Introduction
Edible insects are a sustainable alternative protein source attracting attention due to environmental and food security problems. Consumer interest in healthy foods and a sustainable environment is driving the demand for meat analogs (Ismail et al., 2020). As the demand for food increases due to an expanding global population, developing alternative sustainable protein products that mitigate environmental and food security concerns is needed to replace livestock production (Lee et al., 2019; Van Huis, 2013). For example, edible insects can be further developed as an alternative protein source (food or feed), as they have several advantages, such as cost-effective production, high nutritional value, and the mitigation of environmental pollution (Van Huis, 2013). Therefore, many companies and academics are investigating the use of insect-derived proteins in food.
According to a study by Lee and Kang (2019), disgust (29.6%) was the foremost reason for not purchasing food containing edible insects. In addition, the study showed that consumers were aware that edible insects are high in protein. However, eating insects has a negative perception because of their repulsive appearance. Therefore, most edible insects are ground and processed for use in food so that consumers do not recognize them (Lee et al., 2021; Zielińska et al., 2018). Therefore, it is necessary to study insect protein extracts to expand the applicability of edible insects to food. Additionally, understanding the functional properties of these protein extracts is important for improving food quality (Lee et al., 2019).
Tenebrio molitor (TM) larvae have been utilized as animal feedstuffs because its production efficiency is high compared to imago (Hong et al., 2020). In their study, considering that all parts of the insect are edible, the feed conversion ratio of TM larvae is considered to be higher than those of chicken and pork meat (Wilkinson, 2011). Protaetia brevitarsis (PB) larvae contain a balanced composition of essential amino acids (Histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, and valine) and can be used as a rich source of protein (Chung et al., 2013; Kim et al., 2019). Therefore, TM and PB larvae were used to protein source in this study. This study aimed to investigate the functional properties of salt-soluble proteins obtained from edible insects and examine the interaction between these proteins and porcine myofibrillar protein (MP). Furthermore, the applicability of insect salt-soluble proteins in meat products was reviewed.
Materials and Methods
PB larvae (3rd instar) and TM larvae (10th to 11th instars) fed pumpkin were obtained from Insect company (FG & Bio) located in Jinju, Korea. PB larvae (50–70 larvae in a PVC box) and TM larvae (500–550 larvae in a box) were grown in PVC boxes (490×380×80 mm3) containing fermented sawdust. The insect larvae used in this experiment were fasted for 2 to 3 days before insect protein extraction. After the fasting process, the larvae were rapidly frozen at −70°C. To extract the insect salt-soluble proteins, 400 g of frozen PB or TM larvae and 800 mL of 50 mM phosphate buffer (pH 6.25) were homogenized at 4°C for 90 sec., and then centrifuged at 930×g for 15 min (Model Supra 22K; Hanil Scientific, Daejeon, Korea). This process was repeated three times. The obtained pellet was homogenized (4°C for 90 sec) with 800 mL of 50 mM phosphate buffer (pH 6.25) containing 0.1 M NaCl and centrifuged (930×g for 15 min). After mixing the pellet and 0.1 M NaCl solution (pH 6.25), the insects’ exoskeleton was separated by filtering using a sterile gauze. Finally, the salt-soluble protein was obtained by centrifugation at 930×g for 15 min. A part of the extracted insect salt-soluble protein was used to investigate the insect salt-soluble protein gel properties, and the other part was lyophilized and added to the pork MP gel system.
The protein concentration of the extracted insect salt-soluble proteins was measured using a bicinchoninic acid (BCA) protein assay kit (PierceTM BCA Protein Assay Kit #23227; Thermo Fisher Scientific, Waltham, MA, USA). The final protein concentrations of PB and TM salt-soluble protein were adjusted to 30 mg/mL by mixing 0.05 M sodium phosphate buffer (pH 6.25) containing 0.45 M NaCl. Five grams of the insect salt-soluble proteins was transferred to a vial (Fisher Scientific, Leicestershire, UK), and the samples were cooked from room temperature to 80°C (3°C/min) in a water bath (WB-22, Daihan Scientific, Seoul, Korea). The gels were placed in an ice box immediately after cooking to cool and then maintained at 0°C until use in the experiment.
Pork ham (200 g) was prepared according to followed by Chin et al. (2009) method to extract MP. First, the pork meat was homogenized with 800 mL of 50 mM sodium phosphate buffer containing 0.1 M NaCl (pH 6.25) for 90 sec. The homogenate was centrifuged at 930×g for 15 min at 4°C to obtain a MP pellet. The pellet was mixed with sodium phosphate buffer and centrifuged again; this process was repeated a total of three times. The resulting MP pellet was mixed with 0.1 M NaCl solution (pH 6.25), and then the connective tissue was removed through a sterile gauze. The final MP pellet obtained through centrifugation (930×g for 15 min) was used to measure the protein concentration by the biuret method. Based on the protein concentration, the MP pellet was mixed with 0.05 M sodium phosphate buffer containing 0.45 M NaCl (pH 6.25) to adjust the final MP concentration to 40 mg/mL.
The extracted and lyophilized PB or TM salt-soluble proteins were added to the extracted pork MP. To measure the protein concentration of lyophilized TM and PB salt-soluble protein powders, they were homogenized in double distilled (dd)-water and the protein concentration of diluted solution was measured using the modified method for BCA protein analysis (Wu et al., 2016). The measured protein concentration was multiplied by 100, which is the dilution factor. The protein concentrations of the lyophilized TM and PB salt-soluble protein powders were 436 and 1,122 mg/mL, respectively. Insect protein powder was added to MP and adjusted to 1% or 2% of the total MP protein concentration (40 mg/mL). In this study, five treatments were prepared as follows: MP, MP mixture without insect protein; TM 1%, MP mixture containing TM1%; TM2%, MP mixture containing TM 2%; PB 1%, MP mixture containing PB 1%; PB 2%, MP mixture containing PB 2%. The cooking conditions for the MP mixture containing insect protein powder were the same as preparation of the insect salt-soluble protein gel section.
The viscosity of the TM and PB salt-soluble proteins and the MP mixtures with or without TM or PB proteins were performed using a RC 30 rheometer (RheoTec Messtechnik, Dresden, Germany). The concentric cylindrical probe of the RC 30 rheometer was filled with the protein samples, and a controlled shear stress test was performed. The viscosity was arranged by Excel software version 2016 (Microsoft, Redmond, WA, USA).
A 10% acrylamide separating gel and 4% acrylamide stacking gel were prepared using the Mini-PROTEAN® 3 Cell (Bio-Rad Laboratories, Hercules, CA, USA) according to the method of Lee and Chin (2019). Loading samples containing 1% protein and 2× Laemmli sample buffer (#161-0737; Bio-Rad Laboratories) were loaded with precision plus protein standards (#161-0373; Bio-Rad Laboratories) at 150 V. The acrylamide gel was stained and destained using a staining solution (500 mL of methanol, 68 mL of acetic acid, 1 g of Coomassie brilliant blue, and distilled water to 1 L) and destaining solution (300 mL of acetic acid, 200 mL methanol, and distilled water to a total of 1 L).
The protein surface hydrophobicity and sulfhydryl (-SH) group levels were measured to evaluate the tertiary structure of the proteins in the MP gel systems. The hydrophobicity was determined as described by Chelh et al. (2006), with a slight modification. First, 1 mL of protein (2 mg/mL) or control (20 mM sodium phosphate buffer, pH 6) samples were reacted with 40 μL of bromophenol blue solution (1 mg/mL) for 10 min. After centrifugation (8,000×g for 15 min), the absorbance (A) of the supernatant was measured at 595 nm.
The -SH group level was determined using 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB; Sigma-Aldrich®, Merck KGaA, Darmstadt, Germany) according to the method of Cui et al. (2009). A 0.5 mL of protein sample (2 mg/mL) was reacted with 2.5 mL of Tris-glycine-8M urea and 20 μL of DTNB solution (4 mg/mL) for 30 min, and the absorbance (A) of the sample was measured at 412 nm.
The cooking yield (%) of the protein gel was calculated as the difference in mass before and after cooking. In addition, the gel strength (gf, break force) of the protein gel was measured using an Instron Universal Testing Machine (Model#3344, Canton, MA, USA) equipped with a probe head (speed 500 mm/min).
The MP gels containing the lyophilized TM or PB salt-soluble protein powders were prepared in cube-shape (3×3×3 mm3) for the LV-SEM (Model JSM-6610LV; JEOL, Tokyo, Japan). The cube-shaped samples were fixed using 2.5% glutaraldehyde solution (in 0.1 M sodium phosphate buffer, pH 7) and 1% of osmium tetroxide solution (in 0.1 M sodium phosphate buffer, pH 7). The fixed samples were washed three times with 0.1 M sodium phosphate buffer (pH 7), dehydrated with different levels of ethanol (50%, 60%, 70%, 80%, 90%, and 100%), and then further dehydrated with the acetone. The dried samples were coated with gold using an automatic sputter coater (Model 108; Cressington Scientific Instruments, Watford, England).
Results and Discussion
Fig. 1 shows the viscosity results of the insect salt-soluble protein extracts. The PB salt-soluble protein extract showed higher viscosity than the TM extract. However, the viscosity of the PB protein extract showed higher shear stress than those of the TM extract. Kim et al. (2020a) reported that the viscosity of PB protein was higher than those of TM, similar to the results of this study. In contrast, Kim et al. (2021a) reported that the apparent viscosity of an insect protein-based emulsion was higher when TM protein was used and compared with PB, indicating that emulsified insect proteins may have different properties in the emulsion system depending on insect species.
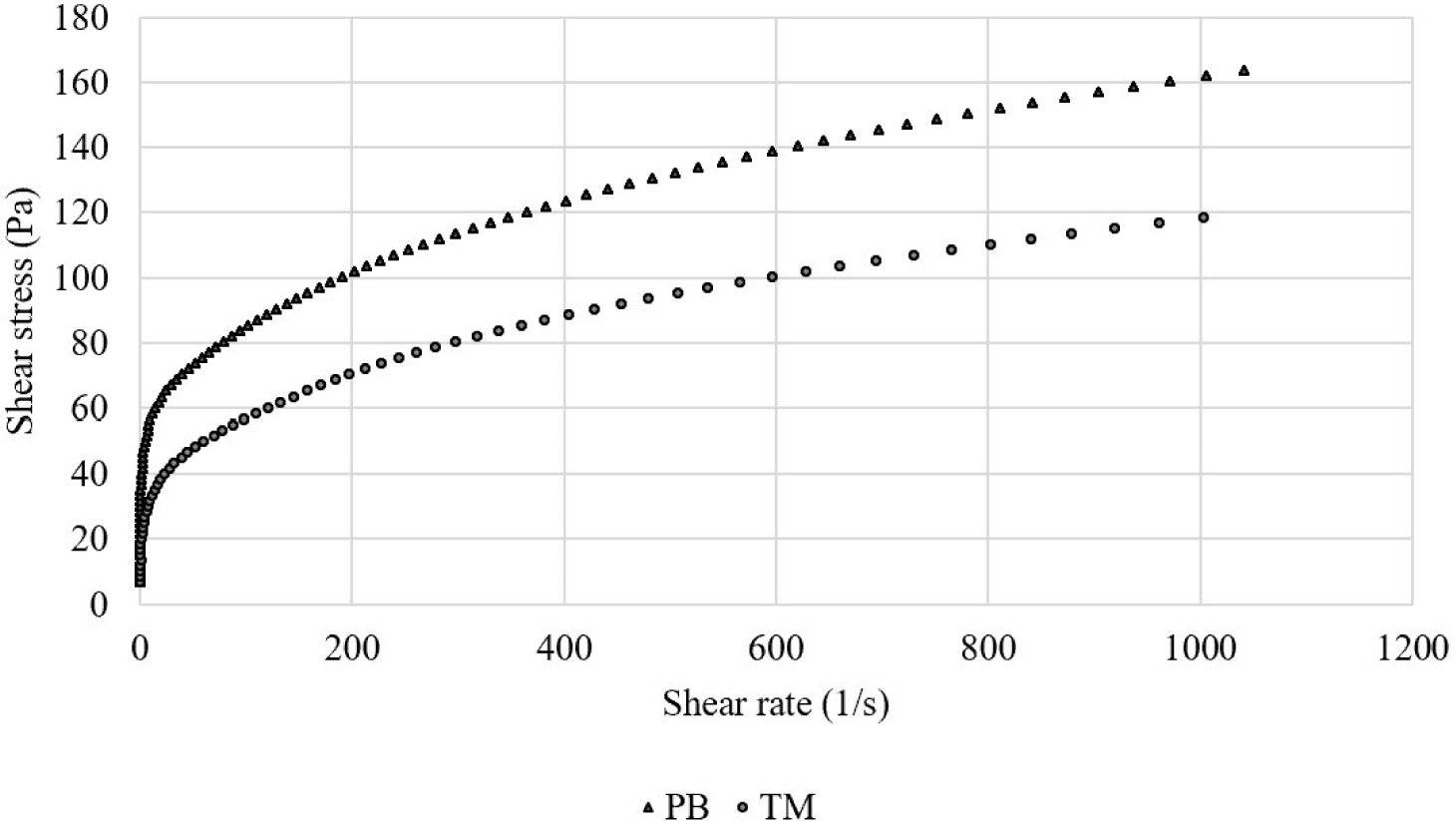
The gel strength results of the insect salt-soluble protein gels are shown in Fig. 2. In contrast to the viscosity results (Fig. 1), the gel strength results showed that insect protein gels extracted from TM had higher than those from PB (p<0.05). In a study by Kim et al. (2020a), heat-induced TM protein gel had harder than heat-induced PB protein gel. Kim et al. (2021a) reported that in the edible insect-based emulsion, TM emulsion had higher hardness than PB emulsion (cooked TM emulsion formed a hard emulsion). It was confirmed that the heat-induced gel strength of salt-soluble proteins extracted from insects varies depending on the insect species used.
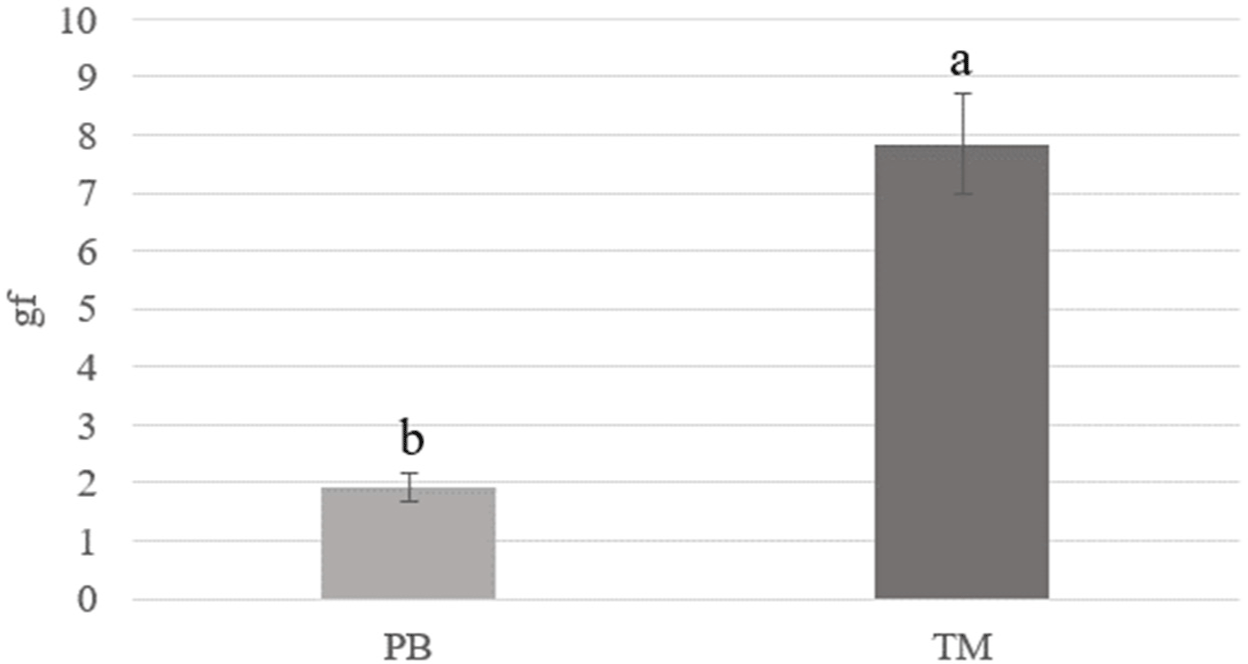
Fig. 3 shows the protein band patterns of the edible insect salt-soluble proteins. Depending on the type of insect, the electrophoretic protein pattern was diverse. Protein bands with various molecular weights ranging from 20 kDa to 250 kDa were observed in salt-soluble proteins (without heat treatment) extracted from two insect larvae. Protein bands with molecular weight above 75 kDa showed similar patterns in TM and PB, but differences in band intensity were found. A biopolymer band was formed at the top of the TM salt-soluble protein, even before heating. Below 75 kDa, differences in protein bands between insects were observed. After heating the PB and TM proteins to 80°C, the protein bands distributed before heating disappeared or faded, and a biopolymer formed on the top of the SDS-PAGE gel. Differences in protein patterns between TM and PB salt-soluble proteins might be due to differences in chitin content, amino acid composition, and types of proteins depending on the edible insect species (Kim et al., 2020b). Therefore, it is necessary to check the protein pattern according to the pretreatment process, extraction process, and insect species.
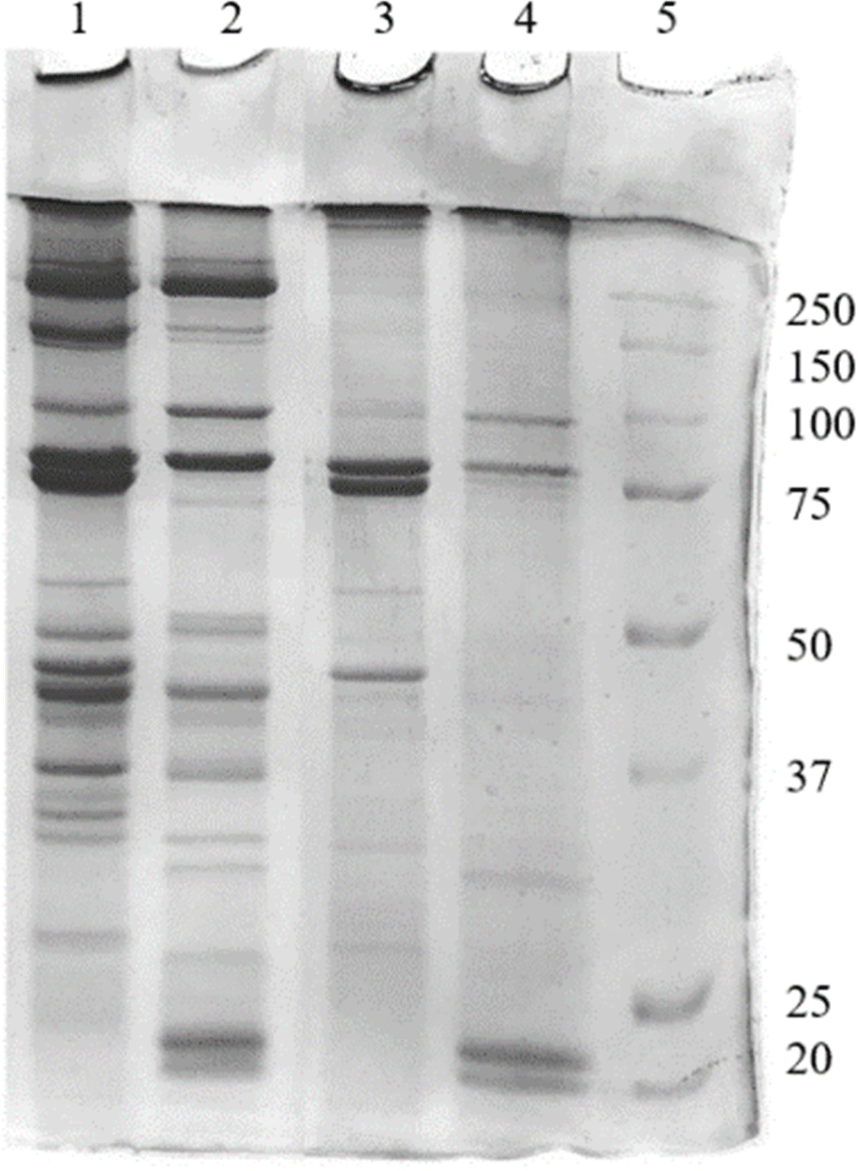
Fig. 4 shows the viscosity results of the pork MP with and without added PB or TM lyophilized salt-soluble protein powder. Comparing the viscosities of the MP showed that the pure MP had a slightly higher viscosity. Adding the insect salt-soluble protein powders tended to decrease the viscosity slightly. Choi et al. (2017) have previously reported that the viscosity of meat batter partially replaced with yellow mealworm was lower than that of pure pork meat batter alone. Furthermore, Kim et al. (2021b) showed a similar trend and suggested that this was possible because the non-protein fraction interfered with the formation of protein–protein cross-linking due to the weakened gel structure.
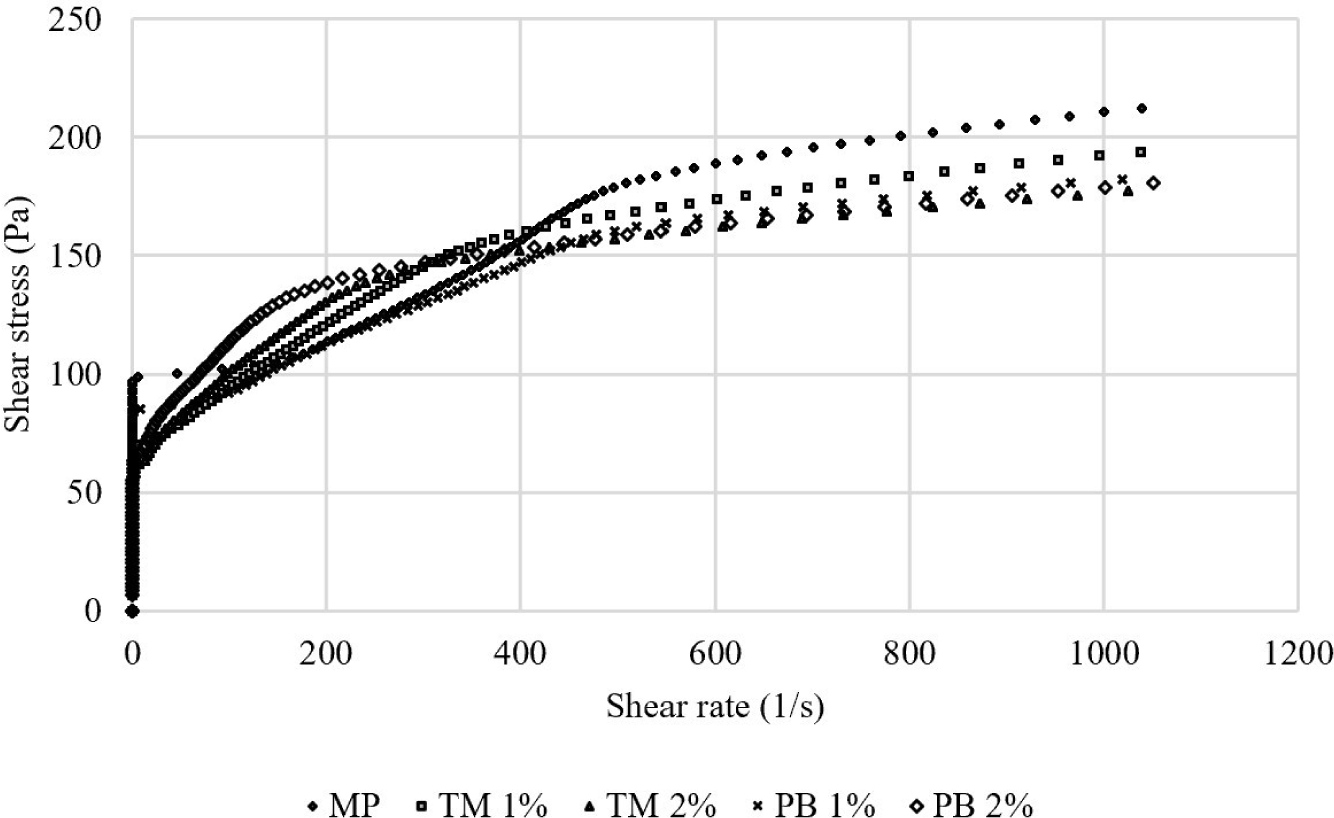
Table 1 shows the pork MP gels’ protein surface hydrophobicity and -SH group levels without and with edible insect protein powder at various concentrations. Conformation and intermolecular bonds of proteins have been reported to contribute to the gelation properties of pork proteins (Liu et al., 2011). Adding the extracted TM and PB protein powders at 1% and 2% did not affect the MP gels’ hydrophobicity and -SH group levels (p>0.05). It has been reported that hydrophobic, disulfide, non-specific, ionic, and hydrogen bonds constituting N-acetyl-D-glucosamine components such as chitin increase when the PB protein to MP ratio increases (Kim et al., 2021b; Pillai et al., 2009). However, there was no effect in this study due to the low content (1%–2%) of insect protein powder in the pork MP gels compared with the study by Kim et al. (2021b), who replaced more than 20% of the MP with PB protein.
Fig. 5 shows the SDS-PAGE results of the pork MP gels with and without insect salt-soluble protein powder. According to Ha et al. (2012), the molecular weight of myosin heavy chain and actin is 222–223 kDa and 42 kDa, respectively. The protein bands of the pork MP gels did not change with the type or amount of insect protein powder added. Kim et al. (2022a) stated that because insects are processed whole due to their small size, uncharacterized and unknown components may affect protein interactions and protein molecular weight. When 1% of PB or TM powder was added to pork MP, changed protein bands were seen on SDS-PAGE analysis. In contrast, no change was observed in this study, which might indicate that the amount of insect protein added to the pork MP gels were too low to affect the protein molecular weight.
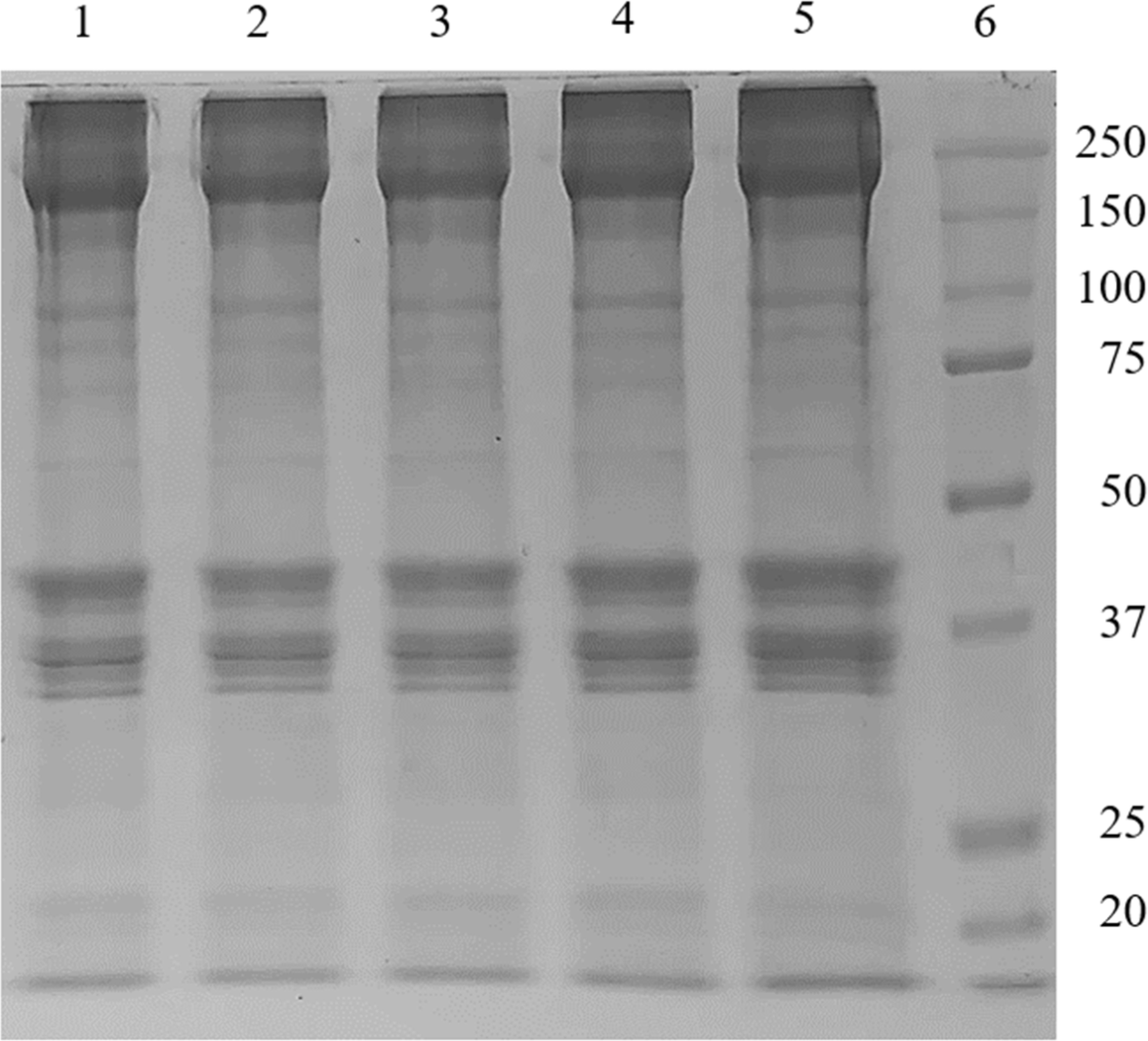
Fig. 6 shows the cooking yield (CY, %) of the pork MP gel alone or various levels of lyophilized insect salt-soluble protein powder. The CYs of the MP gels containing PB or TM protein powder at 1% or 2% were higher than the MP gel without insect protein (p<0.05). Furthermore, in the MP gels that contained insect protein powder, the PB 1% group showed the lowest CY (p<0.05). In the TM treatment groups (1% and 2%), no difference in the CY was observed (p>0.05), although they had a higher CY than the MP gel with PB 1% (p<0.05). In addition, there were no differences in CYs between the PB 1% and 2% treatments (p>0.05). Heating results in the thermal denaturation of proteins, causing water to be expelled from meat protein systems (Palka and Daun, 1999). According to Kim et al. (2022b), pork MP gels added with isolated insect protein may have a negative effect on texture (resulting in a soft texture) due to high cooking loss.
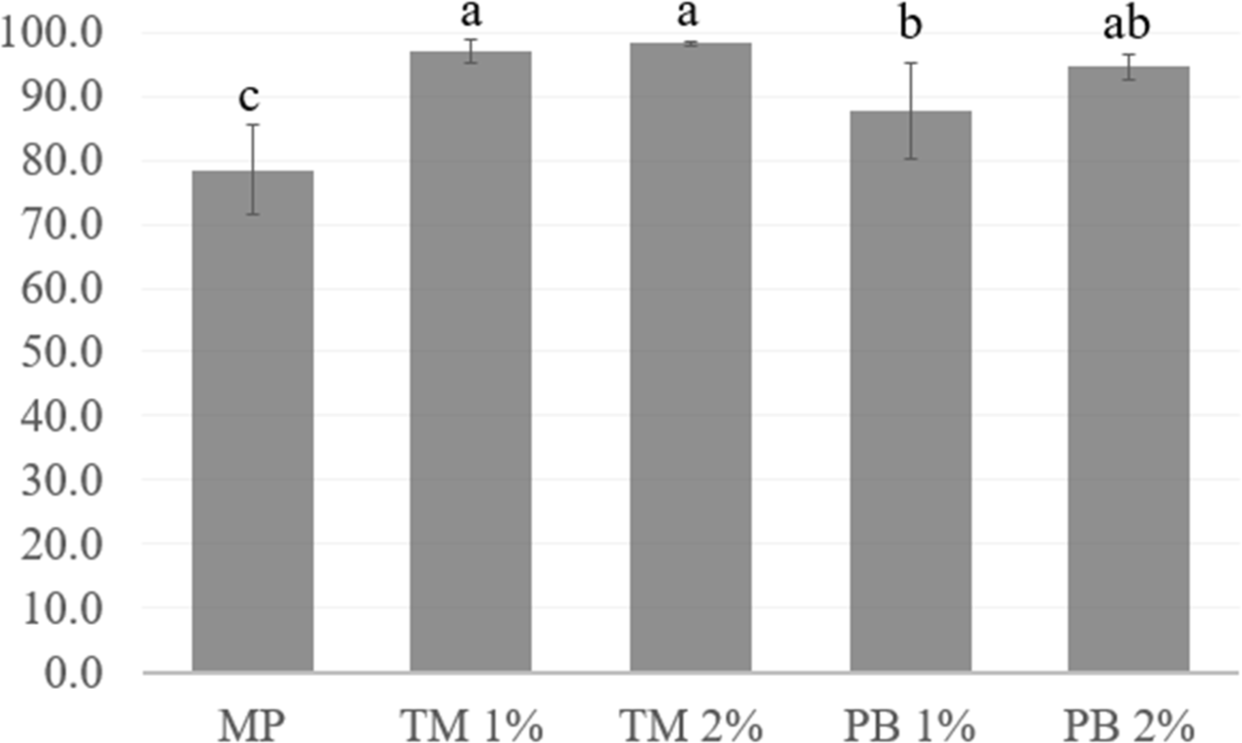
Fig. 7 shows the gel strength of the MP gels without and with added lyophilized insect powder. Regardless of the type of insect protein powder added (PB or TM), the gel strength of MP gels decreased as compared to those without insect protein powders (p<0.05). However, no difference in gel strength with the addition or increased levels of insect powder. Since the gel strength of the pork MP gels was reduced when lyophilized insect salt-soluble protein powder was added, stratedy to solve this problem should be the focus of future studies. A previous study reported by Kim et al. (2021b) demonstrated that partially replacing pork MP with PB protein powder negatively affected textural properties. This trend was similar to the results of studies on other insects, including TM, Acheta domesticus, and Allomyrina dichotoma proteins (Choi et al., 2017; Ho et al., 2022; Kim et al., 2022a). Bang et al. (2015) identified a serine protease in PB larvae. In addition, Janssen et al. (2019) reported that endogenous proteases were present in insects’ mid-gut, which had residual activity when proteins were extracted using a buffer at pH 8. Therefore, these authors suggested that insect proteins could be used as food ingredients by developing a method to control endogenous protease degradation (Janssen et al., 2019). Further studies are needed to determine if the lower gel strength of MP gels with the insect protein compared with the control was due to the poor gelation ability of the insect proteins itself or proteolysis by endogenous proteases present in the powder.
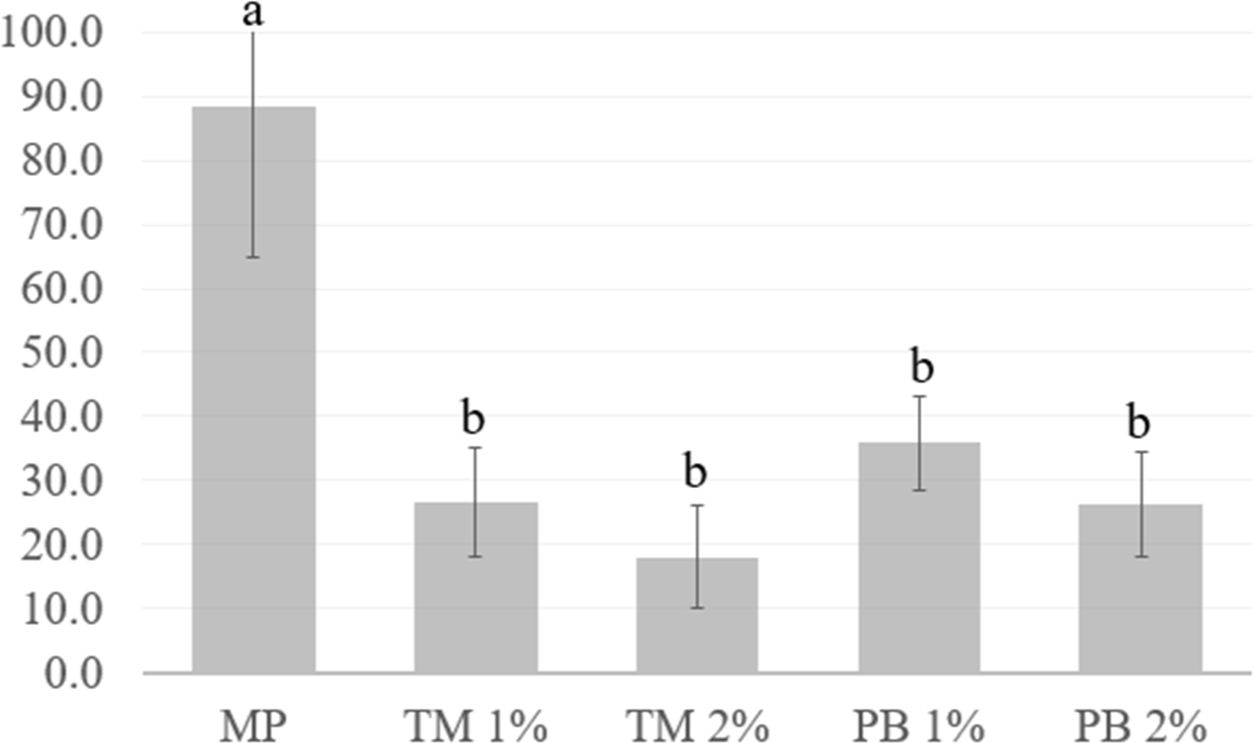
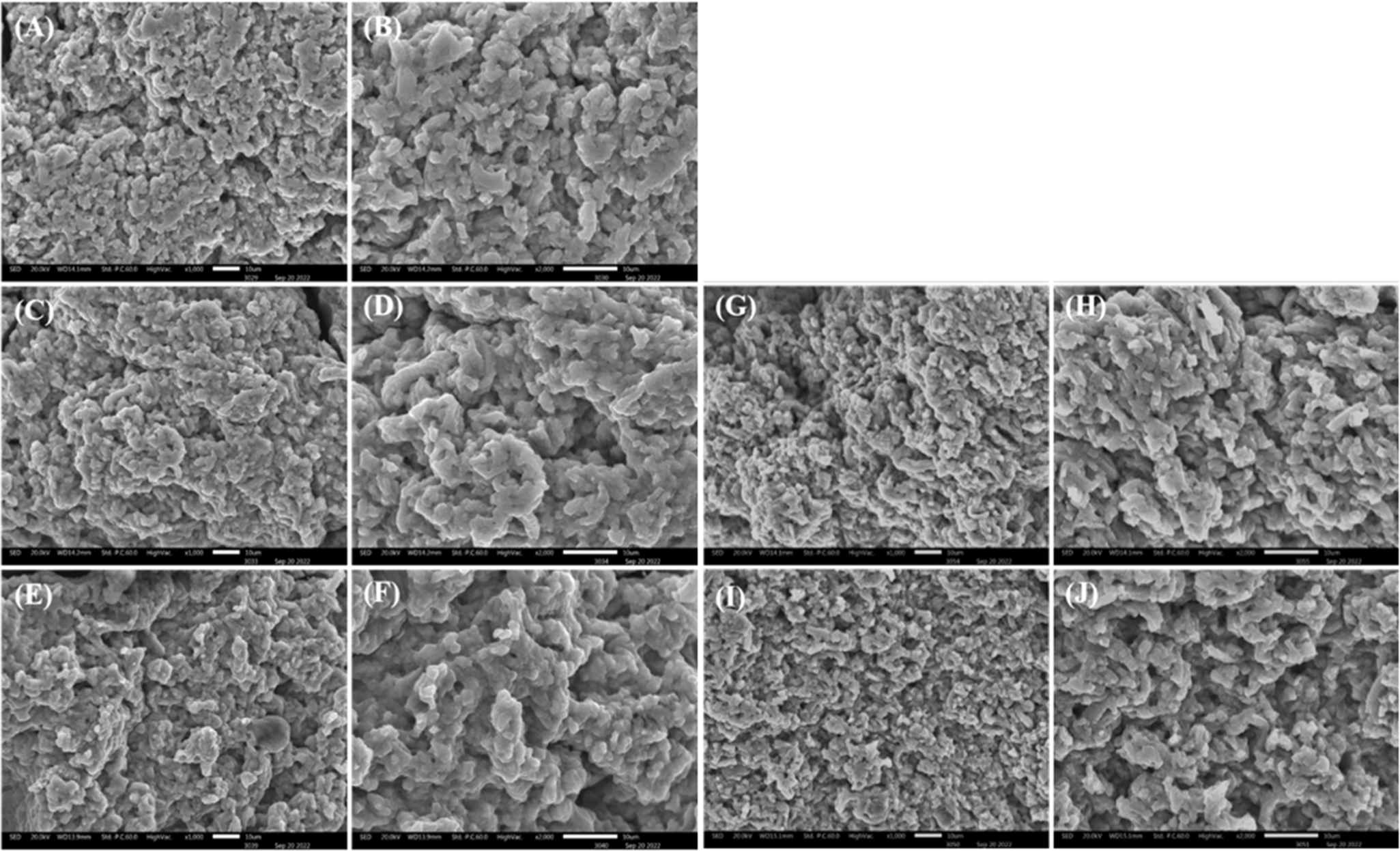
Fig. 8 shows the three-dimensional protein surface microstructure of the MP gels with or without lyophilized insect salt-soluble protein powders. Using LV-SEM is useful for studying muscle structure, and rheological and textural characteristics (Rowe, 1984). The pure pork MP gel (A, B) formed a flatter and denser protein surface microstructure than the insect protein treatment gels (C–J). Regardless of the type of insect, as the amount of insect powder increased, the surface protein structure became rough, and pores were formed between the protein structures. In addition, a rougher protein microstructure and more pores were observed in the PB (G–J) compared with the TM treated MP gels (C–F). Furthermore, this result might be corresponded to the gel strength results (Fig. 7). These results are supported by Kim et al. (2021b), who reported that protein gel structure deteriorated as pork MP was replaced with PB protein; they attributed this to decreased thermal stability and low cross-linking forces.
Conclusion
The gel evaluation of PB and TM salt-soluble protein extracts showed low gelation, and gels were not typically formed. Adding TM and PB salt-soluble protein extract powders to pork MP gels reduced their viscosity and gel strength and made the protein structure rough and loose. Since proteins derived from PB and TM larvae reduced the gelation of pork MP gels, the mechanism to cause weaken gels with the addition of insect protein extracts should be extensively studied, and their application to meat products without these defects will be developed by further research.













