Introduction
Raw milk processing for transportation plays a crucial role in maintaining the quality of raw milk and extending its shelf life. Even when raw milk initially has a low microbial content, storing it above 8°C can lead to a rapid increase in microorganisms (Malacarne et al., 2013). Some of these microorganisms pose a threat to consumers as they can cause diseases like tuberculosis, brucellosis, and gastroenteritis. Moreover, raw milk is highly susceptible to spoilage in unstable environments, and the presence of pathogenic microorganisms can compromise the flavor, texture, and stability of the milk, subsequently affecting the quality of dairy products derived from it. Therefore, it is crucial to rapidly cool raw milk after milking and store it in cooling tanks to prevent microbial growth. However, during transportation from dairy farms to milk collecting centers (MCCs), the duration of transport can promote the proliferation of microorganisms. Unlike larger farms, small to medium-sized dairy farms, particularly those in rural areas, often face several infrastructural challenges in handling and cooling raw milk. These limitations include inadequate or outdated cooling systems, limited access to efficient storage facilities, lack of advanced milk processing equipment, and insufficient transportation resources for maintaining optimal milk temperatures during transit to MCCs. These constraints can significantly impact the quality and safety of milk before it reaches processing facilities.
In Thailand, smallholder dairy farms contribute approximately four-fifths of the country’s raw milk production, often constrained by the high operational costs of advanced cooling systems. Cooling systems play a vital role in preventing bacterial contamination and microbial growth during the transportation of raw milk from farms to MCCs. Furthermore, many of these farms rely on local logistics service providers for milk transportation (Ongkunaruk, 2015). As a result, milk from the first-serviced farms takes longer to reach the MCCs. Smaller farms face a higher risk of milk rejection due to elevated bacterial content, often exceeding the standard plate count (SPC) value of 5×105 CFU/mL specified by the Thai Agricultural Standard (TAS 6003-2010). Consequently, the incomes of these small farmers inevitably decline.
In the food industry, ensuring the safety and quality of food through preservation and processing is of utmost importance. While thermal processing, a conventional food technology, effectively eliminates microbes including bacteria, viruses, and other pathogens through high temperatures, it can lead to undesired side effects such as changes in color, flavor, and loss of active nutrients and bioactive components (Adekunte et al., 2010). To mitigate these alterations in nutritional and sensory properties, alternative non-thermal technologies have been introduced, including ultraviolet (UV) treatment, pulsed electric fields, high-pressure processing, ultrasound, cold plasma, ionizing radiation, and chemical treatment. These methods aim to enhance food safety, extend the shelf life of food products, and maintain their quality (Alexandre et al., 2012; Choi and Nielsen, 2005; Mahalik and Nambiar, 2010; Neoκleous et al., 2022; Roobab et al., 2023; Suthiluk et al., 2023).
Among these non-thermal technologies, UV light treatment is a notable method for inactivating microorganisms, including viruses (Eischeid et al., 2009), bacteria, yeasts, and spores (Atik and Gumus, 2021; Gómez‐López et al., 2005). The UV light spectrum consists of three wavelengths: UV-A (320–400 nm), UV-B (280–320 nm), and UV-C (200–280 nm; Guerrero-Beltrn and Barbosa-Cnovas, 2004; Keyser et al., 2008). Among these, UV-C irradiation exhibits the highest efficiency in germicidal action. Its primary mechanism involves DNA damage, making Gram-negative food-borne pathogens the most susceptible, followed by Gram-positive food-borne pathogens and yeasts (Kim et al., 2017). UV-C treatment also inhibits the growth of lactic acid bacteria, which contribute to food spoilage (Peng et al., 2020) and off-odors (Brugnini et al., 2021). Therefore, UV-C technology holds promise for enhancing the shelf life of food (Brugnini et al., 2021; Lu et al., 2011) and serves as a viable alternative for bacterial inactivation in the food industry. Previous studies on UV-C treatment of raw milk have demonstrated reductions of more than 3 Log in bacterial counts (Reinemann et al., 2006) and a 2.3 Log decrease in viable count compared to untreated milk (Lu et al., 2011). UV-C treatment has also been effective in reducing various milk-borne pathogens such as Listeria monocytogenes, Escherichia coli, Staphylococcus aureus, Salmonella senftenberg, Bacillus cereus, Aeromonas hydrophila, Serratia marcescens, and Yersinia enterocolitica (Bandla et al., 2012b; Crook et al., 2015; Matak et al., 2005). A previous study comparing UV-C powers of 39 W and 48 W demonstrated that 48 W exhibited greater microbial reduction due to its higher energy and larger surface area in the UV-C reactor tube (Makarapong et al., 2020). Research on UV-C application initially concentrated on low flow rates in controlled laboratory settings, exemplified by Oguma et al. (2013) who explored its application in water treatment (Oguma et al., 2013). Complementing this, Atılgan (2013) extended the research scope by investigating UV-C treatment in various liquid food products, including clear and opaque types (Atılgan, 2013). Together, these studies underline the importance of optimizing treatment conditions, such as the residence time of the liquid, to ensure efficient microbial reduction in practical applications, particularly in dairy processing. Furthermore, the implementation of UV-C as a pre-treatment method in dairy farms, particularly at routine milking flow rates, is a novel aspect of our current study.
Our study aimed to develop and validate a UV-C system that is compatible with common milk flow rates in dairy farms. We have established a suitable design and operational dose for UV-C treatment and have assessed its effectiveness in microbial inactivation and lipid peroxidation in raw milk within the context of this study.
Materials and Methods
A homemade UV-C reactor was designed and constructed following the methods described in our previous report (Makarapong et al., 2020). The UV radiation source used was a commercially available low-pressure Hg lamp measuring 114.7 cm in length, as indicated in Table 1. The UV-C reactor used in this study demonstrated an electrical efficiency of 4.68 W. The surface temperature of the reactor was recorded at a maximum of 63.5°C, as recorded by Fluke (Everett, WA, USA). However, it is important to note that this temperature pertains only to the surface of the UV reactor. In our unique setup, milk did not come into direct contact with the UV source. A protective quartz glass sleeve was employed to insulate the milk from the high surface temperature, maintaining the milk’s temperature at a significantly lower 38.5°C during treatment. Furthermore, a cooling airflow inlet was incorporated within the system to regulate the temperature as milk passed through the tube. These features, combined with the notably brief exposure time of the milk to UV-C light, distinctively set our method apart from conventional thermal processing techniques such as low-temperature long-time pasteurization. The design and operational parameters of our UV-C system thus support its classification as a nonthermal process, notwithstanding the high surface temperature recorded.
To construct the UV-C light source compartment, the UV-C lamp was positioned inside a quartz glass sleeve, leaving an approximate air gap of 1.92 mm between the lamp and the sleeve. Four UV-C reactors were arranged in parallel, with each stainless-steel tube of the reactor connected to that of the adjacent reactor, as illustrated in Fig. 1. This arrangement facilitated the flow of raw milk through all four reactors.
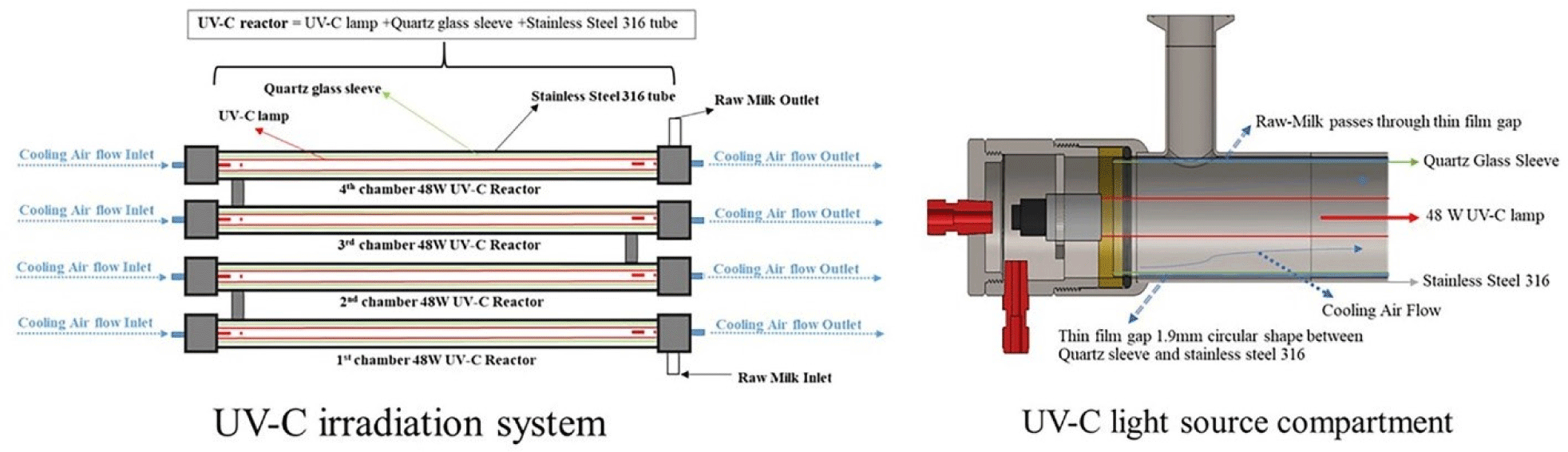
The resulting assembly formed the UV-C irradiation system, allowing the UV light to pass through the compartment as a thin film. Importantly, the system design implemented in this study enables the storage of samples inside the tube, preventing contamination by microorganisms.
The UV-C light sources in this study were conducted using Spectro radiometric methods at the National Institute of Metrology, Thailand. The CAS140CT-154 diode array spectroradiometer with optical probes was used to measure the spectral irradiance at a specified distance of 1.9 mm from the front surface of the quartz glass sleeve.
Computational fluid dynamics (CFD) was used to determine UV-C irradiation by calculating parameters such as velocity, pressure, turbulence, and direction of flow of raw milk in the chamber. The appropriate installation positions of UV-C reactors were also determined by CFD. The integrated UV-C radiance was calculated, using the SpecwinPro® software (Instrument Systems, Munich, Germany), from the spectral irradiance covering the wavelength range from 240 nm to 280 nm.
A key factor that influences the efficiency of UV light disinfection is the fluid turbulence of liquid foods (Koutchma, 2009). In a tube reactor, the turbulence of a liquid is measured by the Reynolds number (Re), which is calculated as follows:
Where ρ is the fluid density or specific gravity (in kg/m3), μ is the dynamic viscosity of the fluid (in kg/m×s), Dh is the hydraulic diameter of the UV reactor (in mm), and ν is the mean fluid velocity (in m/s).
Where L is the length of the UV-C lamp (1,148 mm for 48 W) and T is the flow rate at 2 (0.000033), 4 (0.000067), or 7 (0.000117) L/min (m3/s).
The specific gravity ρ (in kg/m3) was adjusted from the value (D) measured by a lactometer, depending on solids-not-fat and fat percentages in the milk.
For a milk example with 8.5% solids-not-fat and 3.25% fat, ρ is 1,028 kg/m3.
The dynamic viscosity (μ) of raw milk at 30°C was taken from the literature (Geankoplis, 1993) and from the Engineering Tool Box (2012): Food Products–Viscosities. The dynamic viscosity (μ) of raw milk was reported as 0.00149 kg/m×s (Pas).
In this study, we employed the thin-film laminar flow technique to design our UV reactor system. This design approach utilized a specific hydraulic diameter (Dh, mm), which is a function of the cross-sectional area of the UV reactor (A) and its wetted perimeter (P). The dimensions of the reactor were defined with an outlet diameter (Do) of 26 mm and an inlet diameter (Di) of 22.2 mm. The relationship between these dimensions and the hydraulic diameter is further detailed in Eqs. (4), (5), and (6).
Where
The UV-C dose was calculated using Eq. (7) by varying the residence time (or by controlling the flow rates). The UV-C irradiance (221.8±22.2 mWcm2) was obtained by multiplying the UV-C intensity of the lamp at 1.9 mm from the lamp with the transmittance of the quartz glass sleeve (82.35%). Moreover, the transmittance values were measured by the spectroradiometric method using the CAS 140CT-154 Spectrometer (Instrument Systems).
The UV-C lamp was turned on for 5 minutes before pumping raw milk through the UV-C reactor system. Then, the raw milk was run into the UV-C reactors by a magnetic pump, which regulated the specific flow rate. The UV output wattage (144 W) at the germicidal wavelength of 253.79 nm±0.30 nm, with an average flow rate of 3 L/min, was maintained according to the conditions shown in Table 2. The first chamber was designed to hold raw milk (120 L) before passing the milk through the UV-C reactor lamps (pre-UV-C treatment). The second chamber was then used to collect the raw milk after it passed through the UV-C lamps (post-UV-C treatment). Raw milk samples before and after UV-C treatment were collected from both chambers in 3 sterile bottles per treatment (100 mL per bottle) and kept at room temperature before microbiological laboratory testing.
After operation, the UV-C reactors were cleaned in 3 cycles using our standard Cleaning-in-Place (CIP) procedure with a 10% NaOH solution for 10 minutes, followed by a 5% nitric acid solution for 5 minutes. Finally, the reactors were rinsed with water for 5 minutes to complete the CIP process.
For the assessment of UV-C inactivation efficiency on bacteria, bulk milk samples were meticulously chosen from three distinct dairy farms undergoing regular tests at the Bovine Milk Quality Laboratory, Faculty of Veterinary Science, Chulalongkorn University. Each farm had a herd size ranging from 15 to 20 milking cows. The raw bulk milk quality from each farm was graded based on the SPC. Samples with bacterial counts below 3 Log 10 CFU/mL were classified into the low bacteria (L) group, those between 3 Log 10 and 4 Log 10 CFU/mL into the medium bacteria (M) group, and samples exceeding 4 Log 10 CFU/mL were allocated to the high bacteria (H) group. Subsequently, these groups were divided into two portions: one untreated (RAW) and the other treated with UV-C (UVC), forming six subgroups: low bacteria number raw milk (LRAW), low bacteria number UV-C treated milk (LUVC), medium bacteria number raw milk (MRAW), medium bacteria number UV-C treated milk (MUVC), high bacteria number raw milk (HRAW), high bacteria number UV-C treated milk (HUVC). Samples from each subgroup were collected at predetermined intervals before and after UV-C treatment, with each collection involving 30 mL of milk. This procedure was meticulously repeated three times for each subgroup to ensure data reliability. Specifically, these intervals after the treatment process were at the 30th, 60th, 90th, and 120th minute. Directly after each sampling event, comprehensive analyses were conducted, encompassing SPC, coliform bacteria count (CC), coagulase-negative staphylococci count (SC), and lactic acid bacteria count (LBC). Bacterial colonies were enumerated through an automated colony counter (Scan 300®, Interscience, Saint-Nom-la-Bretèche, France), and the pH levels of all milk samples were assessed utilizing a pH meter (Hanna Instruments, Woonsocket, RI, USA).
In this experimental setup, samples with a high bacterial count exceeding 4 Log 10 CFU/mL, referred to as HRAW, were selected for analysis. Immediately following the milking process, the fresh milk samples were promptly transported from the dairy farm. The experimental protocol was meticulously executed within a 30-minute timeframe following milking. The HRAW milk samples were categorized into three distinct groups: the first underwent UV-C irradiation, the second was processed through thermal pasteurization in an open system, and the third remained untreated, serving as the control group. The pasteurization was performed using metal plates and hot water, heating the milk to at least 72°C for a duration of no less than 15 seconds, followed by rapid cooling (Deák, 2014). Following the treatments, milk samples were gathered at 30, 60, 90, and 72-hour intervals. At each interval, samples were obtained from every treatment group, each with a volume of 30 mL, aiming to fortify the reliability of our findings. To guarantee consistency and robustness in our results, this sampling procedure was meticulously replicated three times during the experiment. Immediately after collection, the thiobarbituric acid reactive substances (TBARS) values of the samples were assessed to determine oxidative stability. To ensure the integrity of our experimental setup, all samples were refrigerated at a constant 4°C throughout the experiment. This strategy ensured that the samples were preserved in a controlled environment, thereby reducing any possible variations that might impact the study’s findings.
In a sterile environment, each milk sample was subjected to a 10-fold serial dilution, spanning from 10–1 to 10–8 in phosphate buffer solution. From these dilutions, one millilitre was accurately dispensed into each sterile petri dish. This process was carried out for three samples from every experimental group, with six individual dishes prepared for each dilution step to ensure the reliability and precision of the results.
For the enumeration of microorganisms using the SPC method, one millilitre of each diluted milk sample was plated onto plate count agar in petri dishes. The pour plate technique, as per ISO 4833-1:2013 – Microbiology of the food chain – Horizontal method for the enumeration of microorganisms – Part 1: Colony count at 30°C by the pour plate technique, was followed. The plated samples were then incubated at 35°C for 48 hours to facilitate bacterial growth. Post-incubation, plates exhibiting colony counts ranging from 25 to 250 were selected for enumeration. The number of colonies per millilitre of the sample was calculated and expressed as Log 10 colony-forming units per millilitre (CFU/mL), adhering to the guidelines set forth in the aforementioned ISO standard.
One millilitre from the serial dilutions of the milk sample was precisely transferred into each sterile petri dish. To enumerate coliforms, the pour plate technique was employed using Violet Red Bile agar in accordance with ISO 4832:2006 – Microbiology of food and animal feeding stuffs – Horizontal method for the enumeration of coliforms – Colony-count technique. Following the ISO guidelines, all plates were incubated at 35°C for a period of 24 hours. Post-incubation, plates displaying 15 to 150 colonies characterized by a dark red color and a diameter equal to or greater than 0.5 mm were selected for counting. The number of coliform colonies per millilitre of the sample was then calculated and reported as Log 10 colony-forming units per millilitre (CFU/mL).
Total SC was determined using the spread plating technique on Baird-Parker agar. This method was in accordance with ISO 6888-1:1999 – Microbiology of food and animal feeding stuffs – Horizontal method for the enumeration of coagulase-positive staphylococci (S. aureus and other species) – Part 1: Technique using Baird-Parker agar medium. One millilitre of the diluted milk sample was spread onto each petri dish. Following the ISO standard procedures, all plates were incubated at 35°C for 24 hours. Post-incubation, colonies were confirmed as Staphylococci through Gram staining, catalase testing, and the tube coagulase test. Plates exhibiting 15 to 150 colonies were selected for enumeration, and the number of colonies was calculated and presented as Log 10 colony-forming units per millilitre (CFU/mL).
In accordance with ISO 15214:1998 – Microbiology of food and animal feeding stuffs – Horizontal method for the enumeration of mesophilic lactic acid bacteria – Colony-count technique at 30°C, the pour plate technique was employed using de Man, Rogosa and Sharpe (MRS) agar as the selective medium. Following this standard, 1 mL of the diluted milk sample was pipetted into each sterile petri dish, after which the MRS agar was added. The inoculum and the medium were carefully mixed and then allowed to solidify. All plates were incubated at 35°C for 72 hours. Post-incubation, colonies ranging from 15 to 150 on each plate were counted. The number of colonies was then calculated and reported as Log 10 colony-forming units per millilitre (CFU/mL).
4 N hydrochloric acid was added to 100 mL of the milk sample, and the mixture was filtered. Next, 5 mL of thiobarbituric acid (TBA) solution was added to 5 mL of the clear filtrate and mixed well. The filtrate of TBA mixed solution was placed in a water bath at 100°C for 10 minutes, and after cooling the absorbance at 538 nm was measured. Distilled water was used as a blank sample.
The microbial count (Log CFU/mL), milk pH and TBARS values were compared between groups and within group using R Version 4.0.0 (R Core Team, 2020). The Kruskal–Wallis rank sum test with multiple pairwise comparison by Dunn’s test was used to identify statistical significance. A 95% confidence level was considered statistically significant.
Results
The specific attributes of the UV-C radiation source utilized in this study are detailed in Table 1. We designed and constructed UV reactors specifically to process raw milk samples. The calculations pertaining to the 1.9-mm thin film within the UV-C reactors, which were available in variants of 96 W, 144 W, and 192 W, are outlined in Table 2. The Reynolds number (Re) value observed was 909.48, categorizing the flow within the reactor as a laminar system.
In selecting the optimal power for the UV-C reactor, extensive preliminary testing and literature review were undertaken. These efforts were directed towards finding a balance between effective microbial reduction and the preservation of milk’s inherent quality and flavor. Consequently, a flow rate of 3 L/min was employed for the UV-C reactor set at 144 W for our experiments. This particular power setting was chosen as it offered an optimal equilibrium – sufficient UV-C exposure to significantly decrease bacterial counts while maintaining the natural characteristics and sensory qualities of the raw milk. The exposure time for the milk under this setting was 9.9 s, delivering a UV-C dose of 2.20 J/L. The selection of 144 W over other power settings like 96 W or 192 W was driven by our objective to maintain as much of the raw milk’s original quality and flavor as possible while ensuring food safety through microbial control.
This study assessed the effectiveness of UV-C irradiation at an intensity of 144 W in reducing microbial levels in raw milk. Samples were categorized into three groups based on their initial bacterial loads: high (HUVC), medium (MUVC), and low (LUVC).
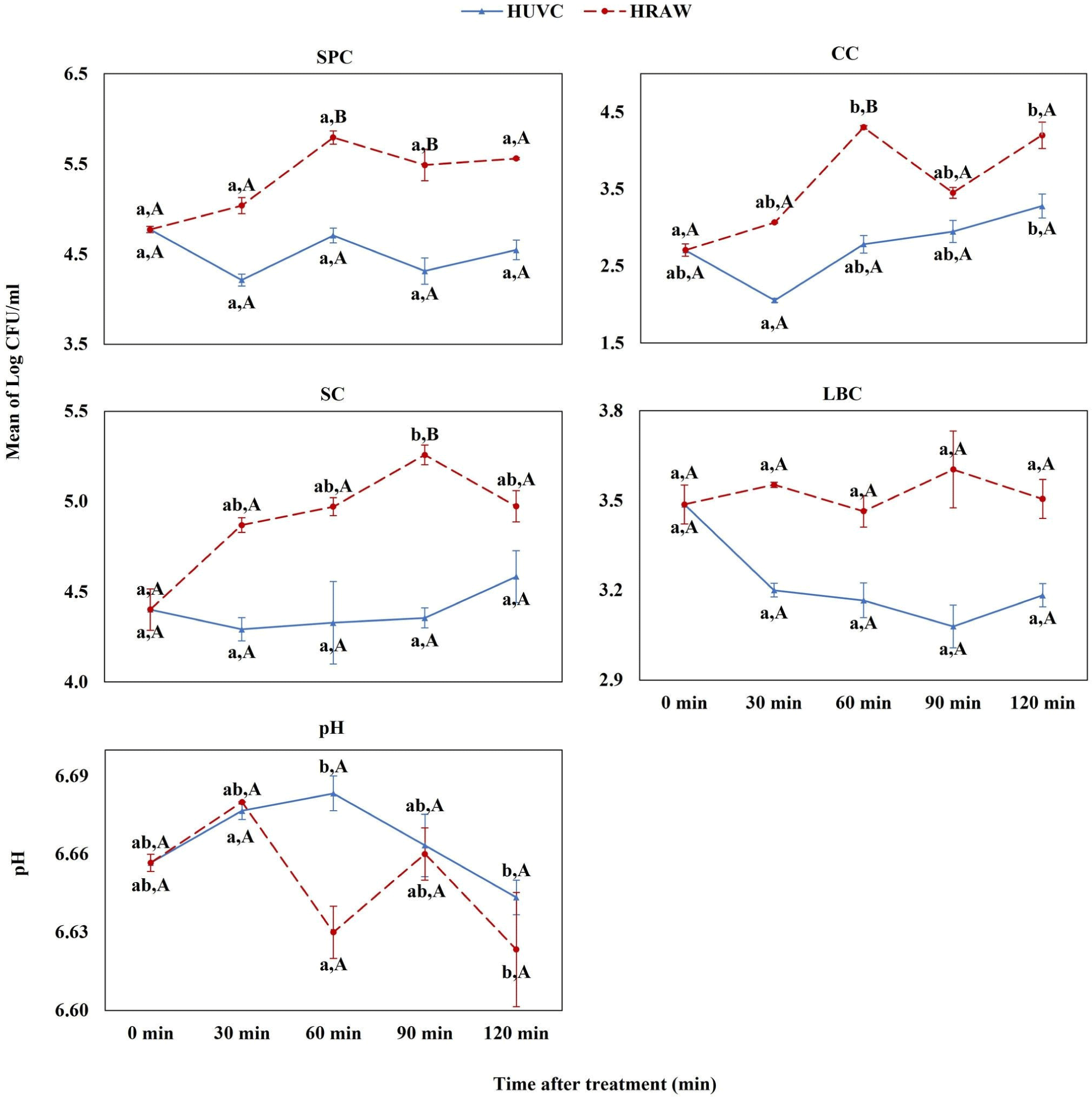
In the HUVC group, variable SPC levels were observed throughout the 120-minute treatment period, generally showing a downward trend from the initial count at time zero (Fig. 2). Notably, the SPC levels at 60 and 90 minutes were significantly lower than those in the raw milk control (HRAW). Additionally, there was a marked reduction in coliform count (CC) at 60 minutes and in SP at 90 minutes, compared to HRAW. A noticeable downward trend was also observed in the LBC beginning at 30 minutes.
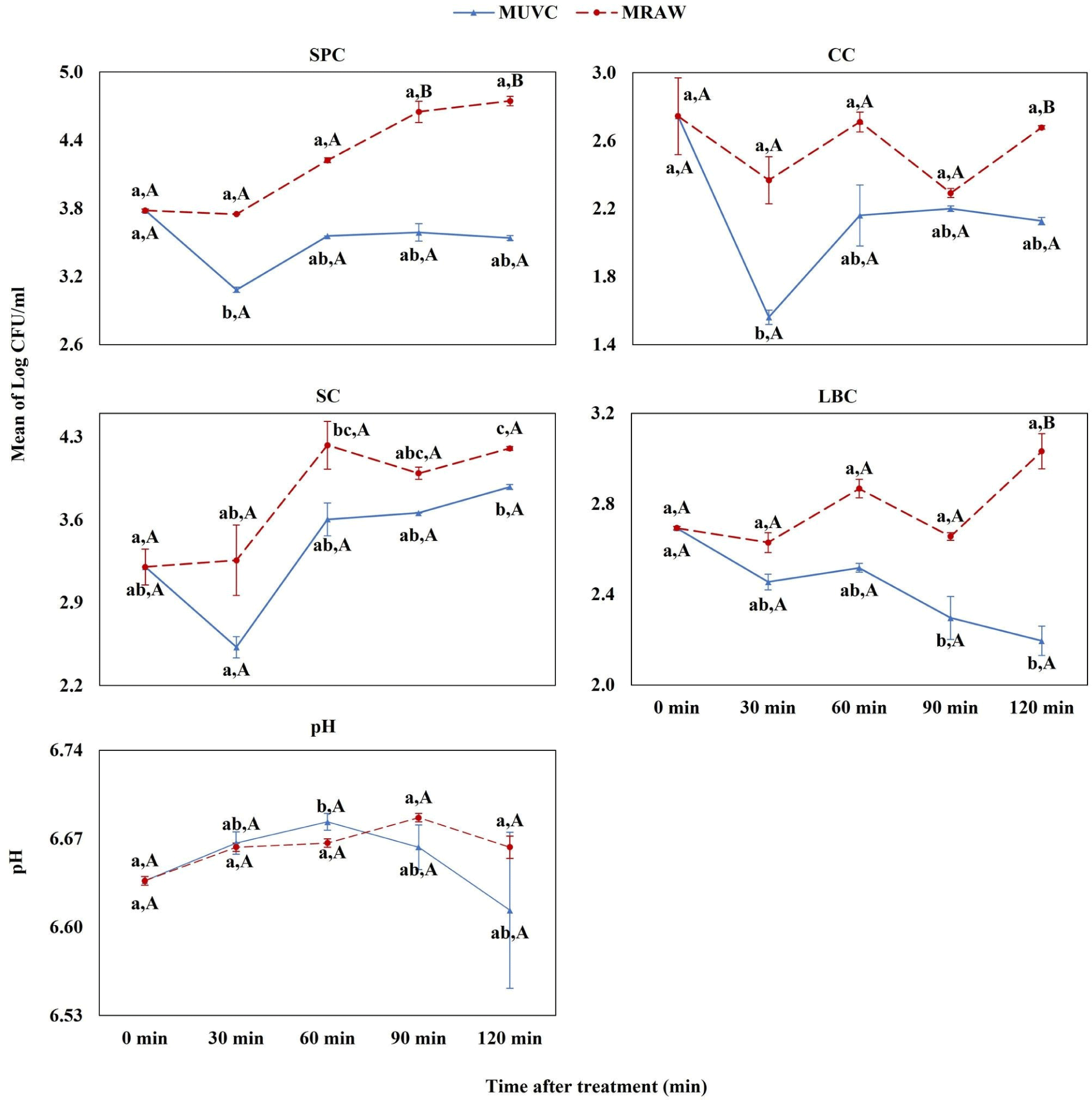
The MUVC group demonstrated a substantial reduction in SPC at 30 minutes compared to the baseline, and this reduction persisted through 60 to 120 minutes (Fig. 3). The UV-C treatment significantly decreased the CC at 30 minutes and appeared to reduce SP at the same interval. However, an upward trend in both CC and SP was recorded at 60 minutes. A significant decline in LBC was noted at 90 minutes, continuing until 120 minutes.
In the LUVC group, there was an initial decrease in SPC levels at 30 and 60 minutes, followed by a significant increase at 90 and 120 minutes (Fig. 4). The UV-C treatment resulted in reductions in CC at 30 and 60 minutes, and in SP at 30 minutes, though these changes were not statistically significant. However, a significant increase in SP was observed at 120 minutes. The LBC did not exhibit any significant inhibitory response to the UV-C treatment within this group.
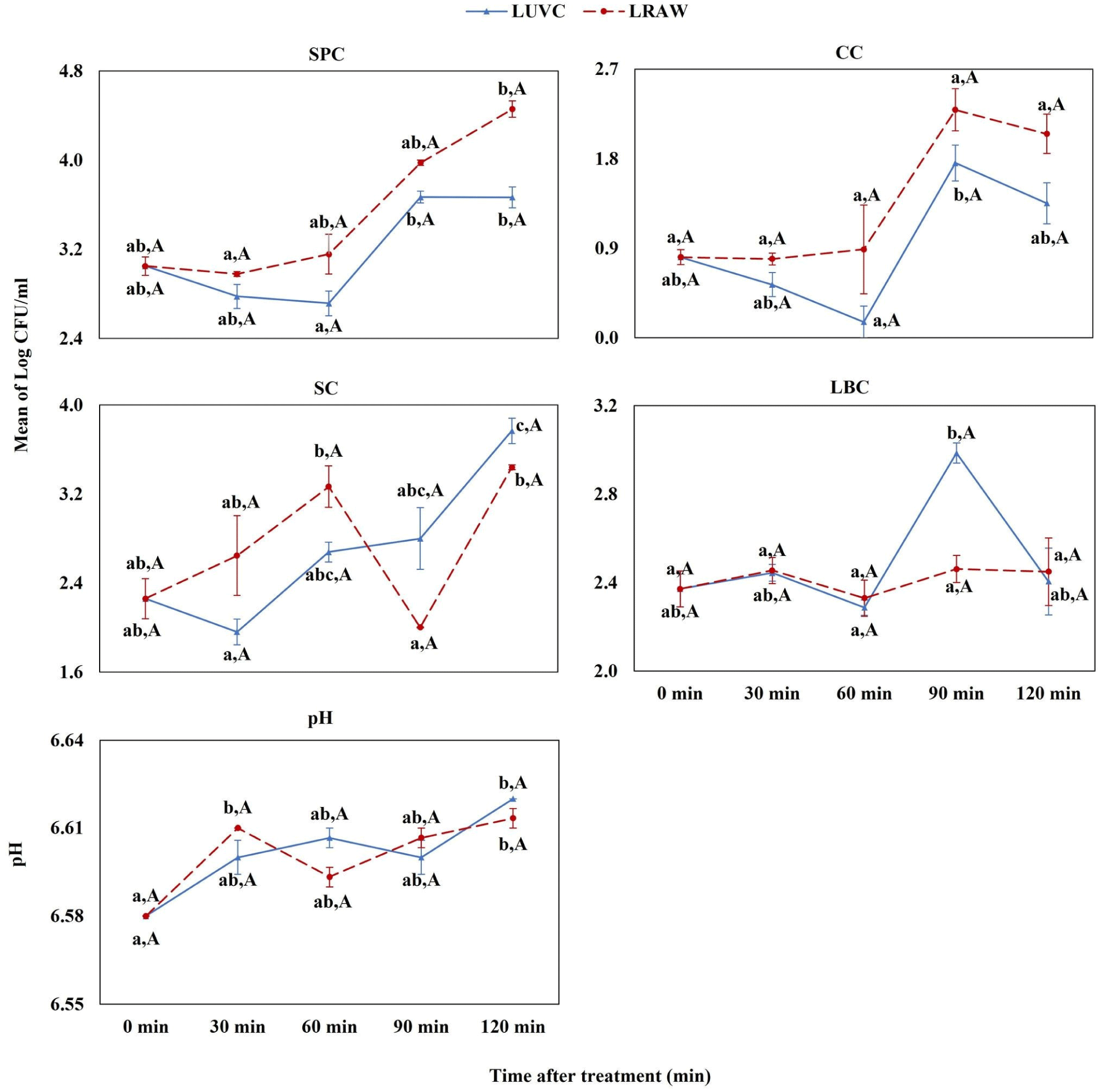
Upon UV-C treatment at 144 W, we observed dynamic changes in the pH of milk samples. In the high (HUVC) and medium (MUVC) groups, there was an initial increase in pH at 30 minutes, which became more pronounced and statistically significant at 60 minutes. This was followed by a noticeable decline in pH levels at 90 and 120 minutes for these groups (refer to Figs. 2 and 3 for detailed trends). Conversely, in the low group (LUVC) and the control raw milk (LRAW), we detected a slight elevation in pH post-treatment, with a significant increase observed at 120 minutes compared to pre-treatment values, as illustrated in Fig. 4.
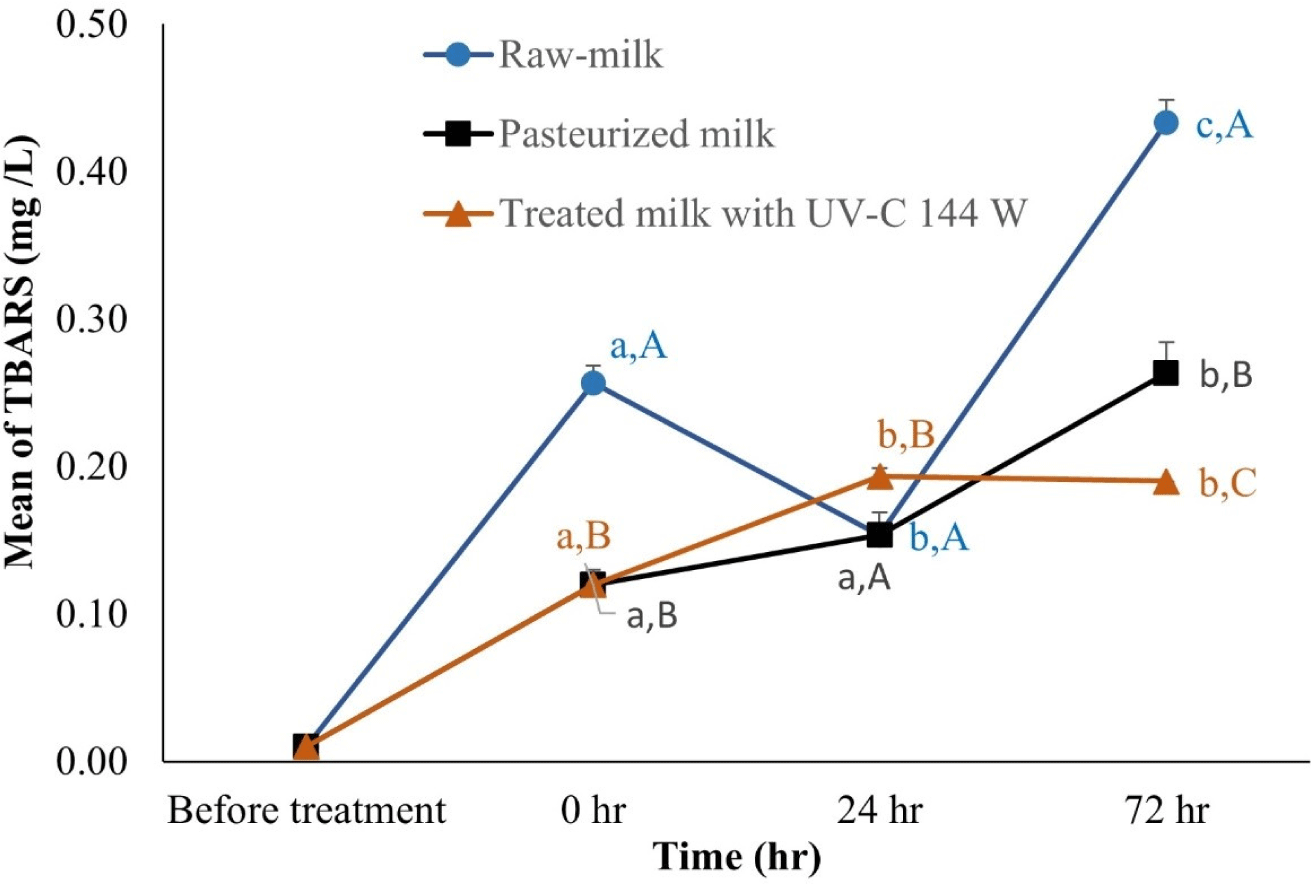
To assess oxidative deterioration, we analysed TBARS in the treated milk. By comparing TBARS values across raw milk, pasteurized milk, and milk treated with UV-C at 144 W (Fig. 5), we noted a correlation between increasing TBARS values and prolonged storage time. Remarkably, the highest TBARS value was recorded in raw milk at 72 hours. In a compelling contrast, milk that underwent UV-C treatment at 144 W demonstrated significantly lower TBARS values than both raw and pasteurized milk after 72 hours. Additionally, the peroxide values in milk treated with the UV-C reactor closely aligned with the observed TBARS trends, although detailed data is not shown. These findings imply that UV-C treatment at 144 W effectively mitigates lipid peroxidation in raw milk within a 72-hour window.
Discussion
Smallholder dairy farmers in tropical regions often face challenges in maintaining the quality of raw milk during transportation to collection centers, primarily due to the prohibitive costs associated with temperature regulation (Sudarwanto et al., 2015). In the current study, efforts were made to optimise the UV-C treatment conditions by using a higher level of UV-C reactors (144 W) in order to maintain raw milk quality while transporting the milk to the MCC. The flow rates and UV-C power used were based on the conditions commonly used in the dairy farm industry environment. Our findings demonstrate that the UV-C reactor, operating at 144 W, exhibited high inactivation efficiency for aerobic bacterial counts. This treatment effectively controlled the growth of microorganisms for a duration of up to 120 minutes. This condition is sufficient to maintain the microbiological quality of raw milk during transportation to collection centres.
The efficiency of UV-C irradiation in reducing various bacterial groups in raw milk, such as CC, SC, and LBC, has been documented in prior studies. For example, several studies have revealed that post UV-C treatment, both CC and SC (Biancaniello et al., 2018; Engin and Karagul Yuceer, 2012), as well as LBC (Atik and Gumus, 2021), became undetectable in raw milk samples. However, our study found that in the high (H) and medium (M) groups, the quantities of CC, SC, and LBC were lower in UV-C-treated samples compared to the untreated group. We observed that these bacteria exhibited varying sensitivities to UV-C light; coliforms were the most sensitive in the H and M groups, while SC was the least sensitive across all groups. This aligns with previous research indicating that UV-C is most effective against coliforms (Reinemann et al., 2006), followed by other microbes like Staphylococcus spp. (Engin and Karagul Yuceer, 2012). Factors such as the nature and type of the microbe, UV-C sensitivity (Crook et al., 2015), initial microbial count (Atik and Gumus, 2021), growth phases, microbial DNA repair capabilities, and recovery conditions play significant roles in the inhibitory efficacy of UV-C (Nurcan et al., 2018; Singh et al., 2021). During our observation period, although the growth of coliforms, Staphylococcus spp., and lactic bacteria was not completely inhibited by the 144 W UV-C treatment, the majority of the milk treated under these conditions exhibited lower levels of these microbes compared to the untreated samples. Notably, the UV-C-treated raw milk in the H and M groups was more effective in reducing microbial populations than in the low (L) group. UV-C treatment has been shown to reduce microbial loads by more than 10-fold (Log 10), particularly in raw milk with microbial counts exceeding 104 CFU/mL (Crook et al., 2015). Utilizing higher-powered UV-C reactors and doses has demonstrated a more pronounced effect on microbial counts (Crook et al., 2015). In our study, we employed the lowest feasible UV-C dose to preserve the quality and flavor of the raw milk. This approach is especially beneficial in tropical countries, where high temperatures and humidity can accelerate microbial growth. UV-C treatment offers a significant advantage in dairy farms with high total bacterial counts in bulk milk, particularly in tropical regions. Future studies are encouraged to broaden their scope by examining the efficacy of UV-C treatment across a wider range of bacterial species, including Pseudomonads spp., spore-forming bacteria, Mycobacterium spp., and Coxiella spp., to provide a comprehensive assessment of its antimicrobial capabilities. Additionally, incorporating an analysis of microbial recovery characteristics will be crucial. This approach will not only enhance our understanding of the resilience and adaptive responses of microbial populations following UV-C exposure but also elucidate the potential long-term effects on food safety and quality. Such comprehensive investigations will significantly contribute to optimizing UV-C treatment protocols, ensuring both the efficacy of microbial reduction and the maintenance of product integrity.
In all the high (H), medium (M), and low (L) groups, we observed consistent directional changes in the pH of raw milk samples, regardless of whether they were subjected to UV-C treatment or remained untreated. Across these groups, the pH values of nearly all samples, both UV-C-treated and untreated (RAW), fell within the range of 6.58 to 6.7. Our findings indicate that UV-C treatment does not exert a significant influence on the pH of raw milk, corroborating the research of Bandla et al. (2012a). They observed that during a 7-day storage period, the pH levels of both UV-C-treated and untreated raw milk samples consistently stayed within the typical pH range for raw cow milk, 6.6 to 6.8 (Bandla et al., 2012a; Choudhary et al., 2011). In a similar study, Orlowska et al. (2013) reported no significant change in the pH (6.68±0.02) of pasteurized milk samples following UV treatment (Orlowska et al., 2013). However, these findings contrast with those of Hu et al. (2015), who noted an increase in the pH of raw milk samples after UV-C treatment, a pattern consistent with our observations in the low (L) group. Hu et al. also posited that the pH alterations in the food matrix could be influenced by the presence of UV-C light-absorbing components within the system (Hu et al., 2015).
Lipid peroxidation is one of the main causes of quality deterioration and unpleasant odour and flavour of milk and milk products (Ajmal et al., 2018; Halliwell et al., 1995; Lin et al., 2018). TBARS are naturally present in biological specimens and include lipid hydroperoxides and aldehydes, whose concentrations increase as a response to oxidative stress (Zeb and Ullah, 2016). TBARS concentration in milk was used as another marker for accumulation of secondary lipid peroxidation products (Hedegaard et al., 2006; Li et al., 2019). Lower lipid peroxidation indicates a high quality of milk. In a previous report, raw milk treated with 39-W and 48-W UV-C reactors showed only a small difference in their TBARS values (Makarapong et al., 2020). In our study, TBARS levels were found to be significant across the three types of milk analysed. Notably, milk treated with a 144 W UV-C reactor exhibited lower TBARS values than both raw and pasteurized milk. This suggests that UV-C treatment effectively reduces TBARS levels, indicative of diminished lipid peroxidation. Furthermore, the stability of this reduction was observed up to 72 hours, highlighting the potential of UV-C treatment in limiting lipid peroxide formation (data not shown).
However, it is important to consider that variations in experimental setups and conditions can influence the extent of photooxidation, thereby impacting TBARS measurements. This consideration could explain the discrepancies between our results and those reports (Johnson et al., 2015; Webster et al., 2009). While their studies highlighted the susceptibility of milk to light-induced oxidation, our findings suggest that under specific UV-C treatment conditions, the impact on TBARS levels can be markedly reduced.
Additionally, our results imply that UV-C treatment might serve as an effective alternative to traditional cooling systems for maintaining milk quality. The observed stability of TBARS levels under our UV-C treatment conditions indicates its potential for preserving milk quality during transportation over extended periods, potentially enhancing shelf life.
Given these findings, further investigation into protein oxidation and the extent of such oxidation under UV-C treatment is warranted. This would provide a more comprehensive understanding of the effectiveness of UV-C treatment for raw milk preservation.
Milk and dairy product management of has greatly benefited from technological advancements. Our study, development and validation of UV-C treatment of milk provides a workable alternative pre-treatment of raw milk in dairy farms prior to the normal pasteurisation process, especially in Thailand and other tropical countries. This study shows that raw milk pre-treatment with UV-C irradiation to reduce bacterial growth and lipid peroxidation is highly effective. After keeping in refrigerator for 24–72 hours, TBARS increased in all groups. Pasteurized milk and UVC-treated milk controlled and reduced a number of microorganisms after treatment whereas the number of microorganisms and lipid oxidation increased faster in the untreated milk group. While the TBARS assay provides valuable insights into oxidative stability, a comprehensive assessment of milk quality post-UV-C treatment necessitates the examination of additional parameters. Future studies should, therefore, extend to evaluating Bile Salt-Stimulated Lipase activity, Alkaline Phosphatase activity, free fatty acid profiles, and vitamin content alongside milk flavour, colour, texture, nutritional values, and bioactive components. Such a multifaceted approach will not only enhance our understanding of UV-C irradiation’s impact across a broad spectrum of quality indicators but also assess its practicality and economic viability for quality control in raw milk production.
Conclusion
Our study addressed tropical dairy challenges through UV-C treatment optimization (using a 144 W reactor and a milk flow rate of 3 L/min) to maintain milk quality during transport. UV-C effectively controlled microbes for 120 minutes, with varying effects on CC, SC, and LBC. Notably, UV-C exhibited significant bacterial reduction across the H, M, and L groups, with the H/M group displaying superior results. Following UV-C treatment, the pH remained stable. Successful reduction in lipid peroxidation led to an enhancement in milk quality. To comprehensively evaluate milk quality, further investigation into protein oxidation and broader quality facets is recommended. Our UV-C approach could revolutionize milk quality control in evolving dairy practices, with economic viability requiring further investigation.













