Introduction
Carnitine, a zwitterion, is an essential nutrient for the human body. However, its role in maintaining the health and in the progression of diseases has not yet been clarified. It plays a key role in lipid metabolism in animals, wherein carnitine is metabolized through β-oxidation for energy (Stefan et al., 2003; Zhang et al., 2020). Carnitine occurs both in its free and esterified forms in tissues and biological fluids (Khoshkam and Afshar, 2014). It is an important nutrient for babies, and hence, should be included in infant formula. Although it is not essential for children and adults, it is recommended that infant formulas (especially those designed for premature infants) should contain the same amount of carnitine as that present in breast milk (i.e., 28–95 μmol/L) (Demarquoy et al., 2004). Many methods have been developed to detect and quantify carnitine such as gas chromatography (GC), capillary electrophoresis (CE), high-performance liquid chromatography (HPLC), CE-mass spectrometry (CE-MS), HPLC/UV, HPLC-MS, and HPLC-tandem MS (Deng et al., 2001; Heinig and Henion, 1999; Jing et al., 2015; Vernez et al., 2004). Because L-carnitine is highly polar and has no chromophore, its quantitative analysis is difficult (Kakou et al., 2005; Park et al., 1996). According to the AOAC (AOAC, 2016; Ellingson et al., 2016), the HPLC method can be used for the quantitative analysis of L-carnitine and choline simultaneously in milk powder and nonformula milk samples. However, HPLC methods are not suitable for the analysis of formulations containing low levels of carnitine (Ahn et al., 2014). The highest amounts of L-carnitine are found in meats and dairy products. Infant powdered milk has a highly complex structure and contains significantly low amounts of L-carnitine; therefore, it is difficult to detect L-carnitine in infant powdered milk (Dąbrowska and Starek, 2014). The Ministry of Food and Drug Safety (MFDS) recommends using a colorimetric titration method as a food additive code test method for the analysis of carnitine; however, the titration method has low accuracy and low reproducibility. Because carnitine is polar, amphoteric, and nonvolatile and does not contain any chromospheres, it shows weak UV absorbance within the short UV-wavelength range (Freimüller and Altorfer, 2002). To resolve these problems, application of chromatographic analysis has recently been proposed to detect and determine carnitine in biosamples such as urine. HPLC is the most commonly used chromatographic analysis method for this purpose (Li and Sun, 2010; Magiera and Baranowski, 2015). If a UV detection method is used without any other pretreatment and derivatization, satisfactory chromatographic separation and detection sensitivity are not obtained. In addition, it is very difficult to apply this method (Seline and Johein, 2007). Therefore, it is necessary to perform precolumn derivatization before such method can be used (Yoshida et al., 1988). The introduction of the synthetically accessible fluorescent active group for derivatization facilitates the separation and identification of carnitine from interfering substances and improves detection sensitivity (Dąbrowska and Starek, 2014; Kakou et al., 2005). The polarity of the sample is reduced through derivatization and a stable derivatization is made for more sensitive analysis. Hence, a fast and accurate derivatization method should be developed to determine the L-carnitine content with a high detection limit in infant powdered milk.
Herein, to increase the sensitivity and efficiency of carnitine in powdered milk, we propose a powerful method with 1-aminoanthracene (1AA) as a catalyst in the presence of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), pre-column derivatization, selection of optimal reagent volumes for derivatization, and high-performance liquid chromatography-fluorescence detectors (HPLC/FLD). Little research has been done except that the presence of carnitine in infant formula has been confirmed (Cao et al., 2007; Ferreira et al., 1997; Sánchez-Hernández et al., 2010). Therefore, in this study, the content of carnitine was analyzed through the pretreatment method of infant formula containing a lot of fat, protein, and starch.
Materials and Methods
The infant powdered milk was obtained from a local manufacturer (Korea) and stored at 4°C. Standard reference material (SRM) 1849 Infant/Adult Nutritional Formula was used (National Institute of Standard and Technology, NIST) for recovery analysis. SRM 1849a contained 136±14 mg/kg of L-carnitine. The L-carnitine reference standard (99.9% pure) was obtained from the US Pharmacopeial Convention. 1AA and EDC were bought from Junsei Chemical and ANKOM (catalog TAHTL-NC24, Macedon, NY, USA), respectively. All the solutions were prepared in HPLC-grade water and all the chemicals used were acquired from Merck (Darmstadt, Germany).
L-Carnitine was used as the reference standard. A stock solution of 1,000 μg/mL of L-carnitine was obtained by serially diluting L-carnitine with water and 0.1, 0.25, 0.5, 1, and 2.5 mg/L working solutions were obtained.
Infant powdered milk contains significant amounts of starch, protein, and/or fat. Therefore, it is necessary to use HPLC methods with derivatization for FLD absorbance detection. The experiments were conducted by applying derivatization with 1AA and modified application using a previously reported sample pretreatment method (Longo et al., 1996). First, 5 g of the infant powdered milk sample was added in a 50 mL flask containing 49 mL water and mixed by sonication. Solid-phase extraction (SAX) cartridges (Thermo fisher scientific, Waltham, MA, USA) were activated using 1 mL of methanol and 2 mL of distilled water. Then, 2 mL of the abovementioned solution was loaded onto SAX cartridges eluted with the same volume of 0.01 M monosodium phosphate solution (pH 3.5). The resulting solution was used as the test sample for the derivatization reaction.
The chromatographic parameters were determined using a previously reported LC analysis. An HPLC equipment (Shimadzu, Kyoto, Japan) was used to determine L-carnitine with an Inertsil ODS-3 column (4.6 mm×250 nm, 5 μm). Here, 0.1 M ammonium acetate adjusted to pH 3.5 and acetonitrile in a ratio of 7:3 (v/v) were used as HPLC-grade solvents. All the solvents were filtered and subjected to ultrasonic treatment. A spectrophotometer was used, with an excitation wavelength of 248 nm and emission wavelength of 418 nm. The flow rate was 1.3 mL/min.
First, 1 mL of the sample was mixed with 40 μL of 1 M hydrogen chloride, 200 μL of 1AA, and 200 μL of EDC sequentially. Then, 5 mL of ether was added to the derivatized sample and mixed. Next, 0.6 mL of the bottom aliquots was mixed with 1.4 mL of 0.01 M disodium phosphate (to adjust the pH to 9.1), followed by the addition of 5 mL of chloroform. The resulting solution was mixed well. In the next step, 0.5 mL of the top aliquot was mixed with 0.5 mL of 0.01 M disodium phosphate (pH adjusted to 3.5) and a uniform solution was obtained. The abovementioned solution was filtered and the resulting solution was used as the test sample.
The developed method was validated for selectivity, linearity, limit of detection (LOD), limit of quantitation (LOQ), and recovery as per the AOAC Guidelines (AOAC, 2002). The selectivity was analyzed by comparing the chromatographic peak of the infant formula to that of a standard sample. The linearity was measured from the average coefficient of determination (r2). The LOD and LOQ values were assessed using the signal to noise ratios by comparing the height of a sample peak and the height of a noise peak. The precision was determined based on intraday and interday repeatability by analyzing the samples (n=3) on three different days. The recovery was determined by adding carnitine to the sample and the results were confirmed by comparing them with the certified values of SRM 1849a.
Results and Discussion
The reaction of HPLC with 1AA gives rise to highly fluorescent single compounds. Although the identity and structure of the derivatized carnitines were not confirmed using tandem mass spectrometry, we attempted to detect L-carnitine in infant powdered milk using FLD radiation. Although the MFDS suggested a simple method for the detection of L-carnitine, it cannot be applied to infant formula because of the complex food matrix. Therefore, improved analytical methods were devised based on the results of Longo et al. (1996) and Minkler et al. (2005) to detect carnitine in infant powdered milk containing large amounts of starch, protein, and/or fat. When these methods are subjected to precolumn derivatization, they can be used for diverse applications. However, these methods still cannot be used with foods containing a large amount of starch, protein, and fat such as powdered milk.
Carnitine is not reactive enough to form an ester. In addition, both sample preparation and chromatography are time-taking processes. Because we used powdered milk as the sample, we made the reagent volume higher than the serum sample. To shorten the time and improve the sensitivity, the reagent volume was increased at each step (Longo et al., 1996). Derivatization was performed using various reagents because it is analyzed with a UV or fluorescence detector instead of a mass spectrometry detector (Cao et al., 2007; Dąbrowska and Starek, 2014; de Andrés et al., 2010). In addition, 200 μL of 1AA, 200 μL of EDC, and 40 μL of HCl were added to the abovementioned solution. Therefore, for the conditions developed herein, the amount of the reagents added for derivatization was doubled because infant powdered milk typically contains a large amount of protein and starch, in contrast to that in serum.
The derivatization result was investigated based on the reaction time (0–60 min) and temperature (25°C–45°C) by integrating the peak areas. The results showed 96.51% of the extraction yield when the temperature was within 25°C–45°C and the time was within 0–60 min. We selected a time of 20 min and a temperature of 25°C to obtain the highest yield (Fig. 1). Longo et al. (1996) used similar time and temperature conditions, whereas Freimüller and Altorfer (2002) used different conditions (45°C and 60 min).
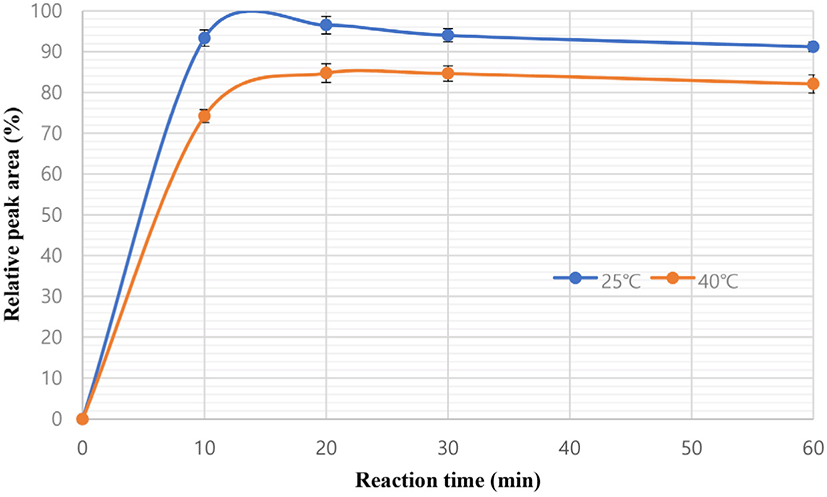
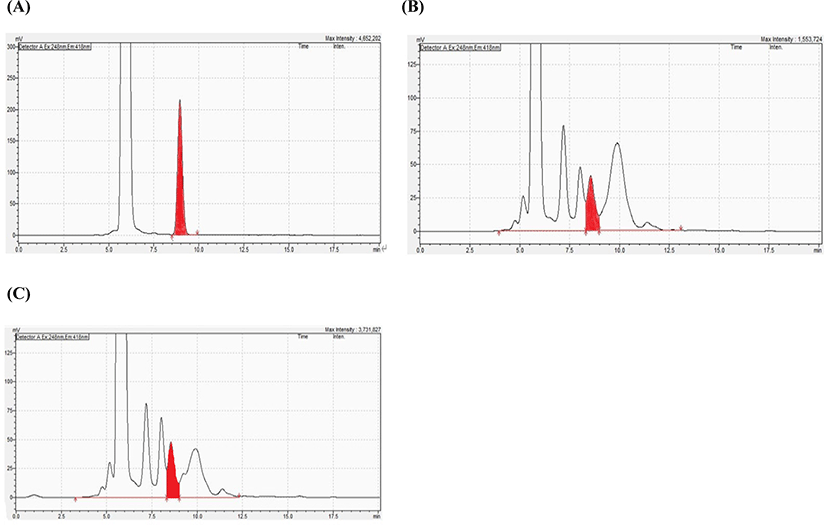
Fig. 2 shows the chromatogram of the standard material, the chromatogram of carnitine on the sample, and the chromatogram of the carnitine on recovered sample. Under the chromatographic conditions used, the derivate formed by carnitine had a retention time of approximately 8 min and the analysis time of approximately 20 min per injection. The linearity results showed that the peak area was linear for the carnitine concentration range of 0.1–2.5 mg/L. The linear equation for the concentration vs. the peak area was y = 8,000,000x – 149,346, with a correlation coefficient of 0.9999. Good linear responses, expressed in terms of a square linear regression coefficient (r2), and slope reproducibility were observed for each compound. The LOD and LOQ were determined to be 0.024 and 0.076 mg/L, respectively, for all analytes, the sensitivity for carnitine detection was satisfactory. The recovery rate for carnitine using NIST Infant/Adult Nutritional Formula 1849a, a standard certification sample, was confirmed to be 92.5% with an relative standard deviation (RSD) value of 1.88%. The result showed that the concentration of carnitine in the infant milk powder investigated in the present study was 125.80 mg/kg, compared to the certified value of 136 mg/kg, i.e., a difference of –8.1%. The recovery rate for the infant milk powder was 97.16%–106.56%. As shown in Table 1, the RSD values, which were used to assess the precision, ranged from 2.53% to 4.72% for intraday precision and from 3.78% to 4.23% for interday precision. Similar results have been reported in literature (Liu et al., 2016). The overall RSD for the infant milk powder was <5%, indicating that this method is accurate and reliable.
| Tested value (mg/kg) | RSD (%) | Recovery (%) | |
|---|---|---|---|
| SRM 1849a | 125.80 | 1.88 | 92.50±2.15 |
Three sample of the infant powdered milk powder were analyzed. The results showed that the content of L-carnitine in the infant powdered milk products that we investigated varied between 8.26 and 16.04 mg/kg. For accuracy, we also determined the L-carnitine contents via LC-MS/MS, L-carnitine results were equivalent as the mean content deviations of HPLC/UV vs. LC/MS were <1.71%, indicating the accuracy of this present method for L-carnitine (Table 1). Even low concentrations of carnitine could be identified through the LC spectrum. Our method can also be used to determine the amount of carnitine present in different food items, to secure food safety, and to improve the overall quality of infant powdered milk. Hence, our method can be used for L-carnitine indication and management for infant powdered milk products available in the Korean market.
We showed that the method developed herein can be used for rapid and accurate determination of the amount of L-carnitine present in infant powdered milk by performing derivatization for 20 min at 25°C. For the HPLC, ammonium acetate and acetonitrile were used as the mobile phase and the run time was within 20 min. Sample pretreatment was performed using 40 μL of 1 M HCl and 200 μL each of 1AA solution and EDC. The HPLC was carried out in combination with FLD treatment to develop an analytical method for extracting carnitine. Although our proposed method requires double the volume of the solvent for derivatization compared to that used in traditional methods, our method has a higher sensitivity than traditional methods, with an LOD of 0.024 mg/mL. A spike recovery test was performed and recoveries of 97.16%–106.56% and RSDs of 2.53%–4.72% were obtained. Our result also showed that SRM 1849a is a suitable certified reference material. Hence, the method proposed in this study can be used for correctly determining the amount of L-carnitine in infant powdered milk products containing a large amount protein and/or fat, which will help in determining the purity of such products. In addition, we hope that this assay would find broad applicability in research on powdered milk and related basic studies.













