Introduction
Obesity is a medical condition in which excess body fat accumulates to the extent that it may have a negative effect on health, leading to reduced life expectancy and/or increased health problems. Diverse approaches to the prevention and treatment of obesity have been reported (Birari and Bhutani, 2007). Among these, both natural and synthetic pancreatic lipase inhibitors are effective in preventing obesity, which is likely to be due to their inhibition of intestinal lipid absorption (Hirose et al., 2013). For these reasons, lipase inhibitors are used in designing drugs for the treatment of obesity (Gupta et al., 2004). Many metabolic products obtained from microbes show potent pancreatic lipase inhibitory activity. Known products include Lipstatin produced by Streptomyces toxytricini (Hochuli et al., 1987; Weibel et al., 1987), Panclicins by Streptomyces sp. NR 0619 (Mutoh, et al., 1994; Yoshinari et al., 1994), Valilactone by Streptomyces albolongus (Kitahara et al., 1987), Ebelactones by Streptomyces buraviensis (Umezawa, et al., 1980), Esteratin by Streptomyces lavendulae (Umezawa, et al., 1978), Caulerpenyne by Caulerpa taxifolia (Tomoda, et al., 2002), Vibralactone by microfungi Boreostereum virans (Liu, et al., 2006), and Percyquinin by Basidiomycete stereum complicatum (Cordula, et al., 2003). Also, peptides that have been found to selectively inhibit fat intake include Enterostatin (Okada et al., 1991; Sorhede et al., 1993) and serotonin (Blundell and Lawton, 1995; Smith et al., 1997). Milk peptides have been shown to have antihypertensive, anti-oxidant and antithrombotic effects, and to influence insulin secretion and glucose control. They also influence lipid concentrations, immune response, inflammation and markers of oxidative stress (Marcone et al., 2017). However, little research has been conducted on the anti-lipase activity of peptide from fermented milk by lactic acid bacteria.
In this study, peptides with a lipase inhibitory effect were separated and purified from fermented milk by Lactobacillus plantarum Q180, a strain isolated from human feces, with a view to developing a new functional anti-lipase activity yogurt product. An ODS-AQ column, a Vydac column, and a Superdex Peptide column were used to separate and purify the peptides.
Materials and Methods
Lactobacillus plantarum Q180 isolated from feces of healthy adults (June, 2012): This strain was selected by screening for probiotic properties such as acid and bile tolerance, antibacterial activity, and antibiotic tolerance (Park et al., 2014). The culture was maintained in MRS broth (Becton, Dickinson and Company, Franklin Lakes, NJ, USA).
Lactobacillus plantarum Q180 was inoculated into 10% reconstituted skimmed milk and incubated at 37°C until the pH of the culture reached pH 4.4.
Ultrafiltration (UF) was performed using a modified version of the method described by Aguilar-Toala et al. (2017). The >10,000-dalton fraction and the <10,000-dalton fraction were separated from fermented milk by an ultrafilter equipped with a membrane having a cut-off molecular weight of 10,000. After that, the fractions were separated into a 1,000-10,000-dalton fraction and a <1,000-dalton fraction by an ultrafilter equipped with a membrane having a cut-off molecular weight of 1,000. They were then evaporated and freeze-dried under vacuum conditions to prepare the peptides.
A modified version of the method of reverse-phase chromatography using an ODS-AQ column reported by Li et al. (2007) was used. The peptide fractions were separated and purified by reverse-phase chromatography using an ODS-AQ column (26×300 mm, Vt: 1,865 mL). Elution was carried out with water and ethanol at a flow rate of 1.0 mL/min. The peaks detected at 215 nm and 280 nm were evaporated and freeze-dried under vacuum conditions.
The peptide fractions were separated and purified by reverse-phase chromatography using a Vydac C18 column (10×250 mm, Vt: 19.6 mL). Elution was carried out with 0.1% TFA in water and 0.1% TFA in acetonitrile at a flow rate of 2.0 mL/min. The peaks detected at 215 nm and 280 nm were evaporated and freeze-dried under vacuum conditions.
The peptide fractions were separated and purified by gel permeation chromatography using a Superdex Peptide column (10×30 mm, Vt: 24 mL). Elution was carried out with water at a flow rate of 0.5 mL/min. The peaks detected at 215 nm and 280 nm were evaporated and freeze-dried under vacuum conditions.
The amino acid sequence of the peptides separated from fermented milk by L. plantarum Q180 was analyzed using a Procise TM (protein sequencing system, Perkin Elmer, Waltham, MA, USA).
A modified version of a method of lipase inhibitory activity determination reported by Lee et al. (1993) was used. The pancreatic lipase activity was measured using porcine pancreatic lipase (Sigma-Aldrich, St. Louis, MO, USA). 0.1 mg/mL of a sample solution dissolved in water, and 0.167 mM p-nitrophenyl palmitate (PNP; Sigma) solution and 0.061M Tris-HCl buffer (pH 8.5) were mixed in the well of a plate, to which 0.3 mg/mL of the lipase solution was then added to start the enzyme reaction. After incubation at 25°C for 10 min, its absorbance was measured at 405 nm.
Results and Discussion
UF was used to separate the peptides from the fermented milk. The cut-off threshold ranged from 3 to 14 kDa depending on the targeted peptides (Aguilar-Toala et al., 2017). Until pH 4.4 was reached, 10% reconstituted skimmed milk was incubated at 37°C using L. plantarum Q180. After UF of the fermented skimmed milk using the cut-off membranes (MW 10,000 and 1,000), the anti-lipase activity of the 1,000-10,000-dalton fraction was slightly higher than that of the <1,000-dalton fraction. These results suggest that low molecular peptides do have lipase inhibitory activities (Table 1). The lipase inhibitory activity in this study was higher than the result obtained for fermented milk using L. plantarum (Gil-Rodriguez and Beresford, 2019). In consideration of the high yield, the <1,000-dalton fraction was separated using the ODS AQ column.
| Molecular weight | Anti-lipase activity (%) | Yield (%) |
|---|---|---|
| 1,000 MW below | 46.83±2.54 | 96.10 |
| 1,000-10,000 MW | 49.85±5.83 | 1.64 |
| 10,000 MW above | 7.73±4.01 | 2.25 |
Reverse-phase chromatography is known to be an effective method of separating and purifying peptides from protein hydrolysates (Herraiz, 1997). After concentrating the <1,000-dalton fraction in a vacuum evaporator and freeze-dried, it was separated and purified by reverse-phase chromatography using an ODS-AQ column. The peaks separated by using the ODS AQ column were named FMO1, FMO2, and FMO3 (Fig. 1). The three fractions were concentrated in a vacuum evaporator and freeze-dried, respectively, and then measured for yield and anti-lipase activity. As a result, the lipase inhibitory activity of fraction FMO1 was the highest at 73.32%, and its yield was also the highest at 89.72% (Table 2).
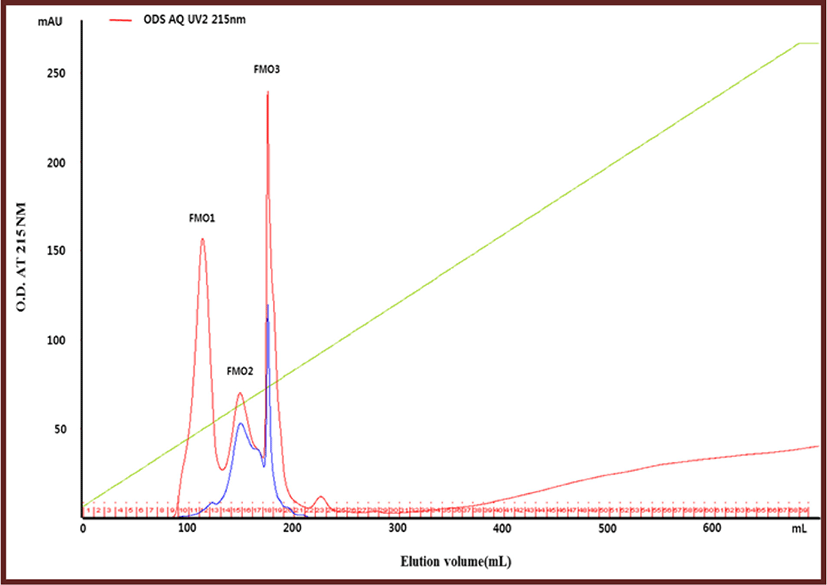
| Peak name | Anti-lipase activity (%) | Yield (%) |
|---|---|---|
| FMO1 | 73.32±0.02 | 89.72 |
| FMO2 | 71.43±0.03 | 5.89 |
| FMO3 | - | 4.40 |
The C18 column is particularly useful for separating peptides of less than 5,000 daltons, and is usually the column of choice for the separation of peptides resulting from protease digestion of proteins (Carr, 2013). Fraction FMO1, which has the highest yield and anti-lipase activity among FMO1, FMO2, and FMO3, was re-separated using the Vydac column. The peaks separated by using the Vydac C18 column were named FMO1V1 and FMO1V2 (Fig. 2). Fractions FMO1V1 and FMO1V2 were concentrated in a vacuum evaporator and freeze-dried, then measured for yield and anti-lipase activity. As a result, the lipase inhibitory activity of FMO1V2 was higher than that of FMO1V2, and the yield of FMO1V2 was 36.40% (Table 3).
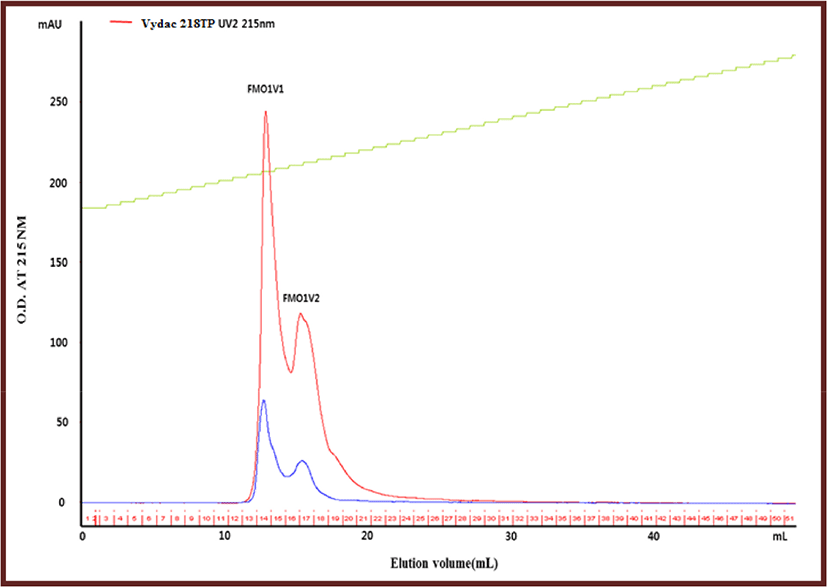
| Peak name | Anti-lipase activity (%) | IC50 | Yield (%) |
|---|---|---|---|
| FMO1V1 | >100% | 3,592 μg/mL | 63.59 |
| FMO1V2 | >100% | 2,880 μg/mL | 36.40 |
The Superdex Peptide HR 10/30 column showed better chromatographic resolution for peptides with an MW in the range of 75-dalton to 12,384-dalton than the TSK gel G2000 SWXL column, especially for peptides with an MW of less than 3,000-dalton (Su et al, 2013). Fraction FMO1V2, which has higher anti-lipase activity than FMO1V1, was separated using the Superdex Peptide HR column. The peaks separated by using the Superdex Peptide HR column were named FMO1V2S1, FMO1V2S2, FMO1V2S3, FMO1V2S4, and FMO1V2S5 (Fig. 3). The yield and anti-lipase activity of these fractions were measured after concentrating and freeze-drying them in a vacuum evaporator. The anti-lipase activity (IC50) value and the yield of FMO1V2S1 were 2,931 μg/mL and 30.54%, respectively (Table 4).
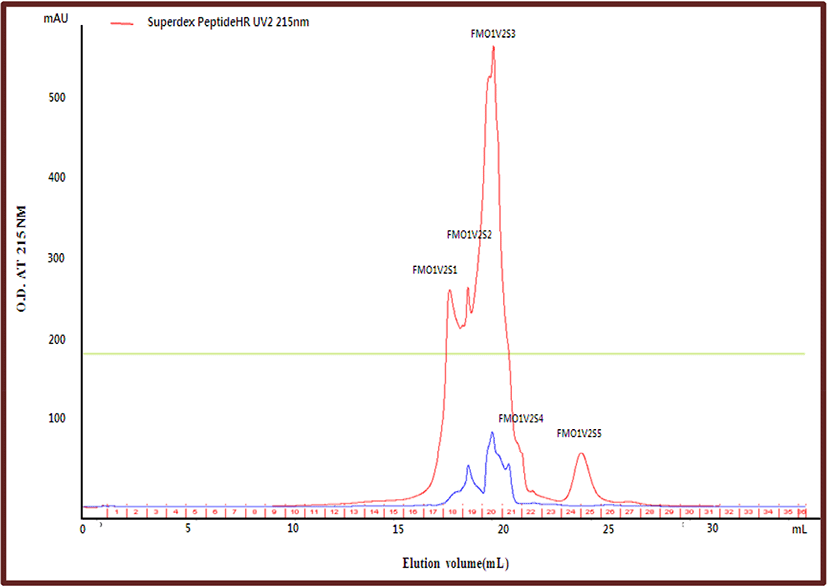
| Peak name | Anti-lipase activity (%) | IC50 | Yield (%) |
|---|---|---|---|
| FMO1V2S1 | >100% | 2,931 μg/mL | 30.54 |
| FMO1V2S2 | >100% | 3,079 μg/mL | 36.21 |
| FMO1V2S3 | 91.42±2.02 | - | 29.21 |
| FMO1V2S4 | 89.12±1.98 | - | 3.63 |
| FMO1V2S5 | - | - | 0.41 |
Fraction FMO1V2S1, which has higher anti-lipase activity than the other fractions, was re-separated using the Superdex Peptide HR column. As a result of separation, there were two peaks; and these fraction were named FMO1V2S1S1 and FMO1V2S1S2 (Fig. 4). FMO1V2S1S1 and FMO1V2S1S2 were concentrated in a vacuum evaporator and freeze-dried, and then their yield and anti-lipase activity were determined. The anti-lipase activity (IC50) value and the yield of FMO1V2S1S1 were 2,817 μg/mL and 73.92%, respectively, which were higher than those of FMO1V2S1S2 (Table 5). Gil-Rodriguez and Beresford (2019) reported that a peptide released from a milk protein hydrolyzed during fermentation by L. helveticus is the active component, and that these fractions exhibited two peaks corresponding to small-sized (<2 kDa) peptides.
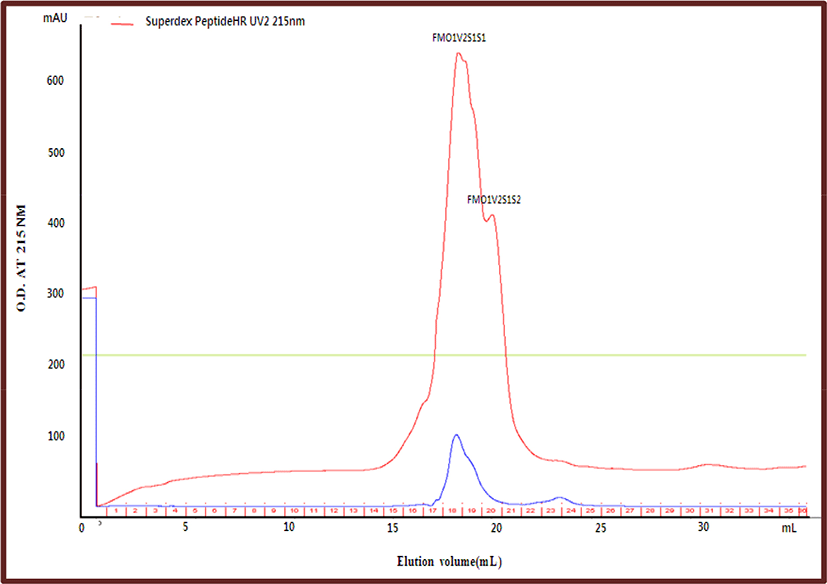
| Variable | Anti-lipase activity (%) | IC50 | Yield (%) |
|---|---|---|---|
| FMO1V2S1S1 | >100% | 2,817 μg/mL | 73.92 |
| FMO1V2S1S2 | >100% | 3,000 μg/mL | 26.07 |
The fermented milk by L. plantarum Q180 was sequentially separated using the ODS AQ column, Vydac C18 column, and Superdex Peptide HR column. The peptide was composed of Asp, Thr, Ile, Ser, Ala, and Gln. The IC50 of the lipase inhibitory activity was 2,817 μg/mL. Okada et al. (1991) reported that Enterostatin (Val-Pro-Asp-Pro-Arg), the activation peptide of pancreatic procolipase, selectively reduces fat intake. In addition, they reported that valine-proline-aspartate-proline-arginine (VPDPR) inhibited fat intake but had no effect on carbohydrate or protein intake.
Conclusion
This study aimed to separate and purify the lipase inhibitory peptide from fermented milk by L. plantarum Q180 for the purpose of developing a new functional anti-lipase activity yogurt product. Lipase inhibitory peptides were gradually isolated by UF, reversed phase column chromatography (RPC), reversed phase high-performance liquid chromatography (RP-HPLC), and gel permeation high-performance liquid chromatography (GP-HPLC) from the fermented milk by L. plantarum Q180.
After the UF of fermented milk by L. plantarum Q180, the anti-lipase activity and the yield of the <1,000-dalton fraction were 46.83% and 96.10%, respectively. When the <1,000-dalton fraction was separated using the ODS AQ column, the lipase inhibitory activity of fraction FMO1 was the highest at 73.32%, and its yield was also the highest at 89.72%. Fraction FMO1 was re-separated using the Vydac C18 column, and the anti-lipase activity (IC50) value and the yield of FMO1V2 were 2,880 μg/mL and 36.40%, respectively. After FMO1V2 was separated using the Superdex Peptide HR column, the anti-lipase activity (IC50) value and the yield of FMO1V2S1 were 2,931 μg/mL and 30.54%, respectively. As a result of the separation of FMO1V2S1, the anti-lipase activity (IC50) value and the yield of FMO1V2S1S1 were 2,817 μg/mL and 73.92%, respectively,
The peptide was composed of Asp, Thr, Ile, Ser, Ala, and Gln, and the anti-lipase activity (IC50) was 2,817 μg/mL. Thus, the results of this study could be applied to the development of a new functional anti-lipase activity yogurt product by Lactobacillus plantarum Q180.













