Introduction
The goat is the most widely distributed livestock species globally, and its numbers have steadily increased over the past decade (Food and Agriculture Organization [FAO], 2020). Global meat consumption is also expected to increase, and the goat industry has the potential for similar growth (Mazhangara et al., 2019). In the past, goats were recognized mainly as a health supplement, but more recently, they have been consumed for their meat (Hwang et al., 2019). While the functions of goat meat were only described in classical Eastern medical literature, the nutritional and physiological activities of goat meat have recently been studied with modern science (Kim et al., 2019).
Muscle atrophy is a reduction in the skeletal muscle and may be caused by various factors, including muscle disuse, aging, and starvation (Jackman and Kandarian, 2004). In muscle atrophy, the fatigue resistance is lower, and the muscle fiber is reduced in diameter (Jackman and Kandarian, 2004). The World Health Organization (WHO) classified sarcopenia, the age-related loss of muscle, as a disease and suggested that it should be managed (Anker et al., 2016; Cao and Morley, 2016). Several meta-analysis studies have found that the prevalence of sarcopenia has increased in older adults around the world (Yuan and Larsson, 2023). To prevent muscle atrophy, adequate intake of dietary nutrients and increasing the protein anabolic capacity of skeletal muscle are necessary (Bowen et al., 2015; Deutz et al., 2014).
Wall and van Loon (2013) found that supplementation with dietary protein and essential amino acids can help preserve muscle mass during disuse-induced muscle atrophy. Similarly, dietary protein supplementation during muscle disuse atrophy in healthy older men did not result in muscle loss during short-term disuse (Dirks et al., 2014). In addition, Thalacker-Mercer et al. (2007) suggested that inadequate protein intake during age-related muscle atrophy may downregulate transcripts encoding essential muscle proteins and protein synthesis. There is also a possibility of gut microbiota being changed by the dietary protein (Albracht-Schulte et al., 2021; Wu et al., 2022).
Therefore, the objective of this study was to evaluate the effect of goat meat treatment on muscle atrophy and changes in gut microbiota in animal models.
Materials and Methods
The 12-month-old female goat meat used in the diet preparation was provided by Gaon Agricultural Corporation (Gangjin, Korea). Each cut of goat meat (forelegs, hind legs, loin, and ribs) was cubed into 2 cm×2 cm×2 cm, and the cube samples were placed into sterilized bags and boiled in an 80°C water bath until the core temperature reached 77°C (Son et al., 2014). The cooked meat was stored in a –80°C deep freezer for one day and freeze-dried for 48 h in a freeze-dryer. The freeze-dried meat was ground into powder, and the powdered goat meat mixture of the four cuts (1:1:1:1) was added to the 2018S (2018S Teklad Global 18% Protein Rodent Diet; Envigo, Madison, WI, USA) at 8%. It simulated the inclusion of goat meat in a regular diet pattern. The concentration of goat meat in the diet corresponds to the average daily intake of red meat per person (69.5 g for a 60 kg Korean), according to the 2016 Korea National Health and Nutrition Examination Survey (Korea Centers for Disease Control and Prevention [KCDC], 2018). The dose was converted for mice according to the clinical trial guidelines of the U.S. Food and Drug Administration (FDA, 2005).
Five-week-old male C57BL/6N mice (Raon Bio, Yongin, Korea) were housed under a 12-h light/dark cycle with a constant temperature (23±1°C) and a humidity (55±5%). After one week of acclimation, the mice were treated with an intraperitoneal injection of dexamethasone (dissolved in saline; D2915, Sigma-Aldrich, St. Louis, MO, USA) to induce muscle atrophy at 10 mg/kg/day (Hah et al., 2020), and the other mice were treated with saline for 14 days. The saline-treated mice were then fed with a normal diet (2018S) for control (CON; n=8), and the dexamethasone-treated mice were fed with a normal diet (DEX; n=7) and goat meat (DEX+G; n=8) for 18 days. The scheme of the animal experiment is described in Fig. 1, and the nutritional information of each diet is shown in Table 1. The animal experiment was approved by the Institutional Animal Care and Use Committee of Sookmyung Women’s University (approval number: SMWU-IACUC-2301-025).
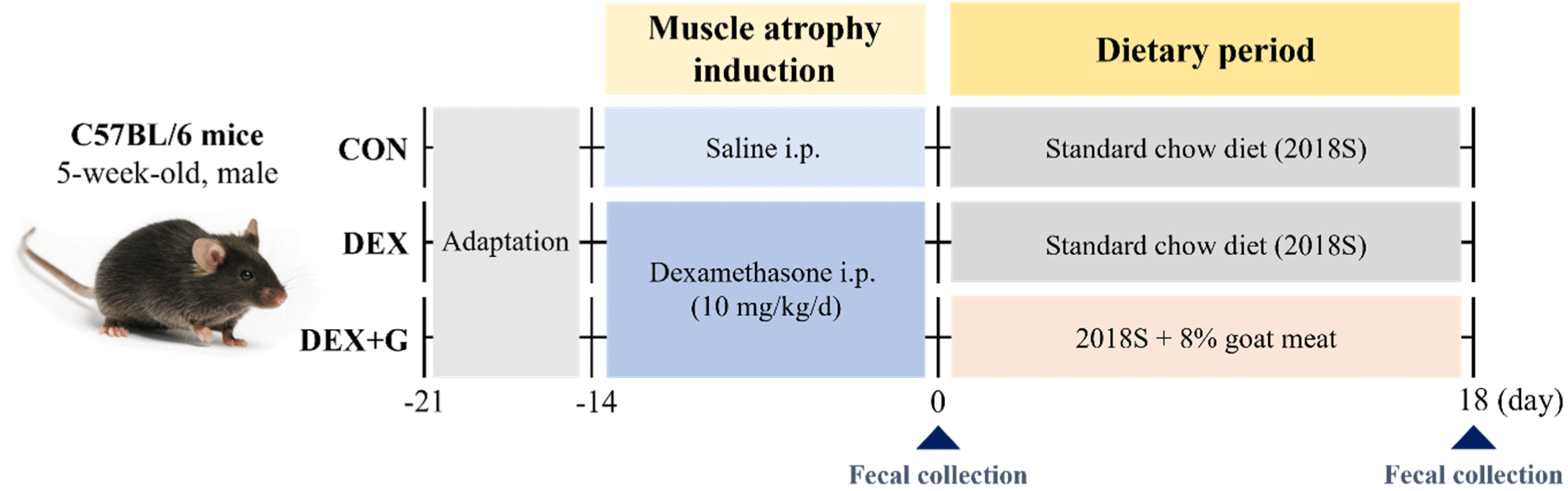
The body weights of the mice were measured daily during the muscle atrophy induction period and weekly during the dietary period. Also, the final weight of the mice was measured on day 18 of the dietary period. After the sacrifice of the mice by inhalation of isoflurane (TerrellTM isoflurane, Piramal Critical Care, Bethlehem, PA, USA) after 18-h fasting, skeletal muscle tissues were collected from the hindlimbs and weighed. The isolated skeletal muscle tissues were gastrocnemius, soleus, and quadriceps femoris. The relative mass of the skeletal muscles to the body weight of the mice were calculated with the following equation: relative mass of skeletal muscle to body weight (%) = skeletal muscle weight (g) / body weight (g) × 100 (Kim et al., 2015). The obtained skeletal muscle tissues were stored at –80°C for further analysis.
Blood samples were obtained from the posterior aorta of mice at sacrifice on day 18. The blood samples were left at room temperature for 30 min and then centrifuged at 2,339×g and 4°C for 10 min to separate the serum. The serum was stored at –20°C until analysis. The levels of serum creatine kinase (CK), lactate dehydrogenase (LDH), and creatinine were measured with Hitachi Automatic Biochemical Analyzer (Hitachi 7180, Hitachi, Tokyo, Japan) in the KP&T (Cheongju, Korea).
The protein of mouse gastrocnemius muscle tissue was extracted with PRO-PREPTM protein extraction (iNtRON Biotechnology, Seongnam, Korea), and protein concentration was quantified with DCTM Protein Assay Kit I (BioRad, Hercules, CA, USA) according to the manufacturer's instructions. The 15 μg of total protein samples were electrophoresed on 12% sodium dodecyl sulfate polyacrylamide gel at 120 V for 1 h, and the separated proteins were transferred to a polyvinylidene difluoride membrane (Cytiva, Marlborough, MA, USA) at 60 V for 2 h 30 min. The membranes were incubated in 5% skim milk in Tris-buffered saline with 0.1% Tween-20 at 25°C for 1 h for blocking. The proteins were then treated with the primary antibodies of glyceraldehyde 3-phosphate dehydrogenase (GAPDH; GTX100118, GeneTex, Irvine, CA, USA), muscle RING finger protein 1 (MuRF1; sc-398608, Santa Cruz Biotechnologies, Santa Cruz, CA, USA), muscle atrophy F-box (MAFbx; sc-166806, Santa Cruz Biotechnologies), and growth differentiation factor 8 (GDF-8; myostatin; sc-134345, Santa Cruz Biotechnologies) at 4°C for overnight, followed by the treatment of goat anti-mouse IgG (H+L)-HRP (Invitrogen, Carlsbad, CA, USA) and goat anti-rabbit IgG (H+L)-HRP (GenDEPOT, Katy, TX, USA) as the secondary antibodies. The antigen/antibody complexes were detected using enhanced chemiluminescence solution (Dongin LS, Seoul, Korea) at room temperature for 1 min and then visualized by a biomolecular imaging system (AmershamTM ImageQuantTM 800, Cytiva). The intensity of the bands was quantified with GelQuant software v.2.7 (DNR Imaging System, Jerusalem, Israel), and the concentrations of each protein were normalized to the expression level of GAPDH.
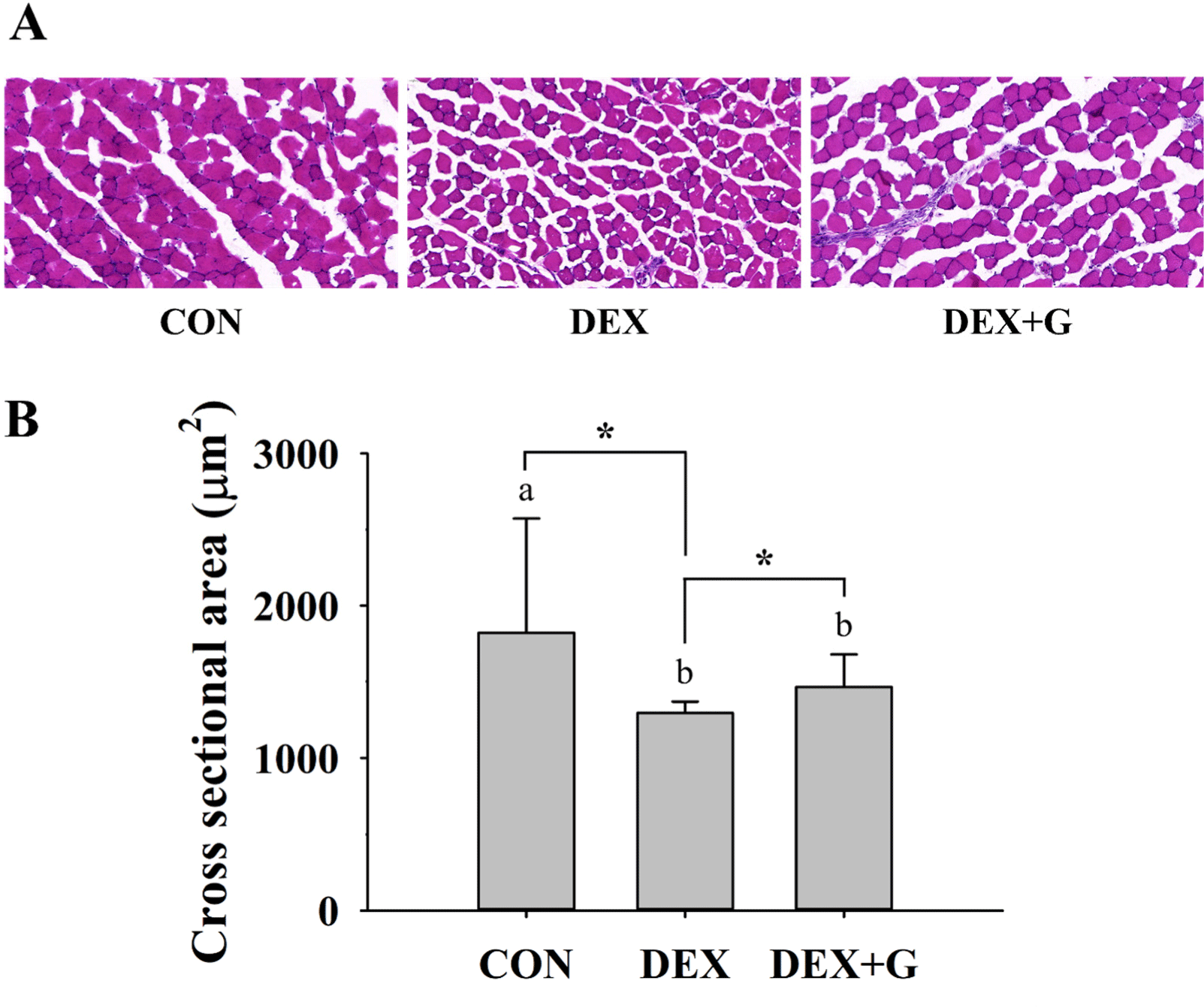
For histological analysis, three mice, which had results closed to the average of relative mass of gastrocnemius muscle and expression levels of muscle atrophy-related proteins in gastrocnemius muscle in each treatment, were selected with comprehensive analysis. The gastrocnemius muscles (n=3/group) were frozen-sectioned and stained with hematoxylin and eosin. The stained tissues were observed using a digital slice scanner (PANNORAMIC SCAN II, 3DHISTECH, Budapest, Hungary), and the cross-sectional area (CSA; μm2) of 100–200 fibers for each sample was measured with the image analysis program (Image-Pro®, Media Cybernetics, Silver Spring, MD, USA). The average CSA of muscle fibers in each group was then calculated. This analysis was performed in T&P BIO (Gwangju, Korea).
Fecal samples were collected directly from the anus into sterilized tubes after immobilizing the mice with a hand on day 0 of the dietary period. The fecal samples on day 0 and fecal samples from the cecum after sacrifice on day 18 of the dietary period were used for DNA extraction with DNeasy PowerSoil Pro Kit Protocol (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. Quant-iTTM PicoGreenTM dsDNA Assay Kit (Invitrogen) was used to quantify the extracted DNA. From the extracted DNA sample, the V3-V4 regions of the 16S rRNA region in genomic DNA were amplified by PCR with Illumina 16S Metagenomic Sequencing Library Preparation protocols, and the pair-end (2×300 bp) sequencing was performed with the MiSeqTM platform (Illumina, San Diego, CA, USA) in Macrogen (Seoul, Korea). The sequencing adapter and primer forward/reverse sequences were trimmed with the Cutadapt (v.3.2) program. The DADA2 (v.1.18.0) package in the R (v.4.0.3) program was used to correct errors in amplicon sequencing. The consensus method of DADA2 was then used to remove chimeric sequences and construct amplicon sequence variants (ASVs). The ASVs sequences were assigned taxonomy information for the most similar organisms based on the reference DB (NCBI 16S Microbial DB) using BLAST+ (v.2.9.0). The abundance and taxonomy information of the ASVs were used to analyze the diversity of the gut microbiota with QIIME (v.1.9). The alpha diversity was analyzed by the Shannon index and Chao1 values (Finotello et al., 2018). The beta diversity was analyzed based on weighted and unweighted UniFrac distance and visualized by principal coordinate analysis plot (Lozupone et al., 2011). The processes above were performed in Macrogen. To analyze the differences in gut microbiota among the groups on day 18, linear discriminant analysis effect size (LEfSe) analysis was performed with the taxonomy information from Macrogen in the phyloseq package in R (v.4.3.1) software at a significance level of p<0.05 and a linear discriminant analysis (LDA) score threshold of >2.0.
Statistical analysis of the data was performed with SAS® OnDemand for Academics (SAS Institute, Cary, NC, USA). For weekly body weight of mice, relative mass of skeletal muscle to body weight, serum biochemical markers, protein expression levels, histological analysis, and diversity and abundance of gut microbiota, differences among the three groups (CON, DEX, and DEX+G) were determined by pairwise t-test comparing least squares means at α=0.05 with the general linear model. In addition, the data between pairs of two groups (CON vs. DEX, CON vs. DEX+G, and DEX vs. DEX+G) were compared with the student’s t-test or Wilcoxon rank-sum test, depending on the normality of the data. A comparison of the relative abundance of gut microbiota within each group between day 0 and day 18 was evaluated using a paired t-test and Wilcoxon signed-rank test, depending on the normality test of the data.
Results and Discussion
Because dexamethasone used to induce muscle atrophy decreased skeletal muscle mass and weight loss (Choe, 2005; Lee et al., 2023), the weights of mice were measured. The weights of mice were significantly decreased (p<0.05) in the muscle atrophy-induced groups compared to the CON group during muscle atrophy induction with dexamethasone. On day 7 of the dietary period, both the DEX and DEX+G groups had similar body weight to the CON group, and all groups had similar body weight at the end of the dietary period (Fig. 2A). This result indicates that dexamethasone administration caused weight loss, but it was recovered during the dietary period.
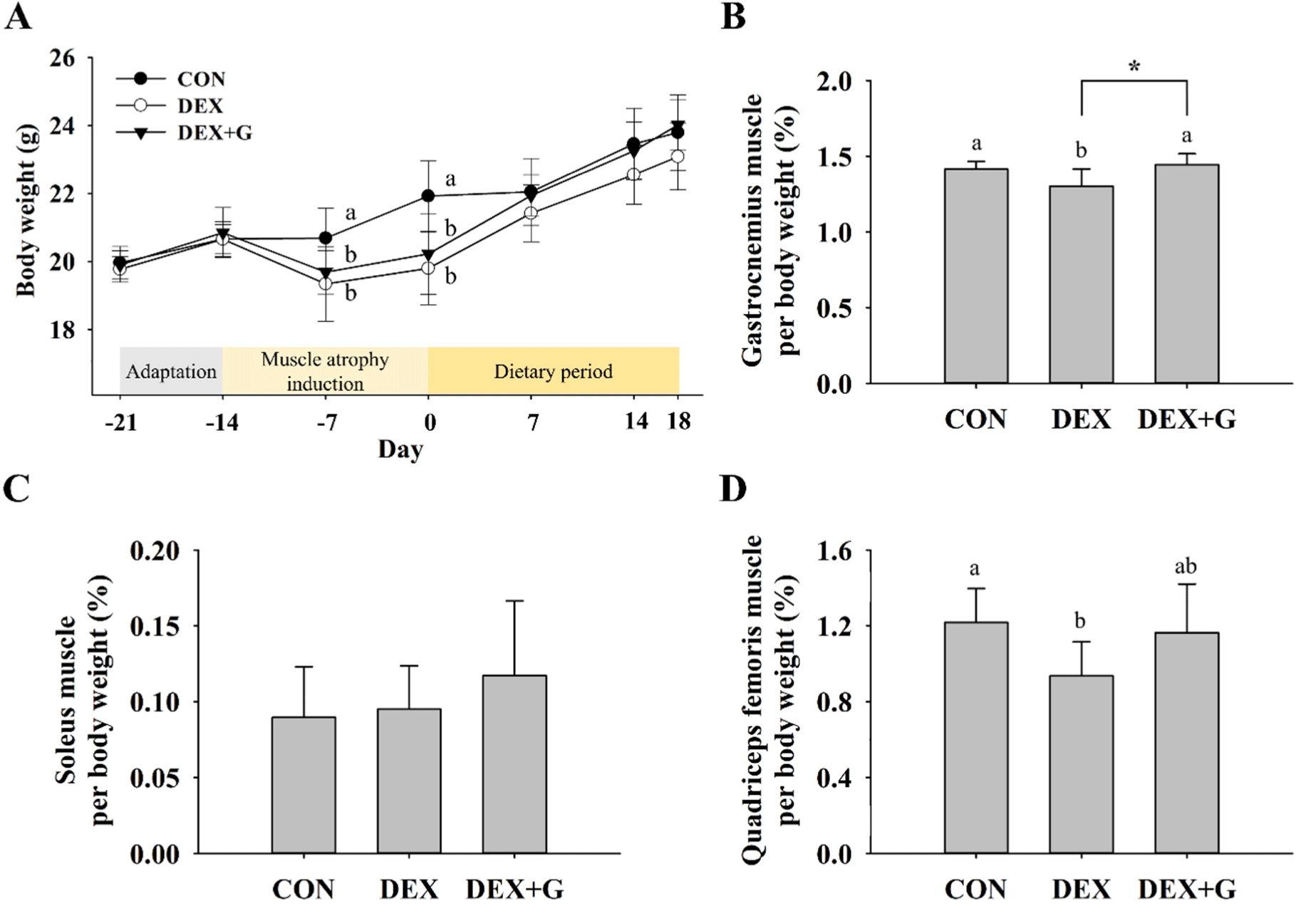
About 40% of the human body mass is composed of skeletal muscle, and it has an important role in physical activity and energy expenditure (Wang and Pessin, 2013). In particular, the gastrocnemius muscle is often analyzed in studies of skeletal muscle function because it could be a representative of the strength and skeletal muscle mass of the body (Edström and Ulfhake, 2005; Xie et al., 2021). Also, the soleus and quadriceps femoris muscles that make up the hindlimb are often analyzed in animal models of muscle atrophy (Sakai et al., 2019; Yamamoto et al., 2010). The relative mass of skeletal muscles (gastrocnemius, soleus, and quadriceps femoris muscle) to body weight are shown in Figs. 2B, C, and D. The relative mass of the gastrocnemius muscle to body weight was lower (p<0.05) in the DEX (1.30±0.11%) group than the CON (1.41±0.05%) group, and it became slightly higher (p<0.05) in the DEX+G (1.45±0.07%) group compared to the DEX group (Fig. 2B). The relative mass of gastrocnemius muscle was similar to the CON (Fig. 2B). For soleus muscle, there were no differences in the relative mass among the treatments (Fig. 2C). The relative mass of quadriceps femoris muscle of the DEX (0.94±0.18%) group was lower (p<0.05) than that of the CON (1.22±0.18%) group, but the relative mass of the DEX+G (1.16±0.26%) group was not different from that of the DEX group (Fig. 2D). However, the relative mass of quadriceps femoris muscle in DEX+G was similar to that of the CON (Fig. 2D). This result shows that dexamethasone induced muscle atrophy in the gastrocnemius and quadriceps femoris muscles, and goat meat treatment in the muscle atrophy-induced group (DEX+G) made the relative mass slightly higher than the DEX only in the gastrocnemius muscle. Therefore, this result suggests that consumption of goat meat might help slightly restore muscle mass only in gastrocnemius muscle, not in the other muscles, in muscle atrophy-induced mice.
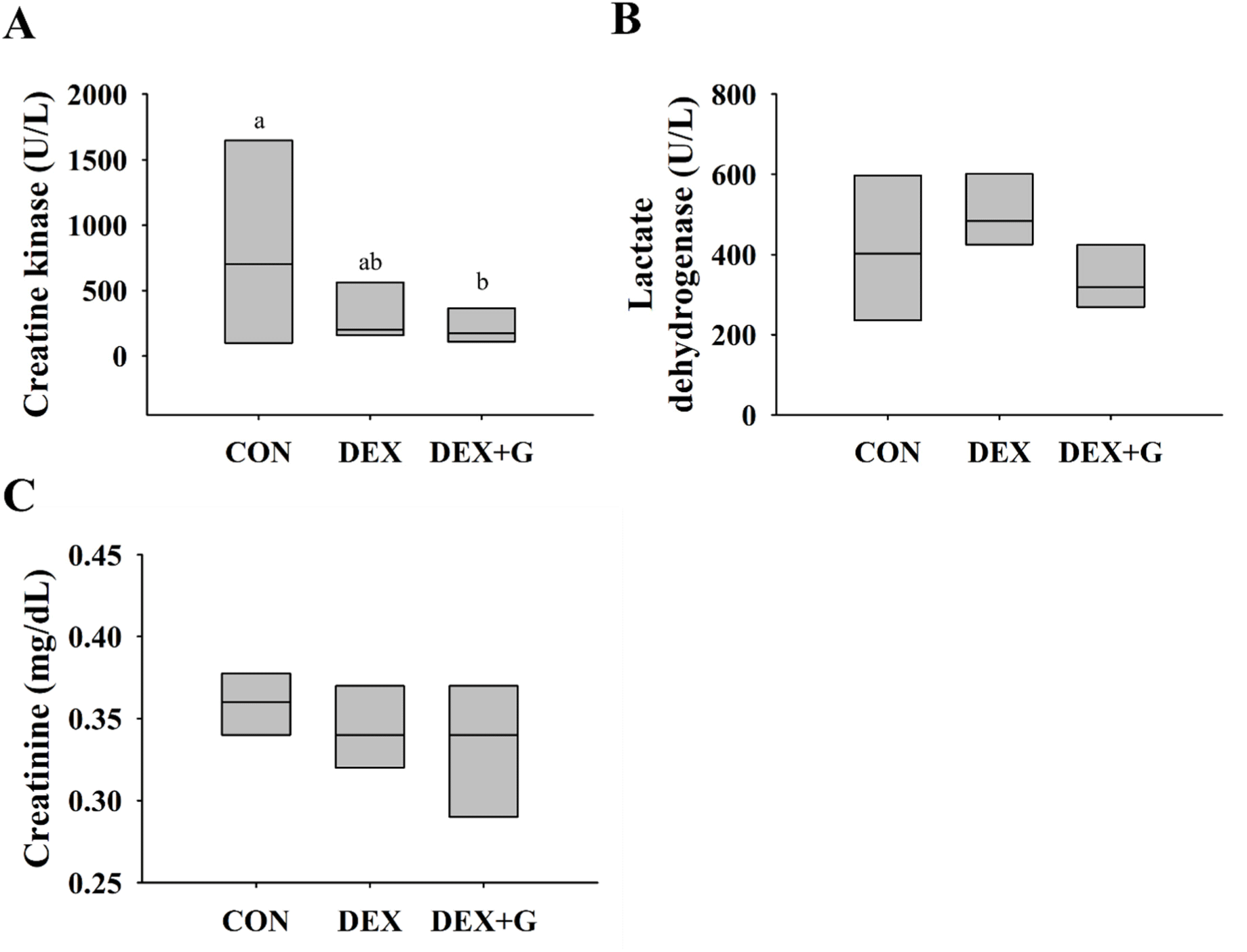
According to Brancaccio et al. (2010), CK and LDH are enzymes that are released into the blood when muscle tissue is damaged and can be used as serum biomarkers to identify muscle damage. Creatinine is a product of the breakdown of creatine phosphate in the muscle (Patel et al., 2013). Thus, the levels of these biochemical markers in blood were measured and are presented in Figs. 3A, B, and C. The levels of CK, LDH, and creatinine in the DEX+G group were not different from those in the DEX group. The level of CK was higher (p<0.05) in the CON group than those in DEX and DEX+G groups (Fig. 3A). This result might be caused by variation among individual mice and hemolysis that occurred in the mice during blood collection. In hemolyzed samples, adenylate kinase released from red blood cells during their destruction might have interfered with the measurement of CK (Greenson et al., 1989). Meanwhile, the levels of LDH and creatinine were not different among the treatments (Figs. 3B and C). Even though the differences in relative mass of gastrocnemius muscle were observed between DEX and DEX+G, the levels of biochemical markers were not different between the groups. It might be caused by variation among individual mice or insufficient muscle damage to cause the production of the biomarkers beyond the threshold.
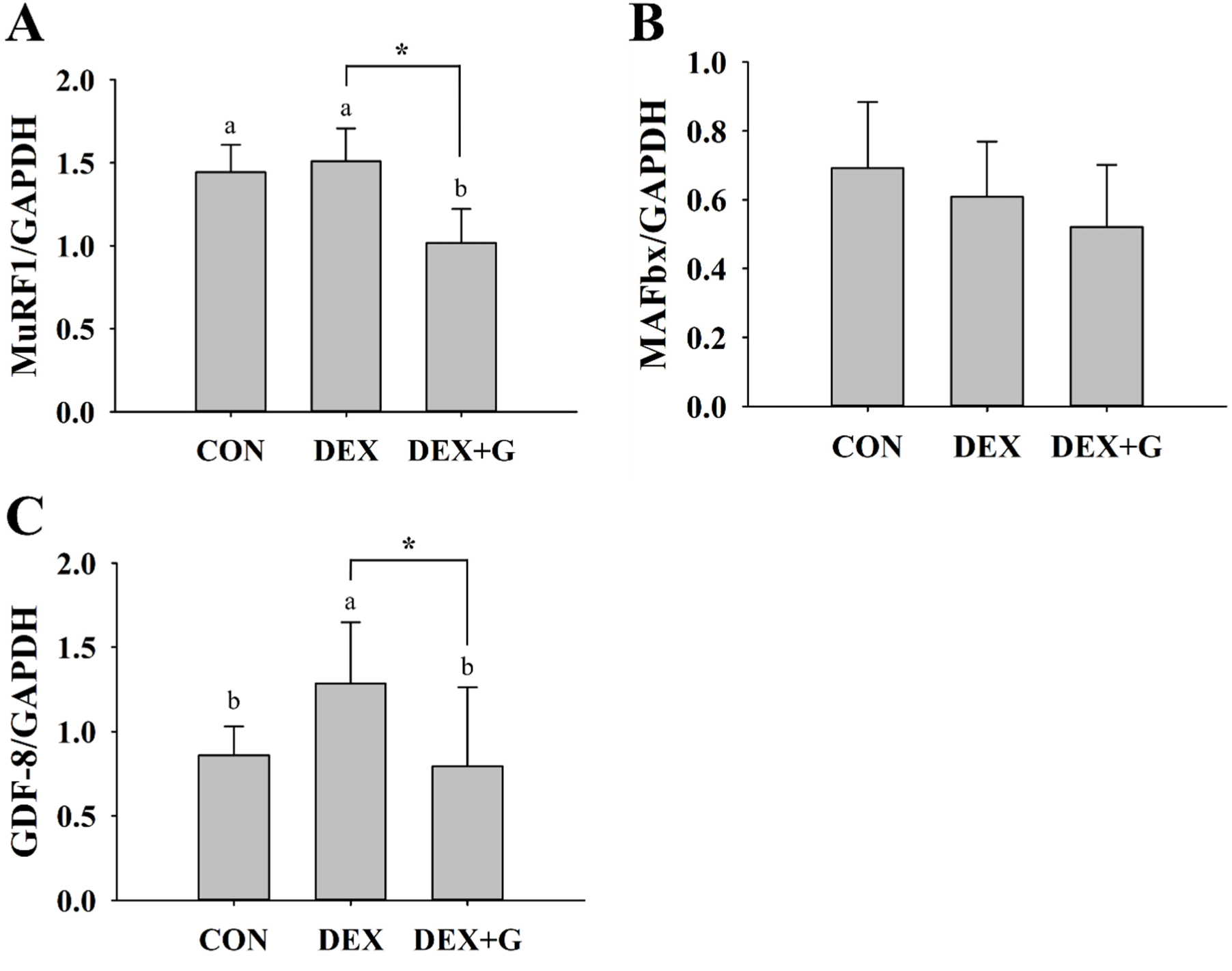
The expression levels of proteins associated with muscle atrophy were analyzed in mouse gastrocnemius muscle (Figs. 4A, B, and C). The expression of MuRF1 was not different between CON and DEX, but it was lower (p<0.05) in the DEX+G group compared to the DEX group (Fig. 4A). The expression of MAFbx was not different among all treatments (Fig. 4B). The expression of GDF-8 protein was lower (p<0.05) in the DEX+G group compared to the DEX group, and it was similar to that of the CON group (Fig. 4C). MuRF1 and MAFbx are E3 ubiquitin ligases that are major factors in causing atrophy of skeletal muscle by promoting the degradation of proteins (Bonaldo and Sandri, 2013; Kang et al., 2023). These proteins promote skeletal muscle atrophy under various stress conditions, including aging, increased glucocorticoids, inflammatory cytokine expression, and oxidative stress at the cellular level (Bodine and Baehr, 2014). Kim et al. (2021) found increased protein expression of MuRF1 and MAFbx in the muscle atrophy-induced group with dexamethasone compared to the control group. However, Alev et al. (2022) showed that rats induced with muscle atrophy using dexamethasone and then subjected to passive recovery tended to regain muscle mass. In our study, the mice in the DEX group were fed a normal diet without continuous dexamethasone treatment during the dietary period, which may have passive recovery and influence the expression of MuRF1 and MAFbx. In addition, the expression of MuRF1 and MAFbx might not be influenced by the applied concentration of dexamethasone. These reasonings might explain why there was no difference in the protein expression of MuRF1 and MAFbx between the CON and DEX groups. GDF-8, a member of the transforming growth factor-β family, is a factor that regulates skeletal muscle growth by decreasing Akt/mTOR/p70S6K signaling (Sharma et al., 2001). Inactivation of GDF-8 can lead to skeletal muscle hypertrophy. In contrast, overexpression of GDF-8 can cause muscle atrophy (Rodriguez et al., 2014).
This result suggests that the slightly improved relative mass in gastrocnemius muscle in the DEX+G group might be related to the regulations of some muscle atrophy-related proteins, such as MuRF1 and GDF-8. However, the interpretation needed to be expanded more fundamentally. Therefore, the CSA of the gastrocnemius muscle was further analyzed to evaluate if the improved relative muscle mass was associated with an increased CSA.
The muscle fiber size was tended to be larger in the DEX+G group than in the DEX group (Fig. 5A). The CSA of gastrocnemius muscle fibers was higher (p<0.05) in the CON group than in the DEX and DEX+G groups (Fig. 5B). In a comparison between the two-muscle atrophy-induced groups, CSA was slightly higher (p<0.05) in the DEX+G group than those in the DEX group (Fig. 5B). The decrease in the number and size of muscle fibers is an important characteristic of skeletal muscle atrophy (Hah et al., 2020; Jo et al., 2021), and thus, the CSA of muscle fibers could be an indicator to determine muscle atrophy and the recovery (Wang et al., 2022). This result suggests that intake of goat meat might increase the CSA of gastrocnemius muscle fiber in muscle atrophy-induced mice. However, this increase was not as large as in the CON group. It may be associated with the slightly improved relative muscle mass as described above.
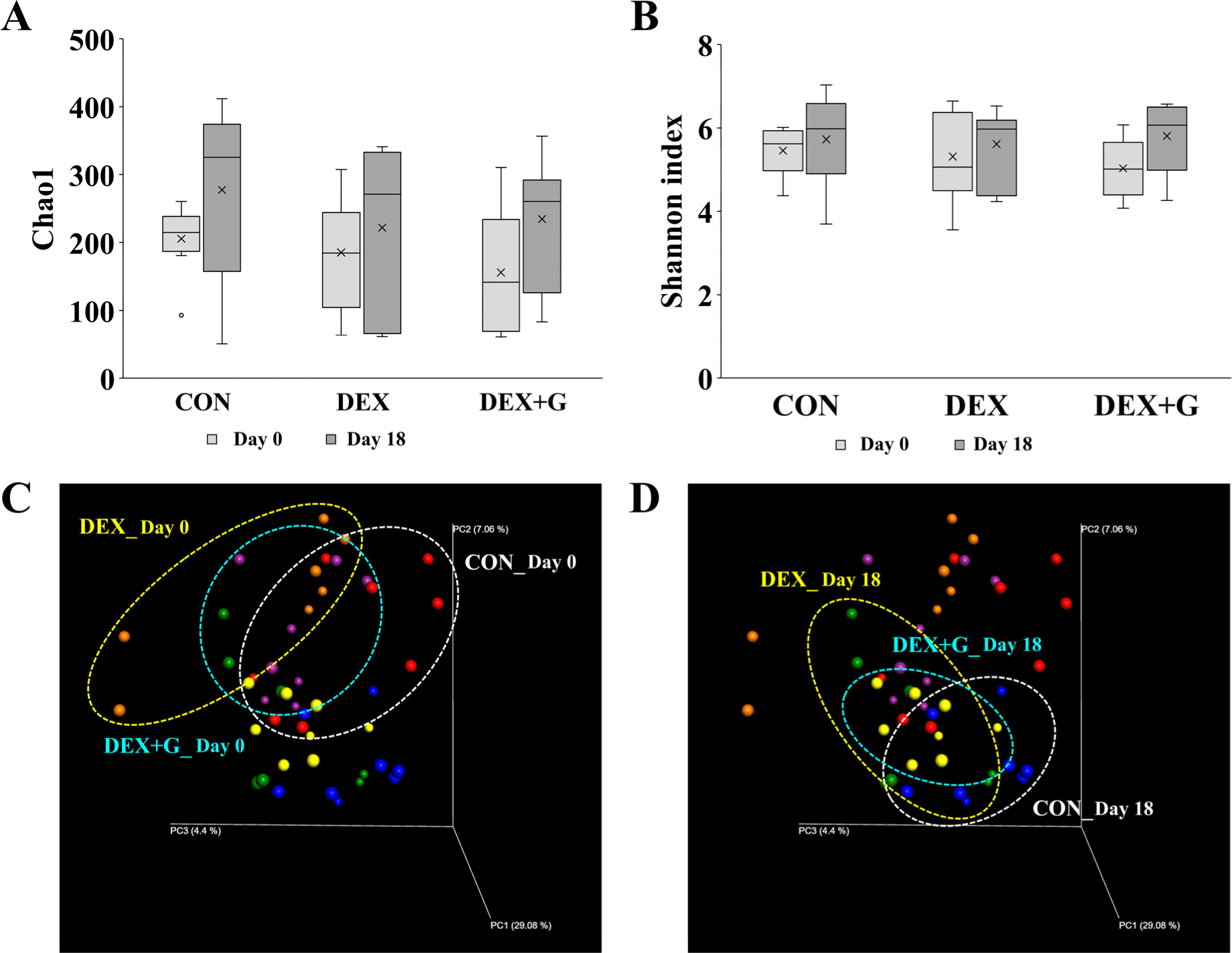
Alpha diversity indicates the diversity of microbes in the community and is represented by Chao1 indicates species diversity, and the Shannon index considers the number of species and species evenness of the gut microbiota (Masetti et al., 2018). On day 0 and day 18, there were no differences in Chao1 and Shannon indices among the treatments. Overall, there was a slight increase in alpha diversity on day 18 compared to day 0, regardless of the treatment group (Figs. 6A and B). This result suggests that the treatment of dexamethasone and goat meat may not have a significant impact on change in the diversity of the gut microbiota. Beta diversity represents the diversity of the microbiota between samples within a comparison group (Koleff et al., 2003). On day 0, clusters of gut bacterial communities appeared similar in all groups (Fig. 6C). On day 18, the communities of the DEX+G group were clustered closer to the CON group than the DEX group (Fig. 6D).
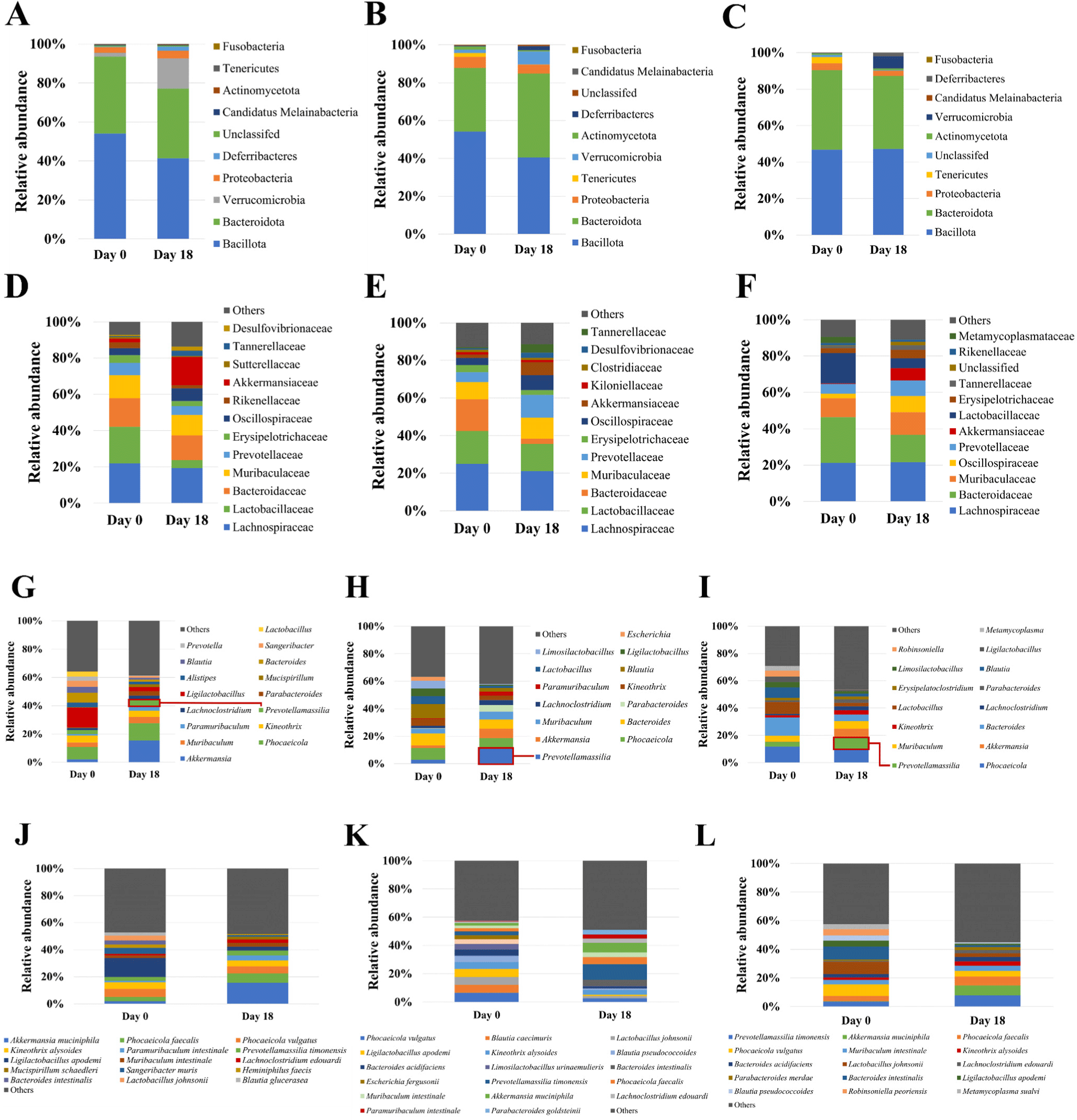
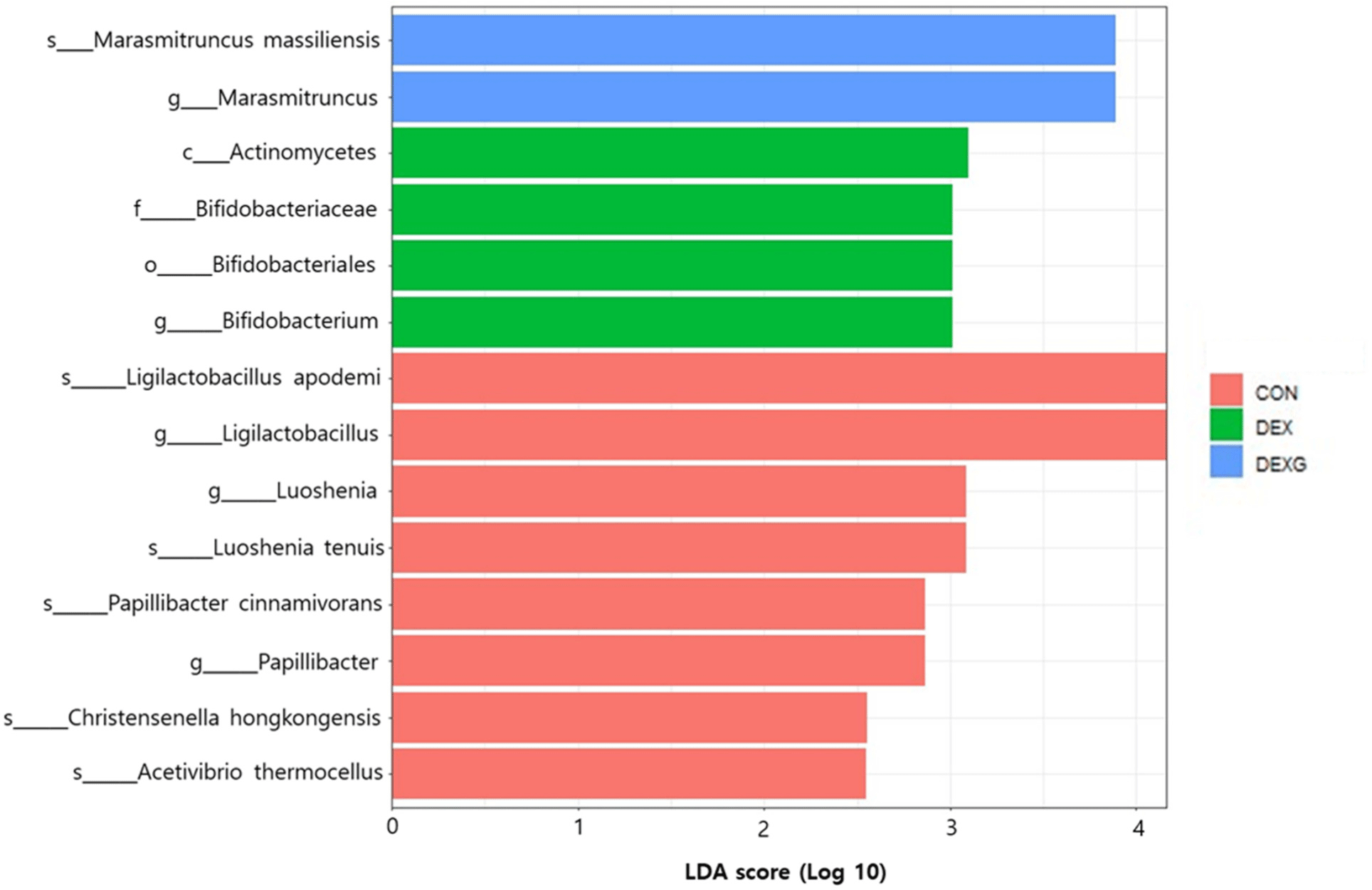
The relative abundance at the phylum level on day 18 compared to day 0 was decreased in Bacillota in the CON group (Fig. 7A). The relative abundance was increased in Bacteroidota and decreased in Bacillota in the DEX group (Fig. 7B). However, no obvious changes of the relative abundance in Bacteroidota and Bacillota were observed in the DEX+G group (Fig. 7C). For the family level, the relative abundance of Akkermansiaceae was highly increased on day 18 in CON group compared to the DEX and DEX+G groups (Figs. 7D, E, and F). Comparison of the DEX and DEX+G groups showed that Lachnospiraceae decreased in the DEX group (Figs. 7E and F). In Figs. 7G, H, and I, the relative abundance at the genus level is depicted. On day 18, the relative abundances of Prevotellamassilia were higher in the DEX and DEX+G groups than in the CON group (Figs. 7G, H, and I). Prevotellamassilia was lower in the DEX+G group than in the DEX group (Figs. 7H and I). At the species level, the relative abundance of Akkermansia muciniphila was higher in the CON group compared to those of the DEX and DEX+G groups on day 18 (Figs. 7J, K, and L). Differences in gut microbiota on day 18 were investigated by LEfSe analysis of gut microbiota (Fig. 8). The LDA score of 14 bacteria was more than 2.0. Actinomycetes (class), Bifidobacteriales (order), and Bifidobacteriaceae (family) characterized the DEX group from CON and DEX+G. For the genus level, Ligilactobacillus, Luoshenia and Papillibacter, Bifidobacterium, and Marasmitruncus characterized the CON, the DEX, and the DEX+G groups, respectively. In addition, five species (Ligilactobacillus apodemi, Luoshenia tenuis, Papillibacter cinnamivorans, Christensenella hongkongensis, and Acetivibrio thermocellus) were identified in the CON group, and one species (Marasmitruncus massiliensis) was identified in the DEX+G group. These results indicate that the taxonomic compositions of microbiota generally varied among the treatments. The composition of the gut microbiota is influenced by various ingredients consumed. The ingredients in goat meat are diverse and might vary among individual goats, sexes, ages, species, feed, etc. Thus, if an analysis is conducted on the relationship between gut microbiota and muscle atrophy without considering these factors in experimental design, using the result for the practical implication may not be appropriate, and the interpretation of the result from the analysis may include too many assumptions. Therefore, the relationship between muscle atrophy and gut microbiota was not analyzed in this study; only the effects of DEX and DEX+G treatments on changes in gut microbiota were analyzed as the optimum approach under given conditions.
Conclusion
In the mouse model, of the three muscles examined, the relative mass of only the gastrocnemius muscle was slightly higher in the DEX+G than in the DEX, and it was similar to the CON group. However, obvious relations between muscle damage-related biomarkers in blood and improving the relative muscle mass were not observed. Of the three muscle atrophy-related proteins in the gastrocnemius muscle, the expressions of MuRF1 and GDF-8 were lower in the DEX+G than in the DEX. The CSA of the gastrocnemius muscle was slightly higher in the DEX+G group than in the DEX group, but it was lower in the DEX+G group than in the CON group. Muscle atrophy induced by dexamethasone and goat meat treatment resulted in changes in gut microbiota. These results show that goat meat treatment might improve slightly relative muscle mass and CSA in only gastrocnemius muscle in muscle atrophy-induced mice not in the other muscles, and it might be related to lowered expression of MuRF1 and GDF-8 in the gastrocnemius muscle, but some biomarkers were not different between DEX and DEX+G. Hence, formulating a general conclusion from the information described above is not appropriate before further study. The current findings are based on a mouse model and indicate that goat meat treatment shows only a slight effect on improving relative muscle mass and CSA only in the gastrocnemius muscle, but not in the other muscles. Additionally, some factors were not different between DEX and DEX+G groups. Therefore, further research is necessary to assess the more apparent effect of consuming goat meat on muscle atrophy, especially in humans.













