Introduction
The per capita consumption of meat (pork, beef, chicken) in South Korea has increased by 74% over the past 20 years, from 33.5 kg in 2002 to 58.4 kg in 2022. This represents a significant increase of 4% in just one year (KREI, 2023). This trend is likely to continue as consumer demand for more convenient and diverse meat products grows. Furthermore, with the introduction of new, more compact cooking appliances and devices, the availability and sales of frozen meat and frozen meat products have also expanded (Havelaar et al., 2010; Moon et al., 2021). As such, the meat and meat processing industry is highly dynamic, and with increasing consumer demand and diverse needs, producers, processor, and distributors are all introducing various safety technologies to ensure the quality and sustainability of meat products. The processing and preservation technologies for meat and meat products require a lot of cost and time, but it is difficult to maintain the fresh form of the raw materials without damaging its quality. In the meat processing industry, various research is being conducted to overcome this challenge, and new technologies have shown advantages and possibilities in their application, but more research is needed for industrial application (Baptista et al., 2022; Dunn, 1996; Fernández et al., 2020; Ganan et al., 2013; Haughton et al., 2011; Hierro et al., 2012; Keklik et al., 2010b; Paskeviciute et al., 2010; Tomašević, 2015).
In order to overcome the thermal degradation of products resulting from heat treatment processes widely used in the food industry, new processes are being researched. One of these processes is the development of technologies utilizing electrical energy. Electrical energy-based technologies include high voltage pulsed electric fields (PEF), intense pulsed light (IPL), nonthermal plasma, cold plasma, high voltage arc discharge, and electron beam irradiation (Choi et al., 2010; Ortega-Rivas, 2012; Shin et al., 2010). These technologies do not significantly increase the temperature of foods, have short processing times, are mostly continuous, and do not significantly alter the physical, chemical, and nutritional properties of the food after treatment. Also, electrical non-thermal processing technology is attracting attention as an environmentally friendly technology that reduces CO2 generation and consumes less energy compare to thermal processing in terms of energy consumption.
IPL technology was initially used in the fields of cosmetics, skin care, and hair care but expanded to food and pharmaceutical areas after Food and Drug Administration (FDA) approval in 1996 (FDA, 1996). Currently, IPL technology is being researched for its application to a variety of food products, including fresh fruit and vegetables, colorless beverage or water, dairy products such as milk and cheese, seafoods like fish and shrimp, and pork, beef, poultry, and processed meat products (Baptista et al., 2022; Chakraborty et al., 2023; Cheigh et al., 2013; Fernández et al., 2020; Hong et al., 2013; Vargas-Ramella et al., 2021). Industrial application for water and beverage is also underway (Mandal et al., 2020). This study aims to present the potential of IPL technology for industrial application in meat processing through a summary of the system configuration of IPL technology and a summary of research results on animal-based products.
Intense Pulsed Light System
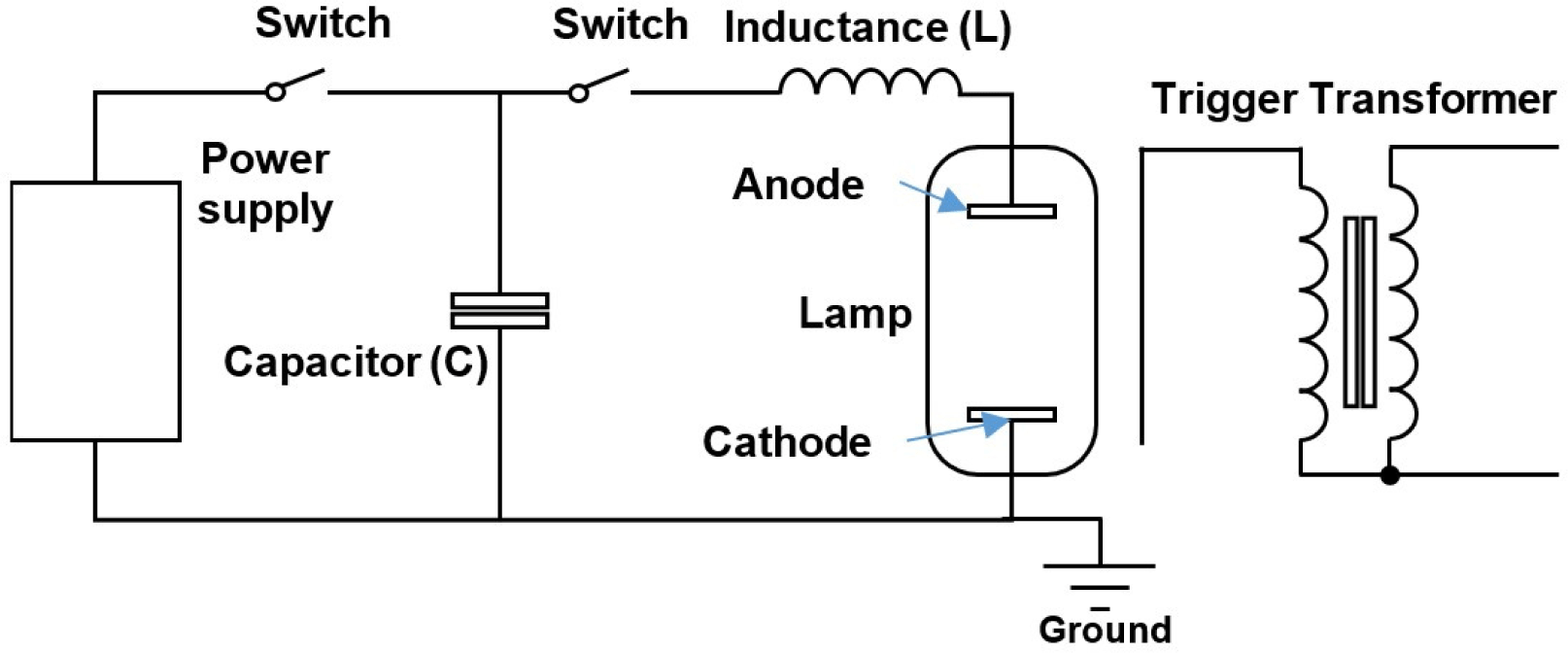
The IPL sterilization technology, one of non-thermal sterilization methods, use a lamp filled with a specific gas (ex. Xenon gas or Krypton gas) to generated light at wavelengths similar to sunlight. IPL technology is also known as high intensity pulsed ultraviolet light, high voltage IPL, pulse ultraviolet (UV)-light, and pulsed white light (Choi et al., 2010; Marquenie et al., 2003; Roberts and Hope, 2003; Sharma and Demirci, 2003; Shin et al., 2010). IPL systems use devices that operate on the same principles as PEF. However, they have several key differences: 1) the voltage range used in the IPL system is lower than that used in PEF, 2) the IPL system uses trigger power, 3) the IPL system use a lamp with a broad spectrum instead of the treatment vessel used in PEF (Fig. 1). The wavelength range used in IPL is similar to that of sunlight, spanning from 100 nm to 11,000 nm. It includes the UV region as well as the near-infrared and visible light regions, setting it apart from UV sterilization method (Dunn, 1996; Heinrich et al., 2016). IPL treatment involves irradiating target materials with pulses of energy density ranging from 0.01 to 50 J/m2 and pulse width ranging from 0.1 μs to 1 ms, occurring from 1 to many times. The light source is typically a clear fused quartz tube filled with xenon gas at a pressure of about 450 torr. Typically, the light source is a clear fused quartz tube filled with xenon gas at a pressure of about 450 torr. The xenon lamp is maintained in an excited state by a constant voltage, and when the trigger voltage is applied, the excited xenon gas emits intense light (approximately 20,000 times the intensity of sunlight) in a large flash.
Inactivation and Repair Mechanism
The most commonly used lamp for IPL treatment is the xenon lamp, with the UV-C part being the most critical wavelength range for microbial inactivation. Mandal et al. (2020) and Rowan et al. (1999) reported on the inactivation of various microorganisms (Bacillus subtilis, Escherichia coli, Aspergillus niger, Staphylococcus aureus, Saccharomyces cerevisiae, Listeria monocytogenes, Pseudomonas aeruginosa, Salmonella Enteritidis, Fusarium culmorum) in agar, media and buffer. They found that conventional UV treatment resulted in a reduction of 1–3 Logs/plate, while IPL treatment achieved a maximum reduction of up to 8.7 Logs/plate. The inactivation effect of E. coli varied depending on the wavelength used. E. coli showed high sterilization effect in the 230–360 nm range, with the maximum inactivation rate at around 270 nm. No significant germicidal effect was observed above 300 nm (Wang et al., 2005). The inactivation mechanism of microorganisms by IPL treatment is thought to be due to a combination of photo-chemical, photo-thermal, and photo-physical effects rather than by any one of these effects (Gómez‐López et al., 2005; Heinrich et al., 2016; Mandal et al., 2020). Many studies have reported that the main inactivation mechanism is due to photo-chemical effects, and some researches have reported that temperature increases during IPL treatment is less than 2°C, indicating that the photo-thermal effect is almost negligible (Kim and Shin, 2014). The photo-chemical effects are thought to be due to DNA damage, including clonogenic death, double-/single-strand DNA breaks, and cyclobutene dimer formation (Cheigh et al., 2013; Takeshita et al., 2003). Some studies have reported that the photo-thermal effect is a possible inactivation mechanism by IPL treatment, even though the temperature of the surface of food matrix dose not increase during IPL treatment. These studies reported that cells absorb light energy, leading to their rupture and subsequent death due to overheating (Dunn et al., 1989; Farrell et al., 2010; Wekhof, 2000; Wekhof et al., 2001). The photo-physical effects are reported to result in the destruction of microorganisms by cell membrane damage, vacuole expansion, and structural changes in cells, as well as the leakage of proteins. This effect is observed when exposed to strong light (with energy levels exceeding 0.5 J/m2) emitted from the lamp (Elmnasser et al., 2007; Wekhof et al., 2001; Wuytack et al., 2003). IPL treatment can leave some bacteria partially damaged rather than completely inactivated. Damaged bacteria may recover from damage through photoreativation or dark repair mechanism (Cleaver, 2003; Jungfer et al., 2007; Setlow, 1992). Photoreactivation is the simplest DNA repair mechanism currently known. Pyrimidine dimers induced by UV irradiation are repaired by the action of photoreactivating enzyme using light in the 310–500 nm range. During/or after IPL treatment, damaged cells can be repaired by a similar mechanism (Farrell et al., 2010; Otaki et al., 2003).
Processing Factors of Intense Pulsed Light Treatment
There are several factors that affect the inactivation of microorganisms by IPL treatment. The process variables include the type of gas filled in the lamp (Park and Shin, 2021; Qaiser et al., 2020), light intensity, the sensitivity of the sample to light, the light transmission medium, the distance between the lamp and the sample, frequency, pulse width, treatment time, and more (Kim and Shin, 2014; Park, 2017; Park, 2021). Lamps used in IPL treatment are filled with inert gas, and the main wavelength range of light varies depending on the type of gas used for filling. In general, xenon gas and krypton gas are used in IPL system lamps. When krypton gas is used, it emits red-yellow light in the range of 476–647 nm, whereas xenon gas filled lamp emit white light in the range of 180–300 nm. Therefore, when xenon gas is used instead of krypton gas, it generates effective UV range light, making it more effective for sterilization. Currently, xenon gas is the only approved for food use by the U.S. FDA (FDA, 1996; Park and Shin, 2021; Qaiser et al., 2020). The closer the distance between the lamp and the sample, the greater the sterilization effect, as the sample directly absorbs a greater amount of light energy emitted by the lamp (Kim and Shin, 2015; Ozer and Demirci, 2005). It has been reported that 1–20 pulses are sufficient for microbial sterilization (Dunn et al., 1989). There is a proportional relationship between the number of pulses and the lethal effect, but the sterilization rate does not increase continuously as the number of pulses increases. In some reports, a single pulse can result in a significant reduction in microorganisms if it has sufficient fluence (Anderson, 2000; Levy et al., 2012; MacGregor et al., 1998). Moreover, it is generally reported that single pulse treatment with higher energy is more effective than several pulse treatments with low energy (Anderson, 2000). The effect of pulse frequency on microbial inactivation is reported with conflicting results. Luksiene et al. (2007) reported that the pulse frequency does not have a significant effect on the inactivation of microorganism in the range of 1–5 Hz, while Kim and Shin (2015) report a proportional increase in inactivation with higher pulse frequencies. Pulse frequency is limited by the safety and efficiency of the device due to the heat generated by the lamp, thus limiting the potential for achieving significant microbial inactivation through only pulse frequency. As a result, pulse frequency is used in the range of 0.5–10 Hz (Ortega-Rivas, 2012). It is expected that more research in the future will lead to a clearer understanding of the effect of pulse frequency on cell inactivation.
The thickness of the sample also affects the sterilization effect, with thicker sample resulting in a reduced effect. This is due to the limitation of light penetration depth, and the effect is even more pronounced in opaque samples (Kim et al., 2013). Since IPL treatment is a method of microbial sterilization by light, the shadow effect can also impact microbial sterilization. When the concentration of microorganisms is high and they form a layer, it can lead to a reduction in the inactivation effect due to the shadowing effect (Gómez‐López et al., 2005). Gómez‐López et al. (2005) reported that when Photobacteriun phosphoreum, L. monocytogenes, and other microorganisms were exposed to a agar plate containing various food components, the presence of protein or fats resulted in a reduction in microbial inactivation, while the addition of water or starch to the medium has a less significant impact on microbial inactivation. Additionally, Shin et al. (2012) reported that there was some difference in the inactivation rate of E. coli depending on the color of the medium, but the difference was not significant.
Meat and Processed Meat Products
IPL technology is mainly used to sterilize the target object surface. Research on the application of IPL in meat treatment aims to reduce the contamination of microorganisms on the surface of meat to extend shelf life. The target meats used in the study are mainly beef, pork, and chicken (Table 1), and there are also many studies on meat products such as ham and sausages (Table 2). Some research has also focused on meats such as venison and rabbit meat. Research on fresh meat is more active in poultry, mainly on chicken breasts, examining the difference in disinfection depending on the presence or absence of skin (Baptista et al., 2022; Haughton et al., 2011; Keklik et al., 2010b; Paskeviciute et al., 2010). The target microorganisms in poultry processing were Salmonella Typhimurium, Campylobacter jejuni, and L. monocytogenes. Various treatment conditions, such as treatment time, irradiation energy dose, and distance from the light source, were applied. The degree of microbial reduction in chicken meat by IPL treatment varied depending on the treatment conditions and the type of microorganism, but it showed values of about 0.4–2.4 Log. Keklik et al. (2010b) reported that IPL treatment of boneless chicken breast inoculated with S. Typhimurium resulted in a decrease of 1.2–2.4 Log for the unpackaged sample and 0.8–2.4 Log for the vacuum-packaged sample. The difference in sterilization degree between the two samples was not large. Paskeviciute et al. (2010) found that IPL treatment at an energy dose of 5.4 J/cm2 reduced the counts of inoculated S. Typhimurium and L. monocytogenes by 2–2.4 Log in skinless chicken breast. Haughton et al. (2011) reported that C. jejuni was reduced by 0.91 Logs, E. coli by 1.51 Logs, and S. Enteritidis by 1.2 Logs in skinless chicken breast when the samples were treated for 30 seconds at a distance of 2.5 cm from the light source. On chicken skin, there was an average reduction of 1.2 Logs. Baptista et al. (2022) also reported a reduction of approximately 0.4 Logs when IPL treatment was applied to poultry meat breast with C. jejuni inoculation using an energy density of 2.82–9.68 J/cm2. There are reports the change in the physico-chemical quality of meat, such as color, thiobarbituric acid reactive substances (TBARS), lipid oxidation, and sensory analysis, due to IPL treatment (Fernández et al., 2020; McLeod et al., 2017; Ojha et al., 2017). IPL technology is a light-based technology that can darken or brown the color of the sample as the processing condition become more severe. Oxidation of lipid can occur, resulting in changes in odor. Tomašević (2015) conducted color and sensory evaluation on various fresh meat, including beef, pork, chicken, turkey, venison, rabbit, and kangaroo, with IPL treatment at energy levels 3.4 J/cm2 and 17 J/cm2, followed by color and sensory evaluation. In this study, there were no changes in color for chicken and rabbit meat, but pork and turkey meat exhibited changes in terms of CIE a* and CIE b*. The sensory evaluation revealed a decrease in the overall preference for odor in all samples, indicating that IPL treatment has the most significant impact on aroma.
| Food product | Microorganism | Treatment condition | Reductions | Reference |
|---|---|---|---|---|
| Boneless chicken breast | Salmonella Typhimurium | Unpackaged or vacuum-packaged 5, 15, 30, 45, 60 s/distances 5, 8, 13 cm | Unpackaged: 1.2–2.4 Vacuum packaged: 0.8–2.4 | Keklik et al. (2010b) |
| Skinless chicken breast | S. Typhimurium Listeria monocytogenes | 1,000 pulses, treatment duration 200 s, 5.4 J/cm2 | 2–2.4 | Paskeviciute et al. (2010) |
| Raw chicken | Campylobacter jejuniEscherichia coliSalmonella Enteritidis | 2, 30 s (7.08, 106.2 J/cm2)/distances 2.5 cm | C. jejuni: 0.46–0.91 E. coli: 1.26–1.51 S. Enteritidis: ~1.5l | Haughton et al. (2011) |
| Poultry meat breasts | C. jejuni | 2.82–9.68 J/cm2 | 0.4 | Baptista et al. (2022) |
Paskeviciute et al. (2010) conducted a triangle test to compare the odor, taste, and flavor of fresh meat, cooked meat, and chicken broth from IPL-treated skinless breast chicken with trained panelists. The panelists could not distinguish the difference in odor, taste, and color from the control samples when IPL was irradiated at 5.1 J/cm2. However, when IPL treatment was increased to 6 J/cm2 or higher, resulting in a surface temperature increase of the chicken surface to 54°C, all panelists were able to discern differences in odor compared to the control samples, but they could not detect any difference in the taste of the chicken broth. These studies suggest that IPL treatment can influence the color, lipid oxidation, sensory attributes, and physico-chemical quality of different types of fresh meat, with the effect varying depending on the treatment conditions and energy levels used.
In summary, IPL treatment of fresh meat is primarily focused on poultry, especially chicken, and research on beef and pork is still limited. The effects of IPL treatment on chicken breast vary depending on microorganisms and processing conditions, but it results in a decrease of 0.4–2.4 Log. After IPL treatment, there is generally no significant difference observed in quality, and no differences ware found even after cooking evaluations.
Research is underway on the reduction of microorganisms and quality changes in processed meat products by IPL treatment (Table 2). The target samples are frankfurter or bologna sausage, boiled ham, cured meat products, and carpaccio (Fernández et al., 2020; Ganan et al., 2013; Hierro et al., 2011; Hierro et al., 2012; Keklik et al., 2009; Kramer et al., 2019; Liu et al., 2019; Wambura and Verghese, 2011), and the target microorganisms are L. monocytogenes, Listeria innocua, and S. Typhimurium. When L. innocua was inoculated onto frankfurter and ham products and then treated with IPL, the packaged samples showed a reduction of approximately 0.1–0.9 Log, while unpackaged samples exhibited a reduction of around 0.3–2.0 Log (Fernández et al., 2020; Hierro et al., 2011; Keklik et al., 2009; Liu et al., 2019). In a study by Kramer et al. (2019), when L. innocua was inoculated onto sliced boiled ham, cured chicken cold cuts, and frankfurter sausage and then treated with energy levels of 0.56, 1.2 and 3.6 J/cm2, sliced boiled ham and cured chicken cold cuts showed a decrease of 1 Log regardless of the initial bacterial inoculation level, while frankfurter sausage exhibited a reduction of 3–4 Log. This is attributed to the smoother surface of sausage, which has less irregularity and no shadow effect, resulting in a higher lethality. Ganan et al. (2013) and Hierro et al. (2012) inoculated S. Typhimurium into beef carpaccio and cured meat products (salchichón and loin) and observed a reduction in microorganisms by IPL treatment. When treated with fluences of 0.7–11.9 J/cm2, beef carpaccio showed a reduction of 0.3–1 Log, while cured meat products showed a decrease of –0.26 to 1.48 Log for salchichón and 0.51–1.73 Log for loin. The result showed that the reduction levels were similar, although there were some differences depending on the type and part of the meat. In addition, it has been reported that there is a germicidal effect on various microorganisms such as E. coli, Micrococcus, Staphylococcus, molds and yeast, and lactic acid bacteria (Hierro et al., 2011; Liu et al., 2019).
| Food product | Microorganism | Treatment condition | Reductions | Reference |
|---|---|---|---|---|
| Chicken-based frankfurters | Listeria monocytogenes | 5, 15, 30, 45, 60 s/distances 5, 8, 13 cm | Unpackaged: 0.3–1.9 Vacuum packaged: 0.1–1.9 | Keklik et al. (2009) |
| Sliced boiled ham, cured chicken cold cuts, frankfurter | Listeria innocua | 0.56, 1.2, 3.6 J/cm2 | Sliced boiled ham: 1 Cured chicken cold cuts: 1 Frankfurter: 3–4 | Kramer et al. (2019) |
| Packaged heat-processed cooked ham bologna | L. monocytogenes | 0.7, 2.1, 4.2 and 8.4 J/cm2 | Packaged heat-processed cooked ham: 1.78 Bologna: 1.11 | Hierro et al. (2011) |
| Cured meat products (salchichón and loin) | L. monocytogenesSalmonella Typhimurium | 0.7, 2.1, 4.2, 8.4, 11.9 J/cm2 | L. monocytogenes salchichón: 0.89–1.81 Loin: 1.01–1.61 S. Typhimurium Salchichón: 0.26–1.48 Loin: 0.51–1.73 | Ganan et al. (2013) |
| Chinese traditional dry-cured meat products (pork) | Micrococcus and Staphylococcus molds and yeasts Lactic acid bacteria Escherichia coli | 8 J, distance 10 cm | Micrococcus and Staphylococcus: 2.39 Molds and yeasts: 1.17 Lactic acid bacteria: increased 0.58 E. coli: not detected | Liu et al. (2019) |
| Iberian ham, serrano ham | L. innocua | 2.1, 4.2, 8.4 J/cm2 | Iberian ham: 2 Serrano ham: 1 | Fernández et al. (2020) |
| Beef carpaccio | L. monocytogenesE. coliS. Typhimurium | 0.7, 2.1, 4.2, 8.4, 11.9 J/cm2 | L. monocytogenes: 0.3–0.9 E. coli: 0.6–1.2 S. Typhimurium: 0.3–1.0 | Hierro et al. (2012) |
The impact of IPL treatment on product color was found to be independent of fluence, storage period, and the specific type of sample in some studies (Ganan et al., 2013; Kramer et al., 2019; Wambura and Verghese, 2011). However, other studies reported changes in color attributes. For example, regardless of packaging, CIE b* increased after IPL treatment in one study (Keklik et al., 2009). In another study, CIE L*, CIE a*, and CIE b* values all decreased over the storage period, signifying color changes (Hierro et al., 2012). Additionally, the response to IPL treatment could differ for similar products based on treatment conditions, as seen in the study by Hierro et al. (2011). In this case, packaged heat-processed cooked ham showed limited color changes even at a high fluence of 8.4 J/cm2, while bologna exhibited significant reductions in CIE L* value and an increase in CIE b* value with no significant change in CIE a* value in treatment groups of 4.2 J/cm2 or higher. This suggests that the effect of IPL treatment on color can vary depending on the specific product and treatment conditions.
After IPL treatment, lipid oxidation in various meat products, including chicken-based frankfurters, packaged heat-processed cooked ham, bologna, dry-cured meat products, Iberian ham, and Serrano ham, was measured. As the storage period increased, the levels of TBARS and peroxide value showed a slight increase (Fernández et al., 2020; Hierro et al., 2011; Keklik et al., 2009; Liu et al., 2019), but the increase was minor.
In terms of sensory evaluation, research results related to appearance, color, odor, and flavor have shown that IPL treatment did not have a significant affect at low fluence. Differences were observed in odor and flavor when the fluence exceeded 4.2 J/cm2, and in appearance and color when it exceeded 8.4 J/cm2 (Hierro et al., 2011). When beef carpaccio was treated with an IPL fluence of 8.4 J/cm2, differences were observed in color and flavor, although these differences were within an acceptable range for panelists (Hierro et al., 2012). Ganan et al. (2013) reported that salchichón and dry loin showed no differences during storage when treated with IPL, but dry loin showed differences in color at 8.4 J/cm2 and in color, odor, and flavor at 11.9 J/cm2, but these differences disappeared as the storage period increased. Processed meat products, unlike fresh meat, have stable pigments and additives such as fat, salt, and spices, which contribute to less quality change after IPL treatment. Therefore, fluences below 8.4 J/cm2 is considered suitable from a quality change and sensory evaluation.
Processed meat products vary in types and materials, and research has been reported on a variety of products. The target microorganisms are also diverse, including L. monocytogenes, L. innocua, S. Typhimurium, E. coli, Micrococcus, Staphylococcus, molds and yeast, and lactic acid bacteria. The degree of microbial inactivation by IPL treatment of processed meat varies in studies but generally falls within the range of 0.1–2.0 Log, with smoother surfaces exhibiting inactivation levels of around 3–4 Log. After IPL treatment, processed meat products also showed no significant differences in both food and sensory qualities. However, prolonged treatment times may result in a slight increase in peroxide value, and differences in color and odor have been observed.
Egg Products
Research on the sterilization of egg products by IPL treatment has been conducted. Microbes were applied to the surface of eggshells, and the sterilization effect was examined by IPL treatment with different fluences (Table 3). The target microorganisms were E. coli, Salmonella, L. monocytogenes, and Enterococcus faecium (Cassar et al., 2021a; Hierro et al., 2009; Holck et al., 2018; Keklik et al., 2010a; Lasagabaster et al., 2011). Depending on the treatment conditions, E. coli was reduced by 1.6–3.7 Log, Salmonella by 0.7–3.5 Log, and Salmonella enterica by 1.3–7.8 Log (Hierro et al., 2012; Holck et al., 2018). In some study, it has been reported that treatment as a fluence of around 4 J/cm2 resulted in non-detectable levels of microorganisms (Dunn, 1996). The extent of reduction varied depending on factors such as fluence, distance between the light source and the samples, treatment time, and treatment system, but it was observed that at least a 1 Log reduction in the same microorganisms occurred. Furthermore, it was reported that IPL treatment did not affect the structural or quality characteristics of the cuticle and internal contents of eggshells (Holck et al., 2018; Keklik et al., 2010a; Lasagabaster et al., 2011). In addition, it has been reported that there was no difference between IPL treated egg and untreated egg when they were hatched and grown into chicks (Cassar et al., 2021a).
| Food product | Microorganism | Treatment condition | Reductions (Log) | Reference |
|---|---|---|---|---|
| Table eggs | Escherichia coli Enterococcus faecium | 1.0, 2.4, 3.1, 4.9 J/cm2 | E. coli: 3.45, 4.00, 3.76, 4.54 E. faecium: 2.03, 2.81, 2.98, 3.52 | Cassar et al. (2021a) |
| Egg shell | Salmonella enterica serovar Enteritidis | 2–12 J/cm2 | Unwashed: 0.14–2.49 Washed: 0.21–1.85 | Hierro et al. (2009) |
| Egg | S. Enteritidis Listeria monocytogenesE. coli | 3.6, 10.8, 18 J/cm2 | S. Enteritidis clean egg: 2.3–3.8 Dirty egg: 0.4–2.2 L. monocytogenes: 1.8–3.7 E. coli: 1.6–3.7 | Holck et al. (2018) |
| Egg | Salmonella enterica | 4 J/cm2 | ND | Dunn (1996) |
| Egg shell | S. Enteritidis | Distance 9.5, 14.5 cm 1, 3, 5, 10, 15, 20, 30 s | Distance 9.5 cm: 2.0–7.7 14.5 cm: 1.3–5.5 | Keklik et al. (2010a) |
| Egg | Salmonella enterica | 0.35, 0.7, 2.1, 4.9, 10.5 J/cm2 | 3.5–4.9 | Lasagabaster et al. (2011) |
| Hard-cooked peeled egg | E. coli K12 | Distance 5.5, 9.5 cm 1, 3, 5, 10, 15, 20, 30 s | Distance 5.5 cm: ~3.54 9.5 cm: ~3.23 | Macias-Rodriguez et al. (2014) |
| Liquid egg white | E. coliS. Enteritidis | Distance 5, 9, 13 cm 20, 30, 40 s | E. coli: 0.23–1.06 S. Enteritidis: 0.57–1.76 | Ouyang et al. (2020a) |
| Liquid egg white | E. coliS. Enteritidis | Distance 5, 9, 13 cm 20, 30, 40 s | E. coli: 0.23–1.06 S. Enteritidis: 0.60–1.76 | Ouyang et al. (2020b) |
Manzocco et al. (2013) measured the quality characteristics of egg white after IPL treatment at fluence levels of 1.75–31.5 J/cm2. As fluence increased, the egg white became cloudy, and aggregates formed due to protein denaturation. However, there was no significant difference in apparent viscosity and gel strength. The foaming formation and stability increased, suggesting that IPL treatment could be used to improve the properties of egg white. Ouyang et al. (2020a) and Ouyang et al. (2020b) reported that when egg white was treated with IPL, the color deepened, or turbidity increased as the treatment conditions became stronger, but the foam-forming ability and stability did not any changes depending on the treatment conditions. IPL treatment proved to be effective for surface sterilization of eggshells. In some cases, the treatment can increase the temperature of egg white, causing protein denaturation. However, its impact on foaming stability and formation shows minimal or even positive effects. Though further research, it is expected that by appropriately setting the treatment conditions, IPL treatment can be applied for both sterilization and quality improvement.
After IPL treatment of the surface of eggs, there were no significant impact on the contents and cuticle layer to the extent that hatching remained possible. The IPL treatment exhibited an inactivation effect of 0.7–7.8 Log levels depending on the conditions. IPL treatment of the egg white did not affect the apparent viscosity or gel strength, but it did increase the foaming performance and foam stability. These results suggest that IPL has the potential for commercial application.
Dairy Products
IPL treatment is applied to dairy products such as raw milk, infant powdered milk, cheese, and whey protein (Table 4). IPL treatment of milk has been shown to be effective in inactivating pathogenic microorganisms, such as E. coli, S. aureus, and L. innocua. The inactivation effect of IPL treatment is dependent on several factors, including fluence, treatment time, flow rate, and processing volume (Innocente et al., 2014; Kang et al., 2021; Kasahara et al., 2015; Krishnamurthy et al., 2007; Miller et al., 2012; Palgan et al., 2011). In general, higher fluences lead to higher inactivation rates. Krishnamurthy et al. (2008) reported that a fluence of 100 J/cm2 was required to achieve a 5 Log reduction in E. coli in milk. Kasahara et al. (2015) also reported that a fluence of 100 J/cm2 was sufficient to inactivate L. innocua in milk. Temperature increase during IPL treatment can also affect the inactivation effect. Chen et al. (2019) reported that the inactivation effect of IPL treatment was lower at higher temperatures. The inactivation effect of IPL treatment is also affected by the milk composition. Milk with lower fat content is more susceptible to IPL treatment than milk with higher fat content. Miller et al. (2012) reported that IPL treatment of skim milk resulted in a higher inactivation rate than IPL treatment of whole milk. The use of a vibratory assisted IPL system can also improve the inactivation effect of IPL treatment. The vibratory assisted IPL system can help to reduce the shadow effect, which is a phenomenon that can occur during IPL treatment when microorganisms are located in areas that are not directly exposed to the IPL. Chen et al. (2019) reported that the use of a vibratory assisted IPL system resulted in a 5.27 Log reduction in Cronobacter sakazakii and a 3.67 Log reduction in E. faecium in non-fat dry milk.
| Food product | Microorganism | Treatment condition | Reductions (Log) | Reference |
|---|---|---|---|---|
| Goat milk | Escherichia coli | 10,000 mJ cm–2 | 6 | Kasahara et al. (2015) |
| Milk | Staphylococcus aureus | Distance 5, 8, 11 cm 20, 30, 40 mL/min | 0.55–7.26 | Krishnamurthy et al. (2007) |
| Milk, milk foam | S. aureus | Distance milk: 8, 10.5, 13 cm Milk foam: 5, 8, 11 cm | 0.16–8.55 | Krishnamurthy et al. (2008) |
| Milk | Total microbial count | 0.26–26.25 J/cm2 | Maximum 3.2 | Innocente et al. (2014) |
| Milk, concentrated milk | E. coli | 2.14–14.85 J/cm2 | Concentrated milk: 0.03–2.05 Milk: (static) 0.35 (Shaking) 3.36 | Miller et al. (2012) |
| Milk | E. coli Listeria innocua | 7, 14, 28 J/cm2 | E. coli: 0.61–1.06 L. innocua: 0.51–0.84 | Palgan et al. (2011) |
| Non-fat dry milk | Cronobacter sakazakii Enterrococcus faecium | 29.36 J/cm2, 1–4 passes | C. sakazakii: 5.27 E. faecium: 3.67 | Chen et al. (2019) |
| Infant powder milk | Listeria monocytogenes | 15 kV, 10 Hz, 1.5 μs, 0–900 s | 3 | Choi et al. (2010) |
| Infant powder milk | Enterobacter sakazakii | 15 kV, 10 Hz, 0–12 msec | 1.5 | Choi et al. (2009) |
IPL treatment reduced the level of Penicillium roqueforti, L. monocytogenes, and L. innocua in cheddar cheese, white American cheese, and ricotta cheese by 1.32 Log, 3 Log, and 3 Log, respectively (Can et al., 2014; Proulx et al., 2015; Proulx et al., 2017). Proulx et al. (2017) and Ricciardi et al. (2021) also found that IPL treatment had no significant effect on the sensory properties, color, or peroxide value of ricotta cheese and cheddar cheese. In a study by Artíguez and Marañón (2015), IPL treatment reduced the levels of L. innocua in whey by 0.5–5.4 Log. When stored at 4°C, the shelf life of the whey was extended by approximately 6 days (Table 5). Furthermore, IPL treatment had no significant effect on the amino acid composition or lipid oxidation of whey protein or whey protein-derived lactalbumin, β-lactoglobulin, and sodium caseinate (Siddique et al., 2016; Siddique et al., 2017). Wihodo and Moraru (2015) reported that IPL treatment of casein films can improve the surface smoothness and uniformity, and increase mechanical strength, by inducing changes in the water absorption, hydrophobicity, and microstructure of the films.
| Food product | Microorganism | Treatment condition | Reductions (log) | Reference |
|---|---|---|---|---|
| White American cheese | Penicillium roqueforti Listeria monocytogenes | Distance 5, 8, 13 cm 5–60 s | P. roqueforti: 0.38–1.32 L. monocytogenes: 1.1–3.08 | Can et al. (2014) |
| Ricotta cheese | Enterobacteriaceae Pseudomonas spp. Yesat | 1.3, 3.1, 7.5, 15.0 J/cm2 | Ricciardi et al. (2021) | |
| Cheddar cheese, white American cheese | Pseudomonas fluorescens Escherichia coli Listeria innocua | 1.02–12.29 J/cm2 | P. fluorescens: maximum 3.74 E. coli: maximum 5.41 L. innocua: maximum 3.37 | Proulx et al. (2015) |
| Cheddar cheese, white American cheese | Pseudomonas fluorescens E. coli L. innocua | 1.02–12.29 J/cm2 | P. fluorescens: 2.19 E. coli: 2–5 L. innocua: 3 | Proulx et al. (2017) |
| Whey (skimmed whey, diluted whey) | L. innocua | 3,000 V (11 J/cm2) | 0.5–5.4 | Artíguez and Marañón (2015) |
Industrial Application of Intense Pulsed Light treatment
The application of IPL treatment technology for sterilizing transparent liquids or food surfaces began to be researched after receiving approval for food use from the FDA in 1996 (Dunn, 1996; Dunn et al., 1989). Subsequently, commercial-scale implementation was realized, with the French company Claranor industrially applying IPL treatment for the sterilization of drinking water in 2004 (Cleanroom Technology, 2023). Since then, IPL treatment has been industrially applied to sterilize food and beverage containers, as well as infant powdered products and packaging containers (Claranor, 2023). Additionally, several companies have recently been developing different models of IPL devices for drinking water treatment and surface sterilization of food, so it is expected that industrial applications in this field will gradually expand (DTX, 2023; Wek-Tec, 2023; Zhongwu, 2023). The industrial application of IPL treatment has been implemented first for fruits, vegetables, and powdered foods, as research on IPL treatment has been conducted on these products (Dunn, 1996; Hong et al., 2013; Lee et al., 2021; Shin et al., 2010). As shown in the previous review, research on the sterilization of meat is currently underway, but industrial application has not yet been commercialized. The application of IPL technology in the livestock industry is currently progressing more rapidly for workbenches and work environments (Cassar et al., 2021b; Gao et al., 2023). Gao et al. (2023) reported the effective inactivation of Salmonella biofilm on stainless steel surfaces used in poultry and livestock workstations. Cassar et al. (2021b) showed that the application of IPL to E. coli on the surface of food-grade conveyor belts contaminated with pathogenic and spoilage microorganisms during livestock processing resulted in a germicidal effect of 3.91–5.04 Log CFU/cm2, indicating the possibility of applying pulsed light treatment to ensure the hygiene of workbenches and conveying devices for processing livestock. Additionally, it has been reported to be effective on food contact materials such as cutting tools, packaging materials, and production surfaces used during processing (Heinrich et al., 2016). An important consideration in the industrial application of IPL treatment is related to heat-sensitive foods and packaging materials. IPL treatment generally does not generate much heat, but excessive treatment can cause a temperature rise of about 20°C. Therefore, a cooling system to reduce temperature rise or a more effective fluence approach is needed. Although the industrial application of sterilization for meat products has not yet been realized, hygienic treatments of working environments, tools, and packaging materials, etc., have been reported to be effective. This suggests that IPL treatment could be used for the sterilization of meat if additional research is conducted on the design, operation, and maintenance costs of processing lines, taking into account the type of meat, the desired level of microbial reduction, and other factors.
Conclusion
IPL technology is an effective method for reducing microbial contamination on the surface of foods. Research findings indicate that IPL treatment has led to substantial reductions in pathogens such as Salmonella, Campylobacter, E. coli, and Listeria, among others. The degree of microbial reduction, however, can vary based on factors like treatment conditions, fluence, and specific product characteristics. Notably, the application of IPL treatment has shown minimal change on the overall quality of the treated food products. While there have been reports of color changes and potential alterations in sensory attributes under specific treatment conditions, these effects are generally within acceptable limits. For instance, the color and sensory aspects of meat, dairy, and egg products have shown manageable alterations when IPL treatment is properly administered, thereby ensuring that the products remain palatable and appealing to consumers. Additionally, the technology holds promise for extending the shelf life of food products, reducing the reliance on chemical preservatives, and enhancing food safety. In the context of the food industry, IPL technology presents a viable option for producers and processors seeking innovative solutions for microbial control and quality enhancement. As the research on IPL applications continues to expand and adapt to specific food products and processing conditions, it is expected that this technology will become increasingly valuable in ensuring the safety and quality of a wide range of food items. While further research and practical implementations are necessary to refine and standardize IPL treatments for specific food products, it is evident that IPL technology has the potential to make a significant impact in the fields of food processing and preservation.