Introduction
Flavor is one of the most important sensory qualities of meat and meat products, influencing consumers’ perception of meat quality and their purchase decisions. Meat flavor results from the interplay of taste and smell with volatile organic compounds (VOCs), and is mainly related to the generation of VOCs (Aaslyng and Meinert, 2017). More than 1,000 VOCs have been identified in meat and meat products, including mainly aldehydes, alcohols, ketones, acids, and others (Kosowska et al., 2017). These compounds are generated by a range of chemical reactions, such as the Maillard reaction, Strecker reaction, lipid oxidation, and lipid-Maillard interactions (Bassam et al., 2022; Liu et al., 2019). The Maillard reaction generates the basic flavor compounds of meat, such as S-, N- and O-containing heterocyclic compounds (Sohail et al., 2022). Lipid degradation typically generates species-specific meat flavor compounds, mainly aldehydes, ketones, alcohols, acids, and esters (Bassam et al., 2022). There is almost no difference in meat flavor when fats are removed from meats indicating that lipids play a critical role in the formation of meat-specific flavor compounds.
Previous studies have suggested that pork VOCs are breed-dependent and correlated with variance in fatty-acid profiles (Wu et al., 2022). For instance, feeding pigs a C18:1-rich diet has been shown to modify lipid compositions by increasing C18:1 levels, improving pleasing flavor attributes in pork (Navarro et al., 2021). Furthermore, the VOC profiles of donkey meat are significantly affected by ageing (Polidori et al., 2022). Specifically, aging increases the release of fatty acids, which are substrates for VOC formation (Meinert et al., 2009). Triglycerides (TGs) and phospholipids containing phosphatidylcholine (PC) and phosphatidylethanolamine (PE) are key lipids for binding and generating VOCs in roasted mutton (Liu et al., 2022). Clearly, the VOCs of meat and meat products are affected by breed, diet, rearing methods, ageing time, and cooking method, all of which are closely related to lipid content and composition. The lipid contents and fatty acid profiles of donkey muscle tissue from different body parts are different (Li et al., 2022a). In addition, the lipid profiles of donkey muscles from different body parts are significantly different, especially in terms of TGs and phospholipids (Li et al., 2021). These differences inevitably lead to the presence of different VOCs in different body parts. Nevertheless, there are relatively few studies that attempt to identify the characteristic VOCs of donkey meat and establish differential VOCs to discriminate meat from different body parts.
The headspace–gas chromatography–ion mobility spectrometry (HS–GC–IMS) is an emerging technology for the visualized detection of VOCs from foodstuffs, including those in meat samples (Liu et al., 2020). HS–GC–IMS allows high-accuracy and -sensitivity separation and qualitation of VOCs with no sample pretreatment, effectively solving the problems of conventional GC–MS technology associated with the loss of VOCs caused by long analysis times and complex sample pretreatment (Wang et al., 2020a). In recent years, GC–IMS combined with chemometrics has emerged as a promising approach for characterizing and visualizing differential VOCs in meat, such as chicken, pork, yak meat, and water-boiled salted duck (Aheto et al., 2020; Huang et al., 2022; Li et al., 2022b). Chemometrics models, such as partial least squares discriminant analysis (PLS-DA), are commonly used for sample classification (Zhu et al., 2023). Moreover, relative odor activity values (ROAVs) can be used to identify and study the contributions of individual VOCs to the aroma of meat (Zhu et al., 2020). However, few studies have combined the use of ROAVs with HS–GC–IMS and chemometrics to determine the VOCs of donkey meat.
Accordingly, in this study, we aimed to (i) obtain the VOC fingerprints of donkey meat using HS–GC–IMS; (ii) identify the characteristic VOCs of donkey meat by ROVA analysis; and (iii) identify differential VOCs for the discrimination of donkey meat samples from different cuts using chemometrics analysis. Thus, this work facilitates a new understanding of donkey meat flavor and provides a basis and method for the control of donkey meat flavor.
Materials and Methods
Meat samples were obtained from 20 Dezhou donkeys (Sanfen, male) in the slaughterhouse of Dong’e Tianlong Food (Shandong, China). The donkeys were provided by a local farm in Liaocheng City (Shandong, China) and reared under the same diet and management conditions. The composition and nutrient levels of the diet are provided in Table 1. The average final body weight of the donkeys was 236±28 kg. Slaughtering procedures were performed according to CAC/RCP 41-1993 and ISO/TS 34700:2016 international standards. The donkeys were electrocuted and bled to death, and then removed the skin. The longissimus dorsi (LD), gluteus maximus (GM), and biceps femoris (BF) were removed and immediately flash frozen in liquid nitrogen before being transported back to the laboratory, where they were stored in a refrigerator at –80°C.
A FlavourSpec®Flavour HS–GC–IMS instrument (Shandong Haineng Scientific Instrument, Shandong, China) was used to analyze the donkey meat samples. Analysis method for the VOCs in samples based on relevant references with few modifications (Man et al., 2023b). Briefly, a 1.5 g minced meat sample was placed into a 20 mL headspace vial and incubated at 60°C for 15 min. Subsequently, a 500 μL headspace sample was injected automatically into the GC–IMS instrument. The temperatures of the syringe and injector were set to 85°C and 45°C, respectively. GC separation was performed using an MXT-5 capillary column (15 m×0.53 mm; 1 μm) at 60°C under isothermal conditions. The GC column temperature was 60°C with nitrogen. Nitrogen (≥99.999% purity) was used as the carrier gas for 0–2 min at 2 mL/min and then 2–20 min at 100 mL/min. The length of the drift tube, linear voltage in the tube, and drift temperature used for IMS were 9.8 cm, 400 V/cm, and 45°C, respectively. Nitrogen was used as the drift gas at a flow rate of 150 mL/min. The retention indexes (RIs) of the VOCs were determined using C4–C9 n-ketones (Sinopharm Chemical Reagent Beijing, Beijing, China) analyzed under the same experimental conditions as references. Four parallel samples were analyzed under the same conditions.
The characteristic VOCs of the samples were evaluated by the ROAV method (Li et al., 2023). The formula for ROAV is as follow:
Where Ci and Ti are the relative percentage content of each VOC and the corresponding sensory threshold, respectively, and ROAVmax is defined as that contributing most to overall flavor and is set at 100. The key characteristic VOCs were defined as those with ROVA≥1, with higher values indicating greater contributions to overall flavor.
The data of VOCs in the donkey meat were performed by analyzed using SPSS 24 software (IBM, Armonk, NY, USA). The NIST and IMS databases in the FlavourSpec®Flavour Library were used to qualitatively identify VOCs of GC-IMS. Differences among samples were analyzed by one-way ANOVA and Tukey test. The results are presented as mean±SEM with a statistical significance difference in p<0.05. Two- and three-dimensional fingerprint maps of the VOCs were constructed by Gallery plug-in and Reporter plug-in on FlavourSpec®Flavour, respectively. Orthogonal partial least squares discriminant analysis (OPLS-DA) and heatmap visualization of the data were used by MetaboAnalyst 5.0 online software (https://www.metaboanalyst.ca/). Variable importance in projection (VIP)>1 and p<0.05 were utilized to screen for differences VOC molecules among samples.
Results and Discussion
The VOCs in different parts of donkey meat are shown in Fig. 1 and Table 2. Of the 40 VOCs detected in donkey meat, 31 were identified in donkey meat (Fig. 1A and Table 2). This number of VOCs is significantly lower than the numbers of VOCs (109 and 122 VOCs) identified in two previous studies performed using GC–MS (Maggiolino et al., 2020; Polidori et al., 2022). This is because, compared with GC–MS, smaller-molecule VOCs are detected by GC–IMS, the number of which is limited. Furthermore, the lack of a GC–IMS library corresponding to the NIST GC–MS library is another reason that GC–IMS identifies a lower number of VOCs in food flavor analysis (Wang et al., 2020b). However, GC–IMS can detect VOCs in a sample (Fig. 1A) with high sensitivity and no pretreatment, so it can be used to supplement GC–MS for better detection of isomers in meat (Table 2).
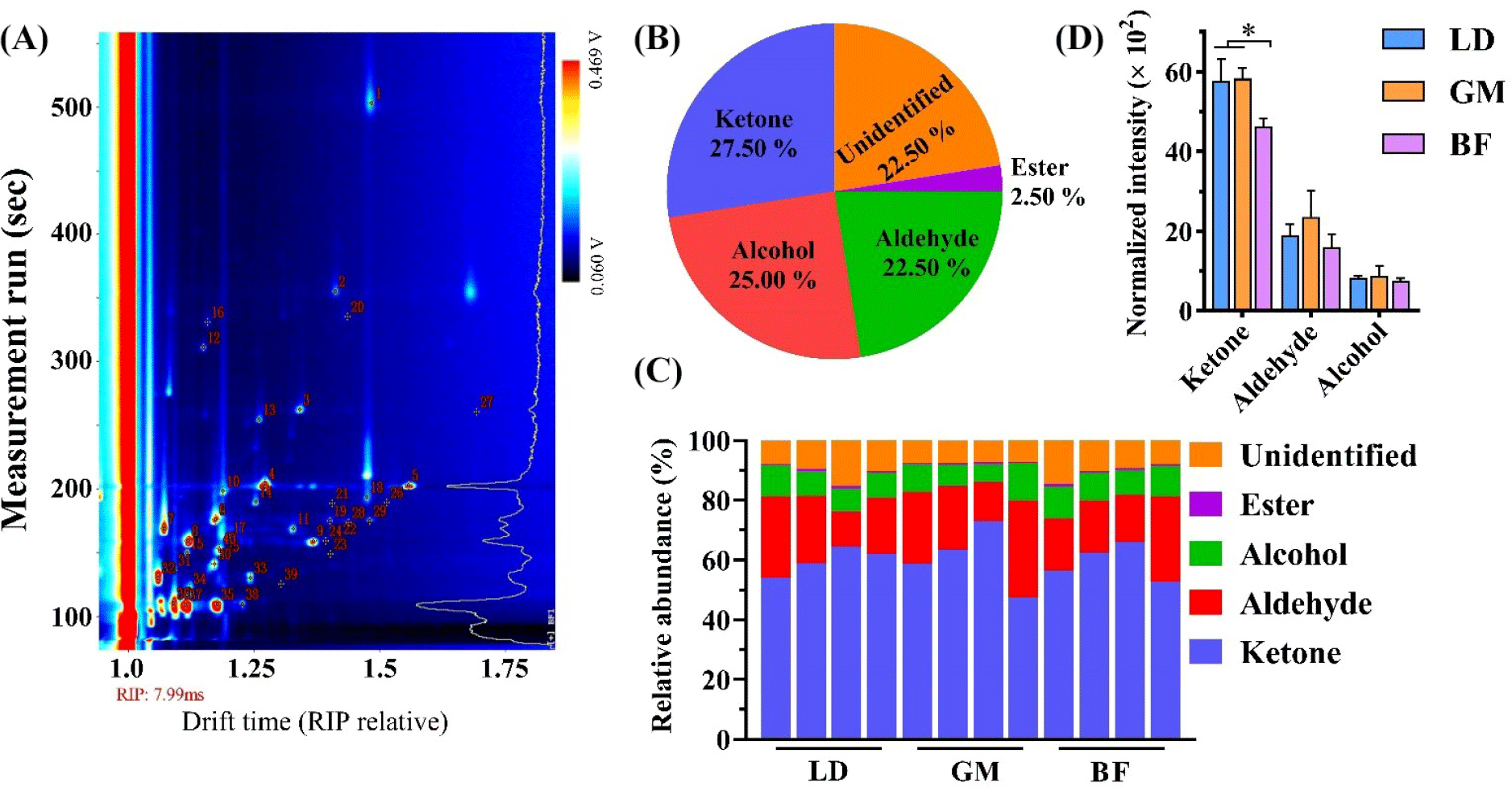
The VOCs are classified into five categories; 27.50% ketones, 25.00% alcohols, 22.50% aldehydes, 2.25% esters, and 22.50% unidentified (Fig. 1B). Thus, ketones are the most abundant VOCs, followed by aldehydes and alcohols in donkey meat (Fig. 1C). This is in agreement with previous studies showing that ketones, alcohols, and aldehydes are the predominant VOCs in donkey meat (Li et al., 2020; Man et al., 2023a). The ketone concentrations are significantly higher in the LD and GM tissue than in BF tissue (p<0.05; Fig. 1D). C20:4 phospholipids can be oxidized to produce ketones (Zhou et al., 2014), and previous studies have shown that the donkey GM is rich in C20:4 phospholipids, including PC (O-18:2/20:4), PC (P-16:0/20:4), PE (19:0/20:4), and PE (18:1/20:4; Li et al., 2021). In addition, aldehydes and alcohols mainly come from the degradation of 18:2 and 18:3 lipids in food (Amanpour et al., 2019). Donkey meat is abundant in polyunsaturated fatty acids compared with beef and mutton, especially 18:2 and 18:3 (Man et al., 2023a). It is well known that aldehydes, alcohols, and ketones contribute substantially to meat flavor (Polidori et al., 2022). The current study confirms that ketones, alcohols, and aldehydes are the predominant VOCs in donkey meat.
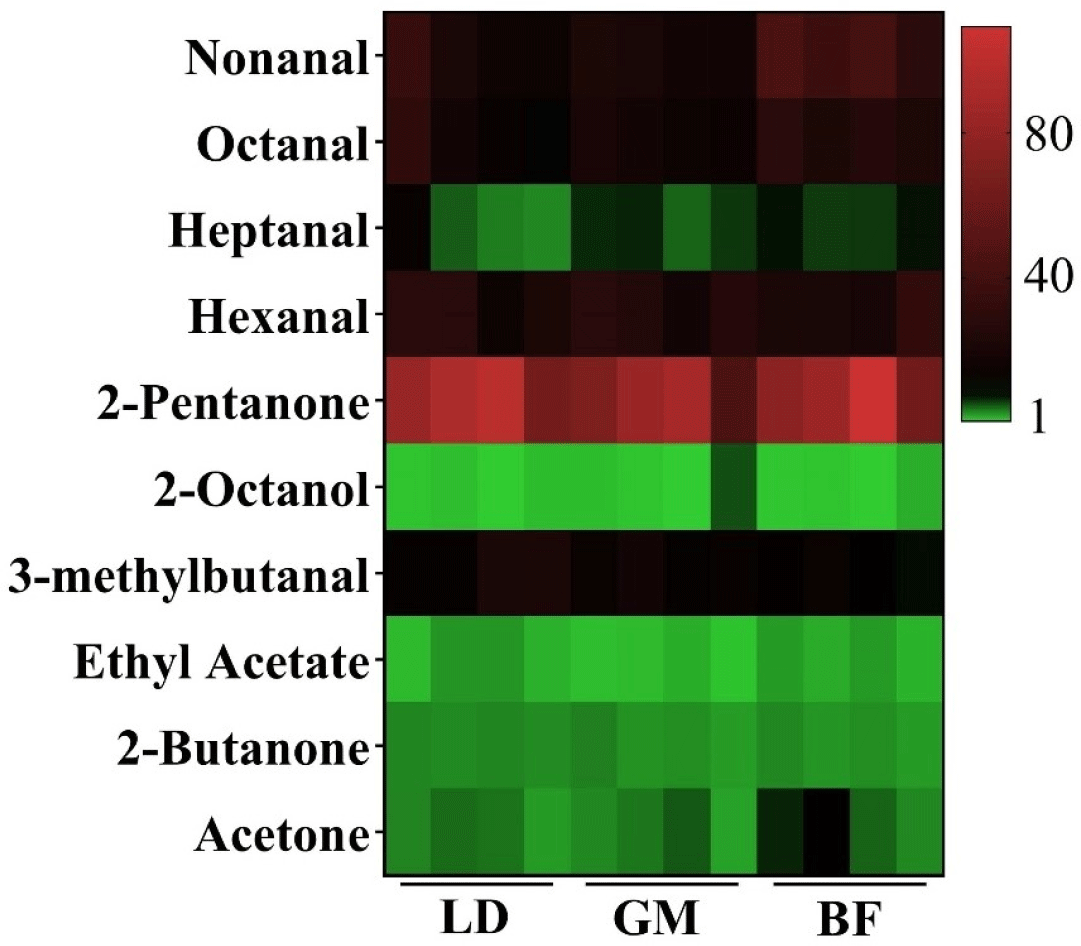
As shown in Table 2, acetone shows the highest abundance in donkey meat, followed by 3-hydroxybutan-2-one, hexanal, and 2-pentanone. The importance of VOCs in meat depends on not only their contents but also their ROAVs (Liu et al., 2022). ROAVs ca be used to determine the contributions of VOCs to overall flavor profiles, with ROAVs≥1 representing key VOCs in molecular sensory science (Xu et al., 2017). In the present study, a total of 10 characteristic VOCs with ROAVs≥1 were identified in the donkey meat (Fig. 2), including 2-pentanone (ROAV=78.21–100.00), hexanal (ROAV=17.35–39.35), nonanal (ROAV=16.95–55.09), octanal (ROAV=15.01–38.63), 3-methylbutanal (ROAV=9.48–25.84), heptanal (ROAV= 3.74–14.98), acetone (ROAV=2.58–10.22), 2-butanone (ROAV=2.82–4.10), ethyl acetate (ROAV=1.08–3.20), and 2-octanol (ROAV=0.59–2.02). This is consistent with our previous study, in which heptanal, 1-octen-3-ol, ethyl acetate, and hexanal with OAVs≥1 were determined to be the predominant flavor compounds in donkey meat (Man et al., 2023b). The characteristic flavors of meats and their products has been extensively analyzed in recent years (Sohail et al., 2022). For instance, hexanal, (E,E)-2,4-decadienal, and 1-octen-3-ol were shown to have the most predominant impact on the overall flavor of sheep muscles (Li et al., 2022c); hexanal, heptanal, and 1-octen-3-ol were determined as the characteristic odorants in roasted mutton (Liu et al., 2022); hexanal and 1-octen-3-ol were found to be the major VOCs in Chinese chickens (Jin et al., 2021); hexanal, nonanal, and 1-octen-3-ol were identified as the main contributors to the overall flavor of boiled pork (Han et al., 2020); and hexanal, heptanal, octanal, nonanal, and 1-octene-3-ol were shown to be the key VOCs in Beijing roast duck (Liu et al., 2019). Interestingly, recent studies have shown that hexanal, heptanal, and octanal are main VOCs in Dezhou donkey meat according to their relative contents without using ROAVs (Li et al., 2020). Furthermore, hexanal and 2-pentyl-furan were shown to have a higher abundance in Martina Franca donkey meat according to their relative contents determined by GC–MS (Maggiolino et al., 2020). Thus, these characteristic VOCs determine the specific flavor of meat and are species dependent, which is why different kinds of meat have unique aromas (Wu et al., 2022). Our results indicate that 2-pentanone, hexanal, nonanal, octanal, and 3-methylbutanal predominantly contribute to the unique flavor of donkey meat.
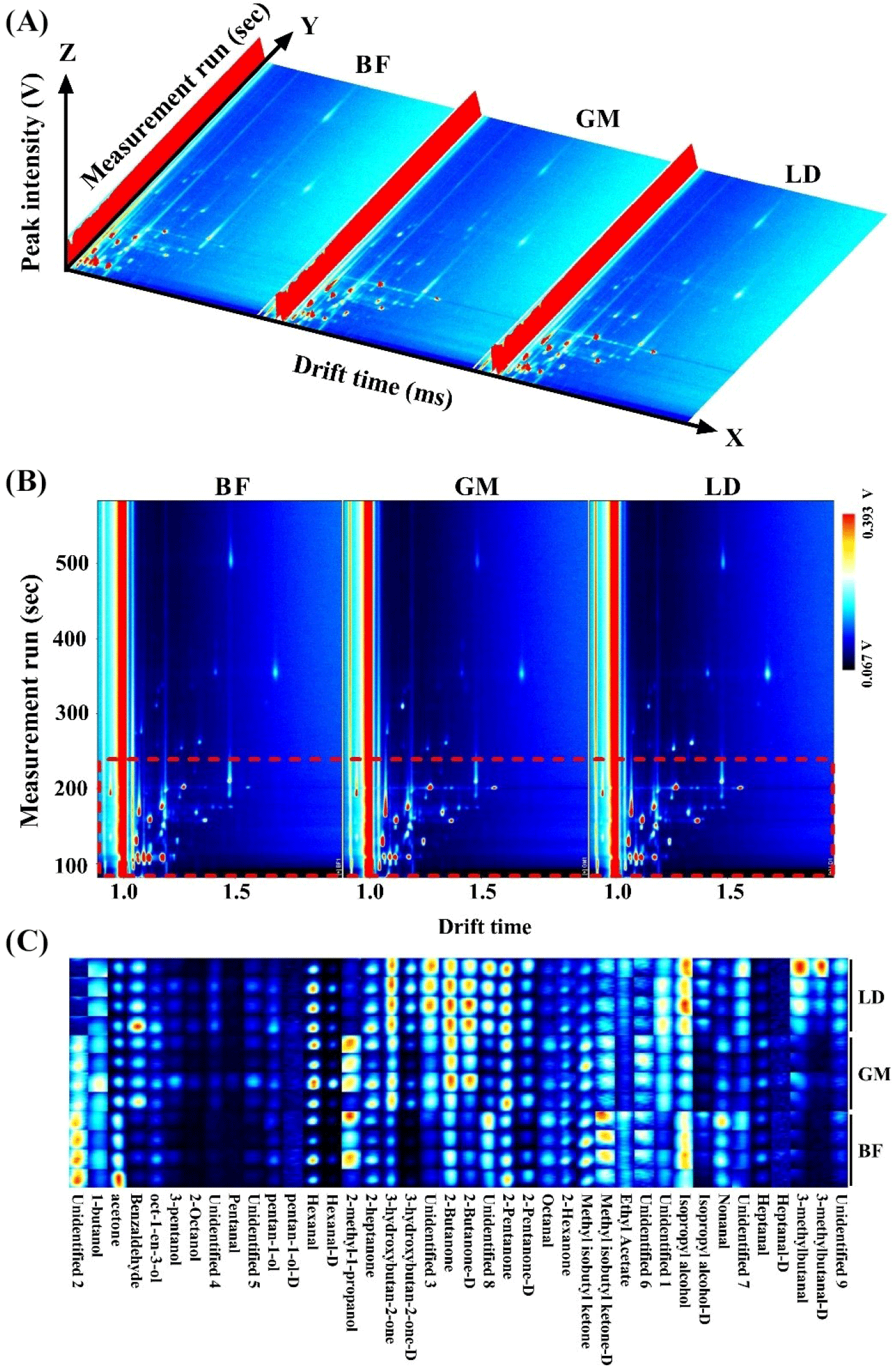
Topographic plots of the VOC fingerprints for different cuts of donkey meat are shown in Fig. 3. The different VOCs in different body parts are shown in a three-dimensional spectrum (Fig. 3A) and a top view (Fig. 3B). The fingerprint gallery plots (Fig. 3C) further demonstrate the differential VOCs in different cuts, including 2-butanone, 2-butanone-D, and 2-heptanone. These results are consistent with those of a previous study in which GC–IMS spectra and fingerprints were shown to intuitively discriminate differential samples (Wang et al., 2020a). This indicates that donkey meat samples from different body parts can be quickly discriminated by GC–IMS analysis through their VOC profiles. To further discriminate the VOCs from different cuts of donkey meat, chemometrics was applied to analyze the GC–IMS data, including supervised OPLS-DA-based, variance, and heatmap analyses. As shown in Fig. 4A, the donkey meat cuts are well differentiated by OPLS-DA. Validation plots show that the OPLS-DA results are reliable and free from overfitting (Fig. 4B). A previous study has demonstrated that OPLS-DA can discriminate different samples (Li et al., 2022d), which is consistent with our results. VIP values represent the weight values of OPLS-DA model variables and reflect the importance of cumulative differences between metabolites for sample grouping. When the VIP of a variable is >1, the variable is important, so it is usually used as a screening condition for potential biomarkers.
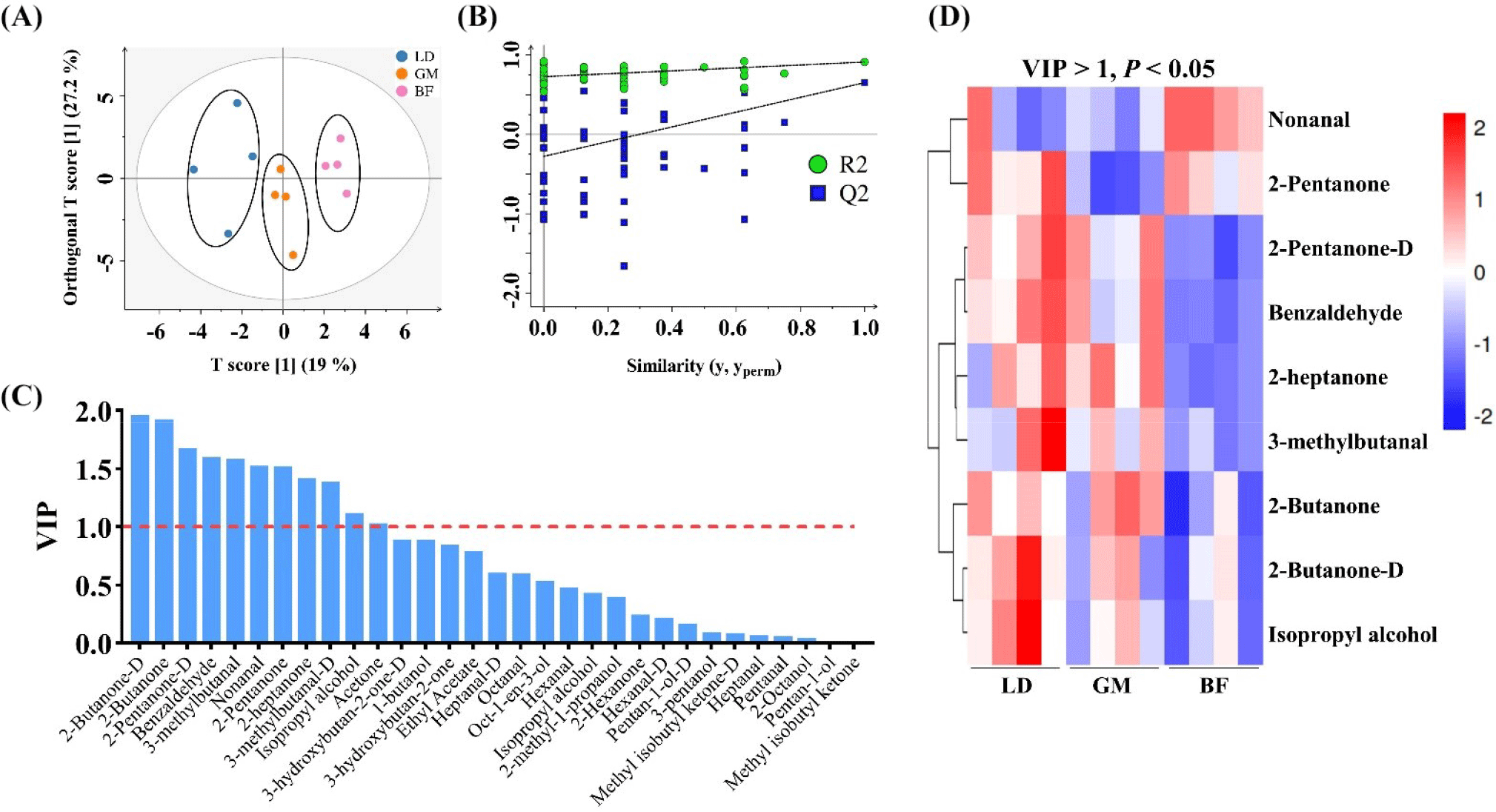
A total of 11 differential VOCs were identified using the criterion VIP>1 (Fig. 4C). In addition, 10 VOCs show significant difference for different cuts by p<0.05 (Table 3). The levels of benzaldehyde, 2-heptanone, 3-methylbutanal, 2-butanone, and 2-butanone-D are significantly higher for LD and GM than for BF (p<0.05), whereas nonanal shows the opposite trend (p<0.05; Table 3). As shown in Fig. 4D, nine differential VOCs were identified for different cuts of donkey meat by setting VIP>1 and p<0.05, which is consistent with the fingerprint results. Previous studies have demonstrated that hexanal, 1-octen-3-ol, and 2,3-octanedione distinguish pork cuts in indigenous Chinese pig breeds (Wu et al., 2022); the concentrations of hexanal and 1-octen-3-ol in mutton are positively correlated with lipid concentrations (Li et al., 2022a); and TGs and phospholipids may be key lipids for binding and generating VOCs in meats, respectively (Liu et al., 2022). Furthermore, our previous findings showed that levels of TG and phospholipid molecules are significantly different in different cuts of donkey meat (Li et al., 2021). In this study, nonanal, 2-pentanone, benzaldehyde, 2-heptanone, 3-methylbutanal, 2-butanone, and isopropyl alcohol were identified as potential markers to distinguish cuts of donkey meat. Lipids are precursors for the formation of VOCs in meat (Bassam et al., 2022). Interestingly, these VOC markers are the products of lipid degradation. For example, nonanal and benzaldehyde are mainly generated by the oxidative degradation of oleic acid and α-linolenic acid (Elmore et al., 2005). Thus, these results indicate that chemometrics analysis of HS–GC–IMS data can discriminate different samples and identify biomarkers.
Conclusion
In this study, characteristic and differential VOCs in different parts of donkey meat were comprehensively investigated by HS–GC–IMS combined with chemometrics. Overall, 31 VOCs belonging to four categories were identified, among which ketones, alcohols, and aldehydes were found to be characteristic VOC categories for donkey meat, and pentanone, hexanal, nonanal, octanal, and 3-methylbutanal were found to be the characteristic VOCs. Nine differential VOCs were identified as potential markers to discriminate cuts of donkey meat, including 2-butanone, 2-pentanone, and 2-heptanone. Thus, HS–GC–IMS combined with chemometrics is a convenient and powerful method for revealing the characteristic VOCs of donkey meat and potential markers to discriminate different cuts. These results revealed the composition of VOCs in donkey meat and the differences in different parts, provide a novel scientific basis and method for the regulation of donkey meat flavor.













