Introduction
Vitamin B12 is a water-soluble vitamin belonging to a family of compounds called cobalamins. Amongst the cobalamins, cyanocobalamin, hydroxycobalamin, adenosylcobalamin, and methylcobalamin are the major forms of vitamin B12 (Anatol et al., 2019; Cho et al., 2019; Pakin et al., 2005). Vitamins are produced by microorganisms and are accumulated in the liver. Thus, they are found in animal products such as meat, fish, egg, and milk products but are present in vegetables in very low concentrations (ng/g). The recommended daily intake of vitamin B12 is 2.4 kg/day for a Korean adult and 2.6 kg/day for pregnant and lactating women (Choi et al., 2008; Jang et al., 2014; Moon et al., 2018). Although the recommended value is very low, vitamin B12 deficiencies have been shown to affect neurodevelopment in infants. Additionally, vitamin B12 deficiency may lead to megaloblastic anemia, nervous system disorders, and/or improper synthesis of DNA (Cho et al., 2019).
The recent advances in this field have drawn the consumers’ attention to minor nutrients, such as vitamins. However, there are only limited reliable databases for vitamin B12 for the evaluation of national nutrition in Korea. The complex structure and multiple possible vitamers render the analysis of vitamin B12 particularly challenging (Fang et al., 2017).
Vitamin B12 has been analyzed using several methods, including spectrophotometry, microbiological methods, and high-performance liquid chromatography (HPLC) (Esteve et al., 2002; Guggisberg et al., 2012). Microbiological assays and chromatographic approaches are the most suitable methods for determining the vitamin B12 content in food (Szterk et al., 2012). Microbiological assays are the oldest assay method and the most commonly used technique for vitamin B12 detection. Although such assays are highly sensitive, they lack specificity as inactive cobalamins in some food matrices may interfere with the microorganism growth. These methods are also time-consuming, as they involve steps such as tissue culture and preservation of strain. Moreover, these methods lack sensitivity and have low precision (O’Broin and Kelleher, 1992).
Numerous methods for the analysis of vitamin B12 have been described by Karmi et al. (2011). Among them, HPLC–mass spectrometry is probably the most frequently used technique for determining vitamin B12 in food and biological samples. To overcome the low sensitivity of the existing techniques, which is a limitation, an attempt was made to obtain food samples with low concentrations of vitamin and analyze them through pretreatment methods such as sample concentration using solid phase extraction or immunoaffinity columns (Heudi et al., 2006; Iwase and Ono, 1997; Sun et al., 2016; Xie et al., 2019). Vitamin B12 exists in free and bound forms in foods. It can be extracted from protein-rich foods using proteolytic enzymes. However, information on the extraction of vitamin B12 from powdered milk is very limited. Especially, powdered milk add starch as well as protein and/or fat to improve its nutrition value (Seo et al., 2018). The presence of these additional components renders the analysis of vitamin B12 extremely difficult (Bito et al., 2016; Lee et al., 2015). Hence, the analysis of vitamin B12 in powder milk must include a pretreatment step. Currently, the methods validated by the Ministry of Food and Drug Safety (MFDS) apply to infant formula, baby formula diet, and milk formulas; however, powdered milk containing starch is not included in this list. In this study, a chromatographic approach involving a pretreatment step and immunoaffinity column purification during the sample preparation of powdered milk containing starch was adopted to remove interfering matrix components and enrich the sample with the target analyte to ease quantitation. This analytical method involving a pretreatment step coupled with immunoaffinity purification and HPLC/Ultraviolet (UV) was validated and applied for the determination of total vitamin B12 content in powdered milk containing starch.
Materials and Methods
The powdered milk used in this study was purchased from a local market and kept at 4°C for further use. An powdered milk standard reference material, SRM 1849a (National Institute of Standard and Technology, Gaithersburg, MD, USA), which is a certified reference material, was used in the recovery tests. Vitamin B12 content in SRM 1849a was 48.2±8.5 μg/kg. Sodium acetate was purchased from Junsei Chemical (Tokyo, Japan), while the enzyme, amylase, was purchased from ANKOM (catalogue TAHTL-NC24). HPLC grade water and acetonitrile were purchased from Merck (Darmstadt, Germany).
Vitamin B12 in the form of cyanocobalamin (Cat. No. 1152009), with a purity of 1.04% (10.4 μg/mg), was bought from US Pharmacopeial Convention (USP, North Bethesda, MD, USA) to be used as the reference standard. The standard material (100 mg) was dissolved in water in a 100 mL volumetric flask to prepare a 10 mg/L stock solution. This stock solution was serially diluted with water to prepare 25, 50, 100, 250, and 500 μg/L working solutions.
A previously reported sample preparation method (Kirchner et al., 2012; Moon et al., 2018) was used to remove protein, fat, and starch from the sample, after a slight modification of the method. Five grams of the cereal infant formula sample was placed on a 55 mL screw cap tube and dissolved in 49 mL of 0.2 M sodium acetate. The pH of the sample solution was adjusted to 4.0 to remove casein, which comprises ~80% of the milk protein fraction. Lowering the pH beyond 4.0 (isoelectric point of casein) resulted in isoelectric precipitation. Following this, 0.5 mL of 1% sodium cyanide was added and mixed, and the sample was extracted ultrasonically at 25°C for 10 min. After the addition of 0.5 mL of α-amylase, the sample was incubated for 30 min at 40°C and then for 30 min at 100°C in an incubator to initiate the reaction. Next, 20 mL of the above solution was filtered by a Whatman paper and transferred to an immunoaffinity column (Easi-Ex-tract Vitamin B12, r-Biopharm, Glasgow, UK). The column was washed with 10 mL water and injected with 40 mL of air by syringe to dry it. The loaded sample was eluted with 3 mL of methanol. The eluate was volatilized to dryness and then reconstituted in 0.5 mL of water. This was used as the test sample.
Chromatographic conditions were determined based on previously reported analogous methods that used LC–UV. A Shimadzu HPLC system (Shimadzu, Kyoto, Japan) equipped with a Shiseido Capcell Pak C18 UG 120 column (4.6 mm×250 nm, 5 μm) was used for the analysis of vitamin B12. Water and acetonitrile were used as the mobile phases for gradient elution. A flow rate of 1.0 mL/min and a column temperature of 35°C were maintained, and the injection volume was 50 μL. HPLC grade solvents were filtered through a 0.45-μm membrane and ultrasonically degassed prior to use. The specific chromatography conditions are A (water): B (acetonitrile) gradient system 0–3.4 min (100:0), 3.5–10.9 min (75:25), 11.0–18.9 (65:35), 19–20 min (90:10), and 20–26 min (100:0) (Table 1).
Selectivity for vitamin B12 detection was determined by comparing the chromatographic peaks of the test sample with those of the standard solutions. Linearity was assessed by injecting 25 to 500 μg/L of vitamin B12 solutions in duplicate. Qualitative parameters were determined by comparing the retention times of the standard solution with those of the samples. The analyte was quantified from the calibration plot equations calculated by the least-squares method. Precision was calculated in terms of intra-day (n=3) and inter-day repeatabilities (n=3) by analyzing spiked cereal infant formula samples and was evaluated by calculating the relative standard deviation (RSD). Accuracy of the method was determined by calculating the recovery and appropriate standard deviation (SD) in cereal infant formula samples spiked with different amounts of vitamin B12. Detection limits were assessed in terms of limit of detection [LOD, signal-to-noise ratio (S/N)=3] and limit of quantitation (LOQ, S/N=10).
Results and Discussion
Analysis using the current method proposed by the MFDS is complex; moreover, it does not yield a desirable peak resolution in the analysis of powdered milk samples. In addition, the MFDS has not yet provided an appropriate method for the analysis of powder milk containing starch. Although the reason for the low peak resolution is not clear, the unstable nature of starch, proteins, and fats during sample treatment has been assumed to be a limitation in this conventional method. In addition, it is difficult to detect vitamin B12 in some food samples using only one pretreatment method (http://foodsafetykorea.go.kr/foodcode/01_03.jsp?idx=324). Since vitamin B12 exists in different forms at very low concentrations in powdered milk containing cereal, the sample preparation methodology is extremely crucial (Lee et al., 2015). In this study, individual pretreatment methods were developed by modifying the Association of Official Analytical Chemists (AOAC, 2002) method to detect vitamin B12 in powdered milk containing starch in high amounts. In the modified AOAC method, samples were purified using an immunoaffinity column and then subjected to HPLC to quantitate vitamin B12 in the samples. Sodium cyanide and α-amylase were used to remove starch, as mentioned in the experimental section. The pre-treatment involving clean-up and concentration using an immunoaffinity column enabled the efficient separation of trace amounts of vitamin B12 from powdered milk samples. As a result of the sample pretreatment, vitamin B12 was eluted at 9.3 min in the HPLC run, suggesting its efficient separation from the degradation products. This method allowed the separation and detection of vitamin B12 within 10 min (Fig. 1). Detection using this approach under the described experimental conditions was slightly more rapid compared to that under the experimental conditions employed in a previous study (Heudi et al., 2006).
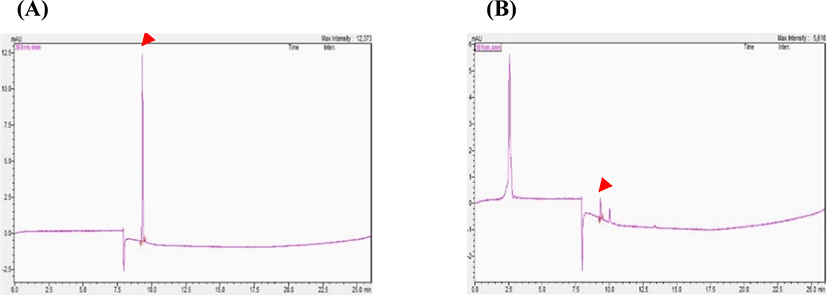
The specificity of the proposed technique was ensured by employing the well-established method of using highly selective immunoaffinity column for sample preparation (Anatol et al., 2019; Nakos et al., 2017). Detection limit and powdered milk containing starch recovery were determined by quantitative analysis, and certified reference material, SRM 1849a, was used to validate our analytical HPLC method. The amount of vitamin B12 recovered in the SRM 1849a reference was 54.10 μg/kg. Compared with value of 48.20 μg/kg (given SRM 1843a certified value), the test represented recovery of the authentication value of 112.24%. The external calibration curve of vitamin B12 standard solutions was linear in the range of 25–500 μg/L, with r2>0.9999. The equation of the calibration curve was y = 53.806x − 150.44, where y represents the peak area of the curve obtained through UV detection, and x is the concentration (μg/L) of vitamin B12. It is evident that the correlation coefficients were greater than 0.9999, which indicated a good correlation between the concentration and peak area of the investigated compounds. The LOD and LOQ were 2.71 and 8.21 μg/L, respectively (Table 2). Accuracy was assessed by adding a known amount of the analyte, followed by calculating the recovery using standards. Accuracy of the method was satisfactory, ranging from 83.41% to 106.57%, which was well within the recovery range reported for other food matrices (Chamlagain et al., 2015; Zironi et al., 2013). Intra-day and inter day variations were used to determine the precision of the established method. As shown Table 3, RSD of intra-day and inter-day variations for compound was less than 5.31% and 3.90%, respectively. These results suggest that the HPLC method involving sample pretreatment, immunoaffinity column separation is precise, accurate and sensitive for quantitative determination of active compounds in powdered milk containing starch.
Four different powdered milk containing starch samples, of which two were manufactured in Korea and two were manufactured in USA, were analyzed using the method developed in this study. The sample pre-treatment was repeated three times for each sample; the results are presented in Table 2. It is evident from Table 2 that the vitamin B12 content in powdered milk products was in the range of 11.03–42.18 μg/kg. As determined from the HPLC analysis, all the products contained trace nutrients that were higher than those displayed on the content labels. Therefore, the vitamin B12 content displayed in powdered milk packaging available in the Korean markets was well verified.
Conclusion
The nutrition labeling system of foods is being strengthened to provide appropriate information to consumers while choosing a food product. Therefore, there is an increasing need for scientifically established analytical techniques to strengthen the national management of foods with high nutritional components. In this work, sample pretreatment, immunoaffinity column separation, and HPLC were employed in combination to develop an analytical method for the extraction of vitamin B12. In the proposed method, starch was removed using a small quantity of α-amylase, unlike the traditional methods. The validation results indicated high sensitivity and good accuracy and precision. The recovery and RSDs were in the acceptable range. Additionally, the value obtained for the certified reference material (SRM 1849a) was within the range of certificated values. The developed method based on HPLC and sample pretreatment for the detection of vitamin B12 could reduce the analysis time and manual labor, thereby proving to be an appropriate alternative to conventional analytical methods. Although, there are several methods for the detection of vitamin B12 in dairy products, powdered milk etc., this is the first study to attempt the rapid detection of vitamin B12 in powdered milk containing starch. Moreover, a beginner can be expected to easily perform this analytical procedure because of its simplicity. This method for the analysis of vitamin B12 may be utilized in industries for micronutrient analysis in dairy products, functional foods, as well as powdered milk.













