Introduction
Fermented meat products have long been regarded as prestigious foods (Leroy and Vuyst, 2016), and they are still manufactured in large quantities, particularly in Europe, owing to their peculiar and unique sensory attributes, versatility, and alleged ties to culinary and traditional culture (Leroy et al., 2013). They are a component of traditional menus in certain parts of the world, where they are regarded as appealing gastronomic entities that contribute to geographical and cultural uniqueness (Leroy and Vuyst, 2016). According to Casaburi et al. (2007), complex physicochemical reactions occur during the fermentation and aging of sausages, resulting in a decline in pH and shifts in microbial communities. Among different requirements, acidification through fermentation by lactic acid bacteria (LAB) and subsequent slow drying/ripening often at room temperature enhancing long shelf-life (Barbut, 2011), more nutritious and substantial and unique flavor of dry fermented meat products.
A real breakthrough in the fermentation of meat products was achieved by the use of pure bacterial cultures in the 1950s (Incze, 1998). The use of starter cultures, along with the processing environment (temperature and relative humidity conditions), are the critical features operated by the fermented sausage industries to enhance the safety and stability of their products, both of which are amongst the major drivers of the dynamic phenomena that occur during ripening (Pasini et al., 2018). During the manufacturing of dry fermented sausage, well-fitting starter cultures must be chosen from microbes that are well suited to the ingredients used in preparation of the meat batter and processing environment, and should be more versatile due to their special metabolic capabilities (Leroy et al., 2006) in order to dominate the process.
LAB and Staphylococcus species are the major microbial groups of technological concern in fermented sausage production (Morot-Bizot et al., 2006). LAB play a key role by producing lactic acid and lowering pH, which affects the ultimate product’s physicochemical properties as well as its microbiological stability (Drosinos et al., 2007). The pH reduction facilitates also the process of ripening which improves the moisture content and delivers the ultimate dry characteristics of fermented meat products. The production of lactic acid and other antimicrobials metabolites by LAB plays a crucial role in organoleptic attributes of aroma, taste, and texture of meat products, and are vital in hygiene of the product by inhibiting the activity of pathogenic and spoilage microorganisms (Villani et al., 1994).
Amongst the LAB species, Lactobacillus sakei, Lactobacillus curvatus, Lactobacillus plantarum, Pediococcus acidilactici, and Pediococcus pentosaceus are widely used starter cultures in the fermentation of meat products (Diguţă et al., 2020; Hugas and Monfort, 1997). Pediococcus species have gained growing attention and are considered as competitive candidates in the fermented sausages industries now days due to their functional efficiency and probiotic features (Diguţă et al., 2020), and their antagonistic impact on other microorganisms, including food pathogens, through the production secretion of lactic acid and bacteriocins known as pediocins (Daeschel and Klaenhammer, 1985).
There has been no comparative research on the performance traits between P. acidiliactici (PE1) and P. pentosaceus (PE2) strains on quality characteristics of dry fermented sausages to date. We believe that assessing the functional characteristics of individual strains aids in the selection and production of high-quality fermented meats on an industrial level, whether they are used as a single strain or as a combination of strains. This research, therefore, investigated the effects of PE1 and PE2 strains of Pediococcus spp. (LAB) as compared to commercial starter culture (COS), Starterkulturen Almi Rohschinken, on physio-chemical and microbial quality characteristics of dry fermented sausage during fermentation and ripening.
Materials and Methods
The two different starter cultures from Pediococcus species of LAB employed in manufacturing of two different types of dry fermented sausages, used as PE1 and PE2 treatments, in the present study were obtained from the Microbial Resource Center, Korea Research Institute of Bioscience and Biotechnology, Daejeon, Korea as lyophilized stocks. The LAB strains were: PE1 and PE2. The COS, Starterkulturen Almi Rohschinken (Almi GmbH & Co KG, Oftering, Austria) was used as the third treatment and positive control (COS). The starter cultures were enriched with MRS broth (Difco, Becton, NJ, USA) by incubating at 37°C for 24 h. The designed suspension incorporated into the sausage batter was one mL/kg (v/w) and each starter culture had approximately 6 Log CFU/g. The viable cell count in the starter cultures suspensions was performed using a hemocytometer (Marienfeld-Superior, Paul Marienfeld GmbH & Co.KG, Lauda-Königshofen, Germany) supported with a computer magnification system.
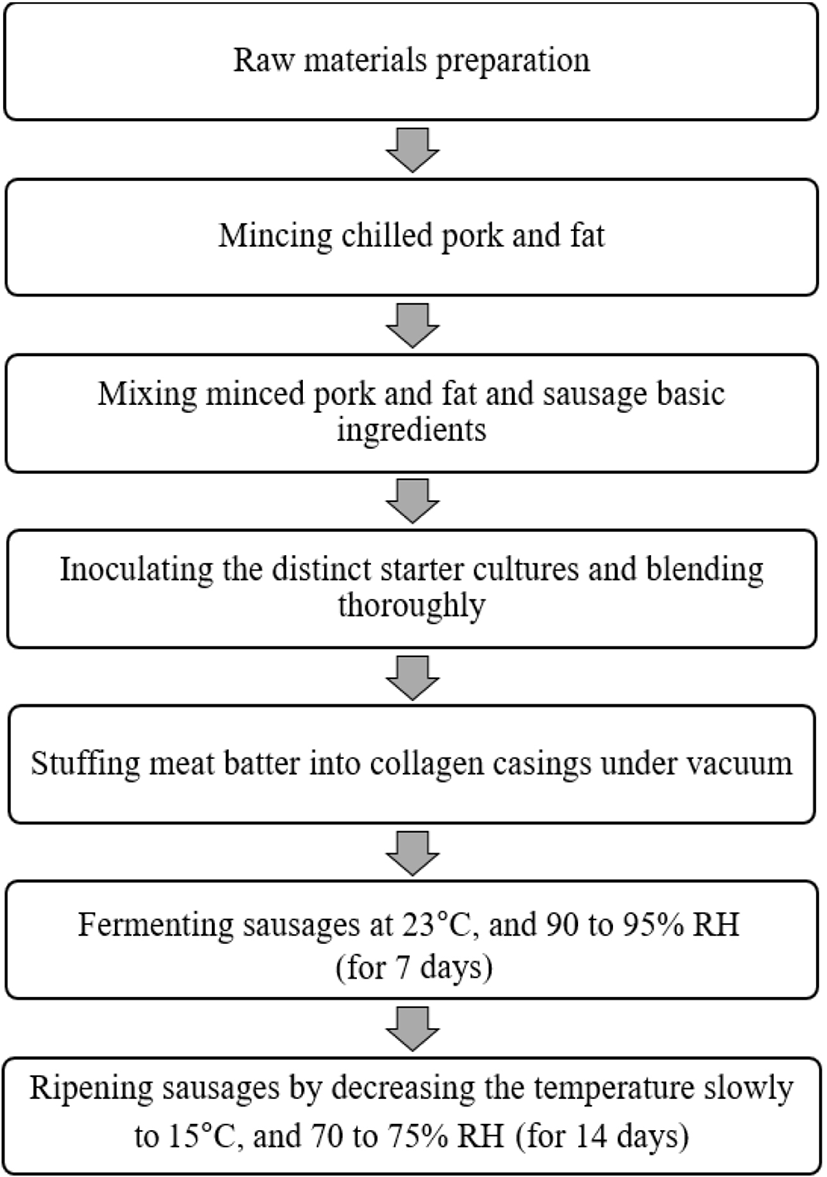
Low-temperature dry fermented sausages were produced in the pilot meat processing center, Animal Resources Department, Daegu University. Fresh loin pork was purchased from the commercial market of Geyongsan, Korea, and used for the study. After trimming the connective tissues and excess fat, the lean meat was kept in a refrigerator for further use. Chilled pork and pork fat were cut into small cubes and minced twice using a meat mincer (M-12S, Hankook Fujee Industries, Suwon, Korea). The basic sausage formulation includes lean pork meat (65%), pork fat (21.5%), ice water (10%), NPS (97:3, blend of NaCl and nitrite; 0.34%), NaCl (1.70%), sugar (0.45%), glucose (0.45%), sodium ascorbate (0.20%), and sausage seasonings (0.36%). After adequately mixing the ingredients with help of a rotary slice cuter (SF-2002, Samwoo Industry, Korea), the batter was divided into three lots (3 kg each) and randomly allocated to 3 distinct starter cultures treatments: COS, PE1, and PE2. The supreme starter cultures (LAB) concentration reached ~106 CFU/g when introduced to the meat batters, and one mL/kg (v/w) starter culture was added for each respective treatment. The batters and corresponding starter cultures were thoroughly blended with the help of a food mixer (SP-100A, Spar Food Machinery MFG, Taiwan) and filled into collagen casings (IKJIN, Seoul, Korea), 2.4 cm diameter and 15 cm length, with vacuum filling machine (RVF 327, Düker-REX Fleischereimaschinen GmbH, Laufach, Germany). The sausages were fermented and ripened in a digital chamber system (SMK-2000SL, Metatek, Nonsan, Korea) with a temperature and relative humidity (RH) control unit. During the fermentation process (the first seven days), the temperature was kept at 23°C and RH was varied between 90% and 95%. Subsequently, the ripening process was performed for 14 days (after the fermentation process) at 15°C (the temperature was decreased slowly from 23°C), and RH oscillated from 70% to 75%. The manufacturing process of dry fermented sausages is displayed by a schematic diagram (Fig. 1). Sampling was done at 1 and 7 days of fermentation and at 7 and 14 days of ripening for water activity (aw), pH, salinity, microbial quality [total plate count (TPC) and MRS counts], and color attributes L* (lightness), a* (redness), and b* (yellowness) of sausages samples. The volatile basic nitrogen (VBN) content, thiobarbituric acid reactive (TBARS), and texture profile analysis (TPA) were performed at 7 and 14 days of ripening. For each analysis time and batches, three replicates samples were withdrawn, and analyses were performed accordingly.
Microbiological quality characteristics were conducted by enumeration of TPC and LAB. About 25 g portion of a sample from each dry fermented sausage was taken aseptically with a sterile spoon, mixed with 225 mL of 0.1% peptone water, and homogenized in a Stomacher Lab Blender (model 400 Circulator, Seward Laboratory, USA) for 2 min. Serial 10-fold dilutions (10–1 to 10–7) were prepared by diluting one mL of the sample in nine mL of 0.1% sterile peptone water. Enumerations of the grown colony of microorganisms were conducted after incubating samples with their respective selective medium: Plate Count Agar (Difco) was used for TPCs and Lactobacillus MRS agar (Difco) for LAB. Plates from different and appropriate dilutions were incubated in triplicate at 37°C for 48 h (Drosinos et al., 2005). The average numbers of colonies per countable plate were counted and the total numbers of colonies per gram (CFU/g) were determined, and then data were presented in Log CFU/g.
Analysis of aw was performed using aw measuring device (LabMaster‐aw, Novasina AG, Lachen, Switzerland) after slicing the core of the samples about 4 mm cubes. The pH values of samples were analyzed after homogenizing three grams of a sample with 30 mL of distilled water using a homogenizer (Model Polytron® PT 2500 E Stand Dispersion Device, Kinematica AG, Switzerland). A digital pH meter (Mettler Toledo, Columbus, OH, USA) was used for reading the values. The same homogenates of samples used in pH analysis were subjected to salt (NaCl) content analysis using a salinity measuring device (EUTECH SALT 6+, Thermo Scientific, Gul Cir, Singapore). Three readings per sample were performed and the percentage (%) of salt content was calculated.
For the determination of VBN contents, the Conway micro diffusion method (Conway, 1950) was followed after some modifications. Briefly, two cleaned Conway’s apparatus were used for each sample and vaseline was applied as sealing agent to the edge of the outer ring. Three grams of the sample and 30 mL of distilled water was homogenized at 1,000×g for 1 min by using a homogenizer. Filtration of the homogenate was performed with the help of Whatman no. 1 filter paper (GE Healthcare Life Sci., Pittsburg, PA, USA). One mL the filtrate was pipetted to the outer chamber of a Conway unit, and 1 mL of 0.01 N boric acid (H3BO3) and 100 μL of Conway indicator (0.066% bromocresol green:0.066% methyl red, 1:1) were pipetted to the inner chamber. Sealing the Conway unit was done immediately after the addition of 1 mL of 50% potassium carbonate (K2CO3) in the outer chamber. Then, samples were incubated at 37°C for 2 h. Finally, 0.02 N sulfuric acid (H2SO4) was added to the inner chamber of the Conway unit, the change in color was monitored, and the VBN contents was measured. Total VBN values were expressed in mg%.
Measurement of lipid oxidation, TBARS content Analysis, was conducted using the method indicated by Pikul et al. (1989) after some modification. Briefly, 5 g of sausage sample, 50 uL of BHA (7.2% in ethanol) and 15 mL of distilled water was homogenized and then centrifuged with the help of a centrifuge (Hanil Science Industrial, Incheon, Korea) at 1,500×g for 15 min. About 2 mL of the supernatant and 4 mL thiobarbituric acid solution (20 mM TBA in 15% Trichloroacetic acid, TCA) was mixed, heating the mixture was performed in a water bath at 90°C for 30 min and then cooled to room temperature. Therefore, extraction of TBARS was done from cooled samples and the absorbance was measured at 532 nm using a spectrophotometer (Multiskan Go, Thermo Fisher Scientific, MA, USA). TBARS content, mg malonaldehyde per kg (mg MA/kg), of each sausage sample was calculated by multiplying the reading of the optical density with a K factor of 5.2.
Instrumental color analysis was performed from the inner surface of the sliced sausages using a portable chromameter (CR-400, Konica Minolta, NJ, USA). The device was calibrated with a standard calibration plate (Y=92.80, x=0.3136, and y=0.3194) ahead of the analysis, and five readings per sample were taken for L*, a*, and b*. Average values were calculated from five readings and expressed as L*, a*, and b* based on the CIE color system (CIE, 1976).
TPA was performed using a TPA measuring device (TA 1, Lloyd Instruments, Largo, FL, USA). During the study sessions, three lots of samples (1 cm in height and 2 cm in diameter) from each batch were compressed 80% vertically for two consecutive compression cycles by exerting a 1 N load cell at a crosshead moved at 2 mm/s. And then, the TPA characteristics were obtained from force-time deformation curves for hardness (kgf), springiness, cohesiveness, and chewiness (kgf) of sausage samples.
Sensory quality analysis was conducted for color, aroma, sourness, and overall acceptability of dry fermented sausages using descriptive sensory analysis (scoring method). The sensory evaluation was performed by seven experienced panelists who are searchers and students in Daegu university’s department of animal resources, meat science laboratory. Ahead of the actual evaluation session, the panelists were trained on sensory characteristics of dry fermented sausages using five-point scale. The intensity scale used to define the quality attributes ranged from 1 to 5 that corresponds to the sensory attributes of samples as follows “extremely pale to very dark,” for color, “very weak fermented aroma to very strong fermented aroma,” for aroma, and “light sour to strong sour” for sourness. Three different types of commercial dry fermented sausage were used during training session, and panel were given 3 slices (5 mm thickness) of samples on white plastic plates during the judgment. To avoid carryover influences, all samples were individually labeled with three digits and provided at random. Before each sample was examined, the panel were provided cold water to rinse their mouths. The sensory analysis method was certified by the life management committee of Daegu University and given an IRB number (1040621-201905-HR-004-02).
Statistical data were analyzed by using Analysis Variance (ANOVA) for the three replicates. SAS software version 9.4 (SAS Institute, Cary, NC, USA) was used for the analysis, and a significance level of p<0.05 was applied for all evaluations. Differences among the means were compared according to Duncans’s Multiple Range Test.
Results and Discussion
The shelf-life of a product is entirely influenced by its aw content, and low aw is well recognized for enhancing the storage stability of foods. Table 1 shows the changes in the aw values of DFS incorporated with different Pediococcus strains (PE1 and PE2) and the control (COS) during fermentation and ripening. The two experimental treatments, PE1 and PE2, revealed no difference in aw values throughout the study period (p>0.05), and they have a similar effect to the control, COS, which is utilized as a starter culture in a commercial application. However, the fermentation and ripening time have a considerable effect (p<0.05) on the aw of all treatments. Treatments exhibited the same trend of declining in aw as fermentation progressed from day one towards an extended period of ripening. In the early fermentation of day one, all three treatments presented the same aw value of 0.95. Consequently, the aw declined and ranged from 0.79 to 0.81 at the end of the ripening process. Based on the final aw values at the end of the ripening period, according to Luecke (1997), fermented sausage can be categorized into “semi-dry” or “dry”. Sausages with aw values in the ranges between 0.90 and 0.95 fall into the “semi-dry” category, whereas sausages having aw less than 0.90 are referred to as “dry”. Considering this classification criterion, all three sausages of the present study can be grouped into dry sausages. Due to the lower aw, such dry fermented sausages possibly have a long shelf life and can also be stored without cold storage.
In sausage production, a lower pH value is crucial because it helps to prevent the growth of adverse microorganisms, improves the redder color (Lorenzo et al., 2014), and enhances the development of the finished product’s distinct flavor and aroma (Hugas and Monfort, 1997). The changes in pH of fermented sausages during fermentation and ripening are presented in Table 1. According to Valyasevi et al. (2001), the production of lactic acid by LAB is mainly responsible for the reduction in pH and rise in acidity during fermentation. At the early stage of day one fermentation, the type of starter culture had no variation in pH value and all three treatments had a similar acidification effect. The pH values between the test samples, PE1 and PE2, were similar (p>0.05) across the fermentation and ripening period. However, inoculation of COS resulted in a considerably lower pH value than two products from the Pediococcus strain of starter cultures during the late stage of fermentation (seven-day fermentation) and throughout the ripening period. According to the Food Safety and Inspection Service of the USA, shelf-stable dry fermented sausages are required to reach a final pH of 5 or less (HORIBA, 2021). In the present study, all three batches achieved a pH of lower than 5 after the completion of the maturation. The lower pH values are often used to confirm the starter cultures’ ability to induce strong acidification (Yim et al., 2017). The exhibited lower pH confirmed that the individual Pediococcus strains’ effectiveness in the acidification process in the production of shelf-stable products. Reduced the pH and increased the lactic acid content were documented earlier by Zhao et al. (2011) in fermented sausages formulated from a mixture of Pediococcus acidilactici, Staphylococcus xylosus, and PE2. The current research revealed that the required pH can be achieved by independent use of Pediococcus strains as starter cultures. Regarding the times of fermentation and ripening, all treatment batches exhibited a substantial drop (p<0.05) in their pH, and the change in the pH range was from (5.15–5.26) at day one fermentation to (4.62–4.76), (4.57–4.72) and (4.49–4.68) at seven-days fermentation, seven-days and fourteen-days of maturation, respectively. According to Baka et al. (2011) investigation, the pH reduction is attributed to the production of lactic acid by LAB, which are increasingly becoming the dominant microorganisms during fermentation and ripening. Whereas, other authors documented that low pH values benefit the starter cultures’ ability to cause extreme acidification (Casaburi et al., 2007; Zhao et al., 2011). In general, the aw and pH values of the fermented sausages in the current study were in ranges that provide stability at room temperature and enhances consumer safety.
Salt (NaCl) plays a crucial role in the production of dry fermented sausages due to its effects on the required salty taste characteristics, enhancing flavor, texture, and overall acceptability. Furthermore, salt plays an essential function in reducing water activity, controlling enzymatic and metabolic activities, and contributing to microbiological safety and stability, all of which are important in increasing product shelf life (Hoppu et al., 2017). Salt promotes the solubility of the myofibrillar protein, improves the binding properties and texture, and increases the viscosity of meat batters, all of which contribute to the technological features of meat (Terrell, 1983). Commonly, fermented sausages are characterized by their high salt content. Based on consumer demands and technological properties, the amount of salt added during fermented sausage production is normally between 2% and 4% (Vignolo et al., 2010). The experimental samples in the current study were manufactured by adding about 2.3% salt (NaCl). During fermentation and early ripening period of 7 days, significant deference (p<0.05) in salt content among the three treatments was exhibited, and the COS, yielded the highest salt content than the two Pediococcus starters culture samples (PE1 and PE2; Table 1) at 7 d of ripening period. However, after the completion of the ripening process, the products didn’t show a difference (p>0.05) in the salt content. As compared to the initial fermentation time, all three treatments had a considerably higher (p<0.05) salt content by the end of the ripening period, ranging from 6.07% to 6.29%. The percentages of salt in the final products of the present study could be considered acceptable as 5.09% to 9.58% (average 7.13%) was reported by Lilic et al. (2011) as normal amount for dry fermented products.
Studies documented that lipid oxidation can alter nutritive values, colors, and flavors of meat products (Kim et al., 2015) and poses health risks to consumers (Grun et al., 2006). The amount of aldehydes formed by lipids’ secondary oxidation is measured using TBARS values. And the malondialdehyde (MDA) is examined as an indicative product. The extent of lipid oxidation is also represented in TBARS values (Chen et al., 2017). Consequently, an investigation of TBARS is considered a standardized method for assessing secondary lipid oxidation products in meat and meat products. The addition of different starter cultures resulted in a considerable variation (p<0.05) in the TBARS values throughout the processing time (Table 2). Samples added with PE1 starter culture exhibited remarkably lower (p<0.05) TBARS content across the study period, and samples from PE2 presented a comparable effect with PE1 treatment during the ripening period. The TBARS content of treatments was in the following order after the completion of the ripening process: PE1<PE2<COS, which is similar to the trend demonstrated in the VBN investigation of treatments. Marco et al. (2006) reported that the TBARS values in the range of 0.6–2.8 mg MDA/kg are reasonable for fermented sausages. The TBARS values of all three batches in the current study did not exceed the described range. Moreover, all three batches showed a decrease in TBARS content from the completion of fermentation towards the ripening process. It may be associated with the similar effect of the starter cultures interference in the oxidation process of lipids. The decline in TBARS contents of all three batches from fermentation to the final ripening stage may be resulted from the accumulation of lactic acid and the drop in pH, the decrease in aw, and the increase in salt concentration observed in three treatments that enhanced the hurdle effect and prevented enzymatic and microbial oxidation. An earlier study by Juncher et al. (2000) documented that low pH suppresses the oxidative activity of lipid. Bingol et al. (2014) reported that lower pH values, caused by the lactic acid activities of starter cultures, had a significant impact on TBARS values. Zhao et al. (2020) noted a decreasing trend in the TBARS values of Chinese dry sausage during the later stages of ripening, which is concordant with the current finding.
Protein degradation can occur from the spoilage bacteria or intrinsic enzymatic activities, and chemical compounds such as NH3, H2S, and CH3CH2SH, which are designated as VBN constituents, could be produced (Huang et al., 2014). Furthermore, protein degradation during fermentation is another important mechanism that leads to the development of non-protein nitrogenous compounds with low molecular weights (Ruiz-Capillas and Jiménez-Colmenero, 2004). Consequently, the VBN content is usually used to assess the freshness of raw meats, as well as the shelf life and microbiological quality of processed meats (Ba et al., 2018). Results for the investigation of the VBN and TBARS contents of the experimental samples are presented in Table 2. At the end of the fermentation period, PE2 and the control treatment COS had similar lower VBN values than PE1 samples. However, both Pediococcus strains, PE1 and PE2, presented a substantially (p<0.05) lower values compared to the COS throughout the ripening period. Based on the VBN contents of treatments at the final stage of ripening, they ordered as follows: PE1<PE2<COS. The VBN values of treatments in the contemporary study ranged from 5.60 to 8.40 mg%, which is considerably lower than the content, 20–25 mg%, identified by Rai et al. (2010) for a similar product type on the 25th day of ripening. The processing time has a profound effect on the VBN contents of samples. VBN substantially (p<0.05) increased in PE2 and COS samples and decreased in PE1 treatment from 7 d of fermentation towards the end of ripening period, and the value was substantially lower (p<0.05) in PE1 than both COS and PE2. This may be resulted from the efficiency of PE1 starter culture to neutralize the VBN content with their organic acids (e.g., lactic acid) or bacteriocin production (Yin et al., 2002) that helps in the stability of the product. In contrast, the increase in the VBN content exhibited in COS and PE2 samples due to extended processing time, according to Egan et al. (1981), could be elucidated by increased protein degradation.
The selection and application of successful starter cultures are crucial factors for the production of high-quality and shelf-stable dry fermented sausages that meet consumer demand. Effect of different starter cultures addition on TPC and LAB (MRS) counts of dry fermented sausages during the fermentation and ripping period is presented in Table 3. Statistical analysis showed that treatments from different starter cultures resulted in a substantial difference (p<0.05) in TPC and LAB counts. Likewise, Essid and Hassouna (2013) found that utilizing a selective starter significantly affects the total viable, LAB, Staphylococci, and Enterobacteriaceae numbers in dry fermented sausage production. At the early fermentation stage of day one, sausages added with COS and PE2 had higher TPC than PE1 samples, and the count ranged from 7.40 to 8.15 Log CFU/g. However, as the processing transformed to final stage fermentation, PE1 samples overwhelmed both COS and PE2 treatments, then resulted in a similarly higher count as the COS at the final stage of ripening. The counts were about 6.84, 6.50, and 6.05 logs CFU/g in control (COS) and treatments added with PE1 and PE2, respectively, in the final products. The TPC showed fluctuation between early fermentation and ripening and steadily decreased subsequently for all the batches after ripening. The decline in the counts for all treatments exhibited in the present study might be associated with the increase in salt concentration, as the drying process advanced, and the lower aw and pH values. Regarding LAB counts of samples, the addition of different starter cultures had a significant (p<0.05) effect during late fermentation and throughout the ripening period. At the final fermentation period, samples added with PE1 showed significantly highest LAB counts compared to both COS and PE2 treatments. The LAB counts were between 7.51 Log CFU/g for the control COS starter culture and 8.72 Log CFU/g for sausage incorporated with a PE1 starter culture. As the processing stage advanced to 7 days of ripening, both treatments added with Pediococcus strains, PE1 and PE2, resulted in similar and substantially higher (p<0.05) counts than a COS or the control COS. However, after the ripening period, the COS batch presented the highest count than other treatments. Treatments are ordered based on LAB counts as follows: COS>PE1>PE2 with corresponding values of 6.71, 6.47, and 5.92 Log CFU/g, respectively, at the end of the ripening process. The LAB counts at the end of the ripening were lower in all samples compared to the end of the fermentation period. The rapid growth of LAB during fermentation is normally expected as incorporated starter cultures induce the LAB to become dominant, resulting in a massive bacterial population (Lim et al., 2008). Similar to the TPC counts, all three batches showed fluctuation in LAB counts due to the effect of the processing stage, and a steady decrease in counts was exhibited between the early fermentation stage and the completion of ripening time for all three batches. TPC and LAB counts showed an almost similar trend (r=0.86, p>0.05: data not presented) that is concurring with the previous finding of Lim et al. (2008).
The color attribute of meat products has a significant impact on consumer perceptions and is the most important component of product quality. Consequently, a product may be rejected solely on the basis of its color before any other attributes are considered. Different factors govern the color of dry fermented sausages, including the sausage’s composition, fat-to-lean ratio, amount and type of spices and additives, and technical operations followed (Perez-Alvarezet and Fernandez-Lopez, 2011). The results for the instrumental color characteristics of dry fermented sausages inoculated with different types of starter cultures during the fermentation and ripening period are presented in Table 4. The addition of different starter cultures showed no difference (p>0.05) in the lightness parameter throughout the fermentation and ripening study. Treatments displayed a similar trend in the redness and yellowness attributes and both parameters were significantly affected by the inoculation of different starter cultures during the initial fermentation period of day one. Samples from PE2 starter culture had markedly increased values than the control, COS, and PE1 treatments in both attributes. However, the difference among samples disappeared, and all batches showed similar a* and b* characteristics since the 7th days of the fermentation period. The fermentation and ripening time have a noticeable effect on L* value of treatments. Substantial decrease in L* was exhibited in all treatments due to extended fermentation and ripening time. A similar finding was reported in an earlier study by Kurt (2016) that L* value is significantly affected due to ripening time. The possible reason for the decreased lightness value might be associated with loss of moisture, especially the surface drying of the sausage samples. The present study exhibited that both a* and b* characteristics were not affected (p>0.05) due to fermentation and ripening times. Redness is the most sensitive parameter, and its intensity indicates the meat products’ stability against oxidation (Ergezer et al., 2018). An earlier study documented that the redness trait decrease during maturation (Cavalheiro, 2013). However, the current study did not exhibit the reported trend, and all batches showed no marked variation due to ripening time. The finding implies that all of the starter cultures utilized excel solely in the manufacture of dry fermented sausages in terms of their effect on color features.
Effects of different starter cultures incorporation on the texture profile analysis (TPA) of dry fermented sausage during fermentation and ripening are described in Table 5. No substantial difference (p>0.05) was exhibited among all batches in springiness and chewiness characteristics across the fermentation and ripening period. The hardness property of all three treatments did not show marked difference (p>0.05) during the fermentation study. However, COS and PE2 samples showed similarly and substantially higher (p<0.05) hardness values than PE1 on the 7th day of ripening. Subsequently, the variation disappeared again, and all the batches showed similar hardness after the completion of the ripening period. Cohesiveness is a characteristic that shows how the sample holds together during chewing (low=fibers break easily, wad disintegrates; high=wad raises in size, compete against breakdown; Lyon and Lyon, 1990). Cohesiveness was not varied in all samples during fermentation and 7 days of ripening time. However, a noticeable difference (p<0.05) was observed in the cohesiveness values amongst the final ripened products, and samples in PE1 and the COS, presented similarly higher values than PE2 treated samples. Fermentation and ripening time didn’t affect the springiness of COS and PE1 treatments and the chewiness of all treated batches. The springiness value in PE2 samples was increased due to the extended time of ripening. Substantially increased hardness and decreased cohesiveness (p<0.05) were revealed in all treatments due to the change in ripening time. As the samples get ripened, the available free water (water activity) is reduced to a significant level (Table 1), and an increase in the hardness values (r=0.85, p>0.05: data not presented) of all treatments was exhibited. The unification of ingredients may be affected due to extended ripping time and resulted in fragmentation of structural components that ended with lower cohesiveness characteristics of all batches studied.
The sensory quality traits of food products are the primary evaluation criteria of consumers before the consideration of any other quality attributes. During the ripening process, starter cultures play an important role in the acidification and generation of fermented sausages with unique and distinct quality characteristics, which contributes to sensory acceptability and physical properties (Bassi et al., 2015). Dry fermented sausages from different strains of Pediococcus spp. and control (COS) were subjected to sensory evaluation and judged by seven trained panelists for four attributes (color, aroma, sourness, and overall acceptability), and the results are presented in Table 6. Among the four descriptors considered in the current study, there were no significant differences (p>0.05) in the aroma and fermented sour taste characteristics of treatments. According to the panel judgments, the sausages added with PE1 and PE2 were perceived to have a similarity in aroma and sour taste as that of COS samples. However, the inoculation of different strains of Pediococcus spp. had a significant effect (p<0.05) on the color and overall acceptability traits of the ripened sausages. Regarding the color, sausages both in PE1 and PE2 treatments showed similar and higher (p>0.05) dark red color intensity than the COS batch. In the overall acceptability attribute, the sensory evaluation panelists generally preferred samples in PE1 than PE1 and COS treatments. In general, the current sensory evaluation showed the inoculation of different strains of Pediococcus spp. presented similar characteristics in aroma and fermented acidic taste as that of the COS and both PE1 and PE2 batches were better in color attributes. Moreover, PE1 scored highest in the overall acceptability attribute while PE2 and COS were perceived to have similarities.
| Attributes | Treatments1) | SEM | ||
|---|---|---|---|---|
| COS | PE1 | PE2 | ||
| Color | 2.47b | 4.31a | 3.46a | 0.85 |
| Aroma | 3.33 | 4.14 | 3.23 | 0.81 |
| Sourness | 4.34 | 3.96 | 3.60 | 0.73 |
| Overall acceptability | 3.69b | 4.47a | 3.57b | 0.52 |
Conclusion
The addition of PE1 and PE2 resulted in a similar beneficial effect in the reduction of pH value and acidification of the products. PE1 inoculation showed a substantially higher LAB count than PE2 after-ripening and pronounced high count than the COS during fermentation and early ripening period. Moreover, PE1 addition led to significantly lower TBARS and VBN contents than COS and PE2. All three starter cultures resulted in similar color characteristics (L*, a*, and b*) since 7 days of fermentation. Moreover, the TPA characteristics of springiness, chewiness, and hardness were the same in all three batches of the ripened products. However, the cohesiveness property was better in PE1 and COS products than in PE2. From the grounds of the present study findings, it can be concluded that the overall performance of PE1 strain was conducive to improving the quality characteristics of dry fermented sausage. The inoculation of different strains of Pediococcus spp. and COS presented similar sensory characteristics in aroma and fermented acidic taste. PE1 and PE2 batches were better in color quality and PE1 preferred in the overall acceptability attribute.













