count (Fig. 3C) and survival rate (Fig. 3D) demonstrated significant differences among the treatment groups during 15-day refrigerated storage (p<0.05). After fermentation (day 0), the viable cell count of B. breve JKL2022 remained the same, exhibiting an inoculum size of 7.53 Log CFU/mL in T1. However, it increased to 7.93, 7.94, and 8.11 Log CFU/mL in T2, T3, and T4 from the initial inoculum of 7.53 Log CFU/mL, respectively. Throughout the refrigerated storage period, all groups exhibited a decreasing trend, with T1 displaying the most significant decline to a final count of 2.26 Log CFU/mL and survival rate of 30%. In contrast, its viability in yogurt supplemented with carbohydrates demonstrated a gradual decline rather than the sharp decrease observed in T1. B. breve JKL2022 viability was maintained at 6.06 Log CFU/mL and an 80% survival rate on day 6 in T3, while T2 yielded 5.40 Log CFU/mL and a 72% survival rate. Ultimately, the viability counts of T2 and T3 decreased to 3.34 and 3.50 Log CFU/mL, with survival rates of 44% and 46% on day 15, respectively. Nonetheless, B. breve JKL2022 exhibited the highest survivability in T4, yielding 6.15 Log CFU/mL and an 82% survival rate on day 9, followed by a decrease to 4.55 Log CFU/mL and a 60% survival rate on day 15. This indicates that TOS-added yogurt exhibited the highest B. breve JKL2022 survival rate among the four groups.
B. breve JKL2022 viability changes in whey and curds during the cream cheese manufacturing process were analyzed (Table 2). The microbial counts of B. breve JKL2022 were significantly higher in the curds than in the whey throughout the entire manufacturing process (p<0.05). This suggests that B. breve JKL2022 is more extensively distributed in the curds during cream cheese production. Moreover, B. breve JKL2022 viability gradually concentrated in the curds, increasing from 7.85 Log CFU/mL after fermentation to 8.15 Log CFU/mL after draining. The final B. breve JKL2022 microbial counts were concentrated to 8.17 Log CFU/mL in the curds after salting from 7.39 Log CFU/mL in the milk after inoculation.
| Samples | Log CFU/mL or g | |
|---|---|---|
| Whey | Curds | |
| After fermentation (pH 5.5) | 6.89±0.02Ba | 7.85±0.25Ab |
| After heating | 6.68±0.07Ba | 8.06±0.03Aab |
| After draining | 6.81±0.17Ba | 8.18±0.17Aa |
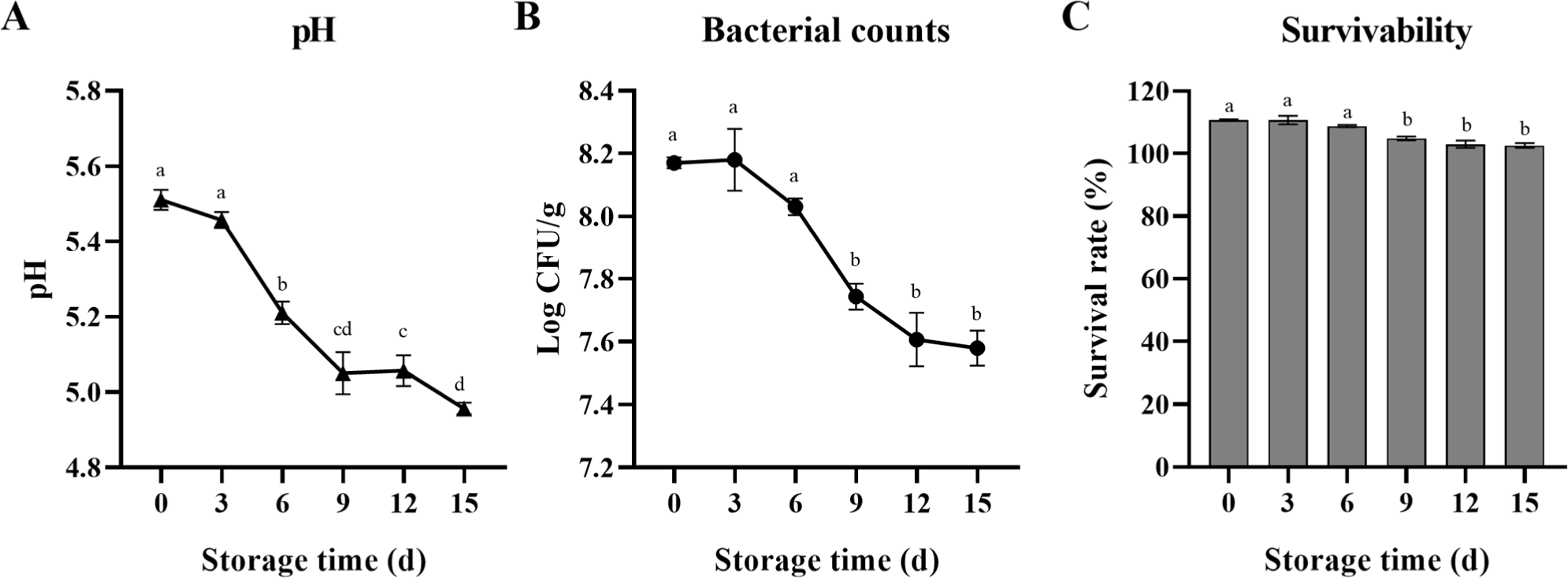
Thereafter, changes in B. breve JKL2022 pH, bacterial count, and survivability (%) in cream cheese were monitored during the 15-day refrigerated storage period (Fig. 4). Overall, pH, bacterial count, and survival rate (%) significantly decreased during refrigerated storage (p<0.05). pH decreased from 5.51 after salting (day 0) to 4.96 on day 15 of refrigerated storage (Fig. 4A). B. breve JKL2022 viability also displayed this decreasing trend during the storage period (Fig. 4B). Cell counts decreased from 8.17 Log CFU/g on day 0 to 7.74 Log CFU/g on day 9, followed by a further decrease to 7.58 Log CFU/g on day 15. Even though the microbial counts of B. breve JKL2022 declined, its survival rate remained between 102% and 111% throughout refrigerated storage (Fig. 4C). This suggests that B. breve JKL2022 can maintain relatively high survivability within the cheese matrix during both the manufacturing process and storage period.
Discussion
This study evaluated the survivability of B. breve JKL2022 in three dairy products: whole milk, yogurt, and cream cheese with the aim of developing probiotic dairy products. Preliminary experiments on the ability of B. breve JKL2022 to grow in milk and its derivative products were performed using 10% RSM. Most independent studies that utilize milk as a culture medium often add glucose, yeast extract, or their combination to promote LAB growth. In this study, B. breve JKL2022 demonstrated favorable growth, fermentation, and CLA production when cultured in 10% RSM supplemented with 0.1% yeast extract or a combination of 0.1% yeast extract and 2.0% glucose. The additional nutritional content derived from these additives is hypothesized to promote the strain’s metabolic activity, allowing better proliferation than that in RSM alone. Additionally, the effects of different sterilization methods were comparatively investigated to assess the minimum treatment required for optimal growth and CLA production. The results revealed that both traditional heat treatment and autoclave methods proved to be efficient means of inactivating contaminants and supporting strain growth. However, in terms of CLA production under aerobic conditions, a higher CLA yield was observed in heat-treated RSM than in autoclaved media. Nevertheless, CLA production under anaerobic conditions exhibited similar outcomes. These results verify that B. breve JKL2022 can produce CLA in milk-derived products under both aerobic and anaerobic conditions. Considering that dairy products are typically produced under aerobic conditions, this suggests that B. breve JKL2022 may significantly contribute to the development of dairy products with enhanced CLA content. Moreover, it was confirmed that significant CLA production occurred only when curd formation (≤pH 5.0) took place as B. breve JKL2022 exhibited a certain level of metabolic activity and growth. This demonstrates a similar result to other reports indicating that bacterial CLA production is correlated with its growth as CLA isomerization serves as a detoxification mechanism. Free LA, which is toxic to bacteria, is converted into less-toxic CLA, thereby protecting the bacterial cells (Jang et al., 2024). Thus, the onset of CLA production is closely linked to bacterial growth, indicating that B. breve JKL2022 must achieve a specific growth level to efficiently produce CLA.
Moreover, B. breve JKL2022 survivability in three dairy products (whole milk, yogurt, and cream cheese) and changes in the chemical characteristics of these products were analyzed. First, this study verified that probiotic whole milk can be successfully developed by applying B. breve JKL2022. B. breve JKL2022 maintained high viable cell counts of 7.44–7.46 Log CFU/mL in whole milk at neutral pH during 15-day refrigerated storage. Moreover, the pH and TA values of whole milk supplemented with B. breve JKL2022 aligned with the standards for normal whole milk, with pH and TA values of 6.60–6.80 and 0.14%–0.18%, respectively (Tadesse et al., 2023). This indicates that it satisfies the standards for probiotic food, maintaining the minimum viable count of 1×106 CFU/mL throughout refrigerated storage without affecting milk quality.
Furthermore, the viability of B. breve JKL2022 in yogurt samples supplemented with three different carbohydrates surpassed that in the control. This is because the additional carbohydrates served as extra energy sources for B. breve JKL2022, enabling longer-lasting metabolic activity (Kamel et al., 2021; Khatami et al., 2022). First, glucose potentially enhances starter culture and B. breve JKL2022 growth in yogurts, serving as the fundamental energy source for numerous organisms (Khatami et al., 2022). This effect was evident in T2, which displayed a higher viable cell count (6.32 Log CFU/mL) than the control group (4.68 Log CFU/mL) on day 3. Next, inulin has been known to promote probiotic viability in dairy products (De Souza Oliveira et al., 2011; Kamel et al., 2021). Although B. breve JKL2022 maintained a survival rate >80% until day 6, it still exhibited a decreasing trend over the 15 day-refrigerated storage. This is because inulin affects the growth of both B. breve JKL2022 and starter cultures by serving as prebiotics that promote the proliferation of these bacteria (Kamel et al., 2021). This increased bacterial growth leads to a decline in yogurt pH, which subsequently diminishes the viability of B. breve JKL2022. In contrast, TOS-supplemented yogurt displayed the highest survivability (>60%) throughout the 15-day refrigerated storage. This is because TOS is a well-known highly selective prebiotic for Bifidobacterium, supporting the metabolic activity and growth of B. breve JKL2022 during storage (Arapović et al., 2024). However, B. breve JKL2022 viability in all yogurts demonstrated low viability, ranging from 2.26 to 4.55 Log CFU/mL on day 15, even though additional energy sources contributed to its enhanced survivability. This result is consistent with that of other studies that evaluated the viability of bifidobacteria in fermented milk or yogurt. Odamaki et al. (2011) observed that the cell counts of six species of Bifidobacterium decreased after 14 d of refrigerated storage, ranging from approximately 1.16 to 4.57 Log CFU/mL. This decline was attributed to two significant challenges: oxygen exposure and a low pH. To overcome these limitations, methods such as microencapsulation and oxygen scavenging, are necessary to enhance B. breve JKL2022 survivability in yogurt (Afzaal et al., 2020; Norouzbeigi et al., 2021).
Cheese has recently been considered a better carrier of probiotics than fermented milk and yogurt owing to its physiological characteristics, such as a high pH and buffering capacity (Rolim et al., 2020). This study observed that B. breve JKL2022 was mainly distributed in curd during the cream cheese manufacturing process and displayed high viability during refrigerated storage, ranging from 7.58 to 8.17 Log CFU/g. This value indicates that B. breve JKL2022 maintained higher viability than other Bifidobacterium spp. in cheese. For example, a previous study found B. longum B1 to survive at 6.30–7.09 Log CFU/g in Argentinian Fresco cheese at 5°C for 60 d (Vinderola et al., 2000). In another study, B. bifidum BB-02 decreased from an initial inoculum size of 7.00 Log CFU/mL to 6.00 Log CFU/g after a 56-d ripening period at 12°C in Canestrato Pugliese hard cheese (Corbo et al., 2001). This indicates that B. breve JKL2022 can be applied to cream cheese for the development of probiotic cream cheese, as the number of living cells exceeded the minimum value required for probiotic benefits. However, B. breve JKL2022 viability was affected by a decrease in pH, indicating that B. breve JKL2022 is particularly sensitive to acidic conditions.
Conclusion
Considering the growing demand for functional foods, developing products enriched with health-beneficial components is important. In this study, we focused on developing probiotic dairy products as functional foods using B. breve JKL2022 as a potential probiotic adjunct culture. This study successfully produced probiotic whole milk and cream cheese, which predominantly possess a higher pH than yogurt. To develop probiotic yogurt, further research into applying microencapsulation and oxygen scavengers, which protect Bifidobacterium spp. from stress conditions, is warranted. Moreover, we generated concrete evidence suggesting that B. breve JKL2022 can produce CLA in milk-derived media when sufficient growth of B. breve JKL2022 is achieved with appropriate amounts of substrates, indicating its potential in the development of CLA-enriched dairy products incorporating B. breve JKL2022.













