Introduction
The presence of free radicals is known to contribute to various chronic and degenerative diseases, such as diabetes, coronary heart disease, inflammation, stroke, and cancer (Cheng et al., 2003). Under normal physiological conditions, the body constantly generates free radicals, but also strictly regulated by the antioxidant defense system, so as to maintain a healthy balance of free radicals and the normal functioning of multiple body functions (Laguerre et al., 2007), however, excessive accumulation of free radicals can occur when the internal defense mechanism is out of balance or external harmful stimuli cause the imbalance of free radicals to occur, then cause a series of diseases such as cancer (Leanderson et al., 1997), cardiovascular disease, and inflammatory disease (Wang et al., 2016).
Antioxidants are defined as any substance that prevent the adverse effects of oxygen. It is a class of substances that can help capture and neutralize free radicals to remove their damage to the human body (Theansungnoen et al., 2014). Meanwhile, antioxidants are increasingly used in the field of dietary supplements and should be widely used for disease prevention purposes. However, due to the disadvantages of synthetic antioxidants such as harm to health, high cost, and single mechanism of action (Shi et al., 1996), the development of green, pollution-free and high-safety antioxidants that can replace artificial synthesis has development and application prospects.
Bioactive peptides (BAPs) refer to peptide compounds which are beneficial to biological activities or have physiological effects. As a basic nutrient, active peptides have high bioavailability and are more easily absorbed than amino acids. Meanwhile, they have the function of regulating human physiological function, which is not possessed by original proteins and amino acids composed of them (Hartmann and Meisel, 2007). They have a variety of biological functions, such as hormone effect, immune regulation, anti-hypertension, antitumor effect, antioxidant effect, cholesterol lowering, inhibition of bacteria and viruses, etc. (Samaei et al., 2021; Sarabandi and Jafari, 2020). Among them, antioxidant peptides act as antioxidants by scavenging free radicals and relieving oxidative stress (Giordano et al., 2017; Pandya et al., 2019; Yi et al., 2022). It is found that certain BAPs do indeed have antioxidative properties and can be used as a natural substitute to synthetic antioxidants to improve health (Nadalian et al., 2019). Due to their low toxicity and high efficiency, antioxidant peptides are widely used by the product processing industry and in health care products (Tadesse and Emire, 2020; Wen et al., 2020).
Food protein from dairy products by fermentation method is a source of antioxidant peptides (Anukam and Reid, 2009). The fermentation method uses dairy products as raw materials, inserts protease-producing microorganisms, and utilizes protease produced in the fermentation process of microorganisms to hydrolyze milk protease into peptides with different amino acid sequences and molecular weights under aerobic or anaerobic conditions (Agbor et al., 2011; Chandrasekara and Shahidi, 2011), which are microbial cells themselves, or direct metabolites or secondary metabolites (Ali et al., 2019; Li et al., 2012). Lactic acid bacteria (LAB) are the dominant group of protease-producing microorganisms, among which Lactobacillus spirochetes, Lactobacillus bulgaricus and Lactobacillus paracasei have been reported (Sun et al., 2010). The abundance and characteristics of antioxidant peptides released from milk and dairy products are strain-dependent (Ayyash et al., 2020; Rubak et al., 2020).
Due to LAB’s ability to release BAPs in fermented dairy products, and the fact that it is “generally recognized as safe” (GRAS) for use in food, LAB has become more common for certain strains to produce fermented dairy products with certain functional properties (Kim et al., 2017). Therefore, the objective of this study was to obtain antioxidant peptides using Lactobacillus rhamnosus B2-1 from fermented whey protein. It has important practical significance for developing new whey protein products and improving the comprehensive utilization rate of whey protein.
Materials and Methods
Dimethyl Sulfoxide (DMSO) was purchased from Sangon Biotech (Shanghai, China). 2,2-amino-di (2-ethyl-benzothiazoline sulphonic acid-6) ammonium salt (ABTS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and Trolox were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum, penicillin, streptomycin, and dulbecco’s modified eagle medium (DMEM) were purchased from Thermo Scientific (Waltham, MA, USA). Macroporous resin D101 was purchased from Amicogen Biopharm (Jining, China). Sephadex G-15 gel was purchased from GE healthcare AB Bio-Sciences (Concord, MA, USA). All other chemicals and reagents used were of analytical grade.
Whey protein-MRS medium: According to the modified MRS (DeMan Rogosa-Sharpe) medium, the specific components are as follows: Desalinated whey powder 10 g, beef extract 10 g, yeast extract 5 g, glucose 20 g, Twain’s 80 1 mL, dipotassium hydrogen phosphate 2 g, sodium acetate 5 g, sodium citrate 2 g, magnesium sulfate 0.2 g, manganese sulfate 0.05 g, add water to 1 L, 121°C, sterilization for 15 min.
L. rhamnosus B2-1 was obtained from the soaking liquid of corn. The soaking solution was inoculated into 5% desalted whey powder medium and cultured for 48 h, centrifuged at 1,600×g for 10 min, and the supernatant was taken for preliminary antioxidant activity screening. After initial screening, the bacteria fermentation broth was expanded and enriched at 37°C for 48 h. Single colonies were isolated and selected after spreading the serially diluted fermentation broth on MRS agar plate and incubating them at 37°C for 48 h. The isolated strains were streaked on MRS agar plate and observed by Meilan staining.
By extracting the genome of the strain with properties, and identifying the 16S rRNA by Bioengineering Biotechnology (Shanghai, China), the data was submitted to National Biotechnology Information Center by basic local queue search methods for homology comparison.
L. rhamnosus B2-1 was cultured in 1 mL of sterilized whey protein-MRS medium and incubated under anaerobic conditions at 37°C for 48 h. After continuous expansion for 2 times, the culture was inoculated into 5% whey protein-MRS medium for 48 h, centrifuged at 4,600×g for 10 min, and the supernatant was freeze-dried.
Peptides for whey protein fermentation (PWPF) were preliminary isolated by macroporous resin D101. PWPF (20 mL, 150 mg/mL) were loaded on macroporous resin D101 column (25 mm×460 mm), and stepwise eluted with a series of gradient ethanol solutions at a flow rate of 2.5 mL/min. Automatic collectors were used to collect the eluate (2 min/tube). In order to obtain the elution curve of the sample solution, the absorbance of the fractions was measured at 214 nm, 254 nm, and 280 nm. Besides, the peptide content and ABTS radical clearance rate were measured in every three tubes. The absorption peaks were collected, pooled, rotary evaporation and lyophilized. The purified fractions, labeled F1, F2, F3, and F4, were stored at –20°C until needed.
A Sephadex G-15 chromatography was used to purify the fraction with strong radical scavenging activity. The column was pre-equilibrated with deionized water. The purified fraction was formulated into a 0.7 mg/mL solution with deionized water, and then the peptide solution (4 mL) was loaded onto the column (16 mm×100 cm). Deionized water was used to elute the column at a volume of 4 mL/tube, with absorbance monitoring at 214 nm, 254 nm, and 280 nm. The fractions were lyophilized for further use.
The concentrations of acrylamide in the resolving and stacking gels were 16% and 4%, respectively. The sample was mixed with one volume of 2×sample buffer [12% SDS (w/v), 6% mercaptoethanol (v/v), 30% glycerol (w/v), 0.05% G-250 (w/v) and 150 mM Tris/HCl (pH 7.0)]. After boiling for 3 min, the samples were cooled and loaded onto polyacrylamide gels, which were pre-run at 60 V for 20 min before the sample was applied. The electrophoresis was performed by stacking and breaking down the gel at constant voltages of 60 V and 100 V, respectively. After electrophoresis, the gel was removed, the fixative was fixed for 30 min, stained for 1 h, and it was decolorated.
The sample was mixed with 10 mL of 6 mol/L HCl (containing 0.1% phenol), and nitrogen was filled into the prepared hydrolysis tube to protect the samples from oxidation during the hydrolysis process. The hydrolysis tube was hydrolyzed at 110°C under vacuum for 24 h. After drying, sodium citrate buffer solution (1 mL, pH 2.2) was added into the test tube and dissolved. The absorbed solution was transferred to a sample bottle for detection by the amino acid analyzer after passing through a 0.22 μm filter membrane.
Antioxidant activity was measured by measuring the radical-scavenging ability of ABTS. The sample (40 μL, 1 mg/mL) was mixed with the ABTS reagent (160 μL) and reacted at room temperature for 6 min in the dark. The absorbance was measured with a microplate reader at 734 nm. As a blank control, 50 mmol/L of Tris-HCl buffer was used, and Vc as a positive control. The calculation formula is:
To determine the oxygen radical scavenging ability, ORAC method was used. The sample (20 μL) was added to FL working solution (80 μL), followed by 200 mmol/L AAPH added quickly. The fluorescence intensity of each well was measured every 6 min at 37°C at excitation wavelength of 485 nm and emission wavelength of 538 nm using a microplate analyzer. The measurement time is usually set after the fluorescence attenuation has reached the baseline. With Trolox as positive control, the calculation formula is as follows:
AUCsample and AUCblank in the formula represent the area under the curve of sample solution and blank solution respectively.
The hydroxyl (·OH) radical scavenging activity was determined using the salicylic acid method. The sample (2 mL) was incubated with ferrous sulfate solution (0.5 mL, 9 mmol/L), salicylic acid solution (2 mL, 9 mmol/L in absolute ethanol) and H2O2 solution (8.8 mmol/L) at 37°C for 30 min, and the absorbance was measured at 510 nm. In the control, deionized water was used as a substitute for H2O2, while deionized water was also used for the blank, and Vc was used as a positive control. The calculation formula is:
LoVo human colon cancer cells, KB human oral cancer cells and Cal-27 human oral cancer cells were purchased from the Cell Resource Center, Peking Union Medical College which is the headquarter of National Infrastructure of Cell Line Resource (NSTI). Three types of cells were cultivated in DMEM containing 10% FBS, ampicillin sodium, and streptomycin sulfate. All cells were cultivated at 37°C in a humidified atmosphere containing 5% CO2.
MTT assay was used to evaluate the effects of samples on cell proliferation. Cells were harvested during logarithmic growth phase and seeded at a density of 2×104 cells per well in 96-well plates. During overnight growth, the culture medium was replaced by various concentrations of the sample for 48 h. MTT was added to each well for 4 h. The supernatant was removed and dissolved in 150 μL of DMSO. ELISA plate readers were then used to measure absorption at 570 nm. Cell inhibition rate (percentage) was calculated using the following formula:
Results
In the experiment, the fermented bacterial strain was isolated from the soaking liquid of corn. The strain isolate was genotyped by using 16S rRNA gene sequences. The 16S rRNA gene sequences alignment of the isolated strain showed 99.15% identity with Lacticaseibacillus rhamnosus strain NBRC 3425. L. rhamnosus B2-1 was identified and named based on these results. All the raw reads have been submitted to the NCBI SRA under the accession number SRR19749980.
The lyophilized powder from the fermentation broth of whey protein metabolites of L. rhamnosus B2-1 was separated by macroporous resin D101 column, and its elution was detected by ultraviolet detector. As can be seen from the Fig. 1A, four peaks were successively separated, namely F1, F2, F3, and F4. The yield of each component was 6.03%, 21.50%, 16.66%, and 9.69%, respectively.
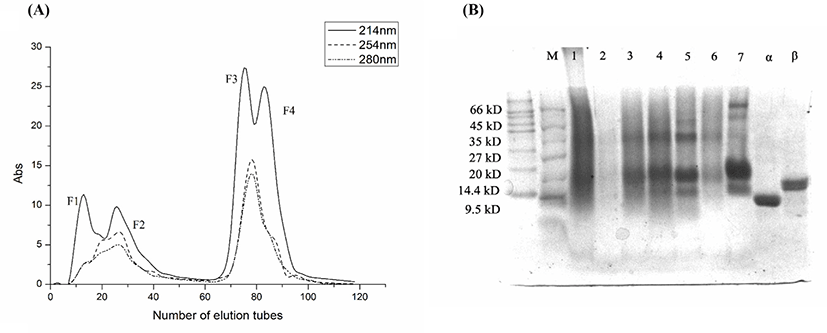
The results of Tricine-SDS-PAGE electrophoresis were shown in Fig. 1B. Controlling with α-lactoglobulin and β-chylous protein, the results showed that the molecular weight of F1 mainly concentrated around 14.4–66 kD, while the molecular weight of F3 and F4 mainly concentrated around 14, 20, and 35 kD, showing obvious bands in these three molecular weights.
The amino acid compositions of F1, F2, F3, and F4 are represented in Table 1. Of the four fractions, the highest amino acid content was F4, followed by F3, F2, and F1. F4 was found to contain both essential and non-essential amino acids, with the main amino acids being Glu, Pro, Thr, Asp, and Ile. Hydrophobic amino acids (Ala, Val, Met, Ile, Leu, Phe, Pro, and Tyr) were 8.738% of F4 (Qian et al., 2008). Thus, F4 contained the highest proportion of hydrophobic amino acids.
Antioxidant activity of protein fractions was determined by ABTS radical scavenging activity, oxygen radical absorbance capacity and ·OH radical scavenging activity.
Fig. 2A shows that ABTS radical scavenging activity of protein fractions. As shown in Fig. 2A, the ABTS radical scavenging activity of F4 was 34.46±1.71%, which was significantly higher than other fractions at the same concentration. The scavenging rate of ABTS radical was F4>F1>F3>F2. These results indicated that the F4 and F1 has potential antioxidant abilities.
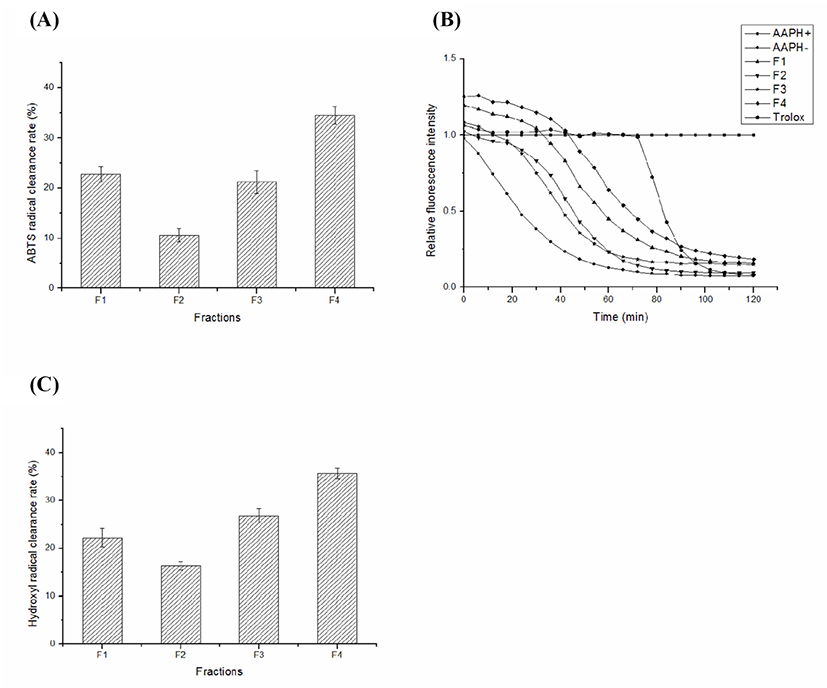
Fig. 2B shows that the results of the fluorescence natural decay curves of protein fractions. Based on Fig. 2B, the antioxidant capacity of ORAC was measured for different fractions of the same concentration and compared with the positive control Trolox that was used to calculate ORAC value. The relative ORAC values of F1, F2, F3, and F4 were 1,060.36±1.52 μM Trolox equivalent/g, 704.82±1.55 μM Trolox equivalent/g, 631.00±0.96 μM Trolox equivalent/g and 1,388.80±2.34 μM Trolox equivalent/g, respectively. The results showed that F4 and F1 have good oxygen radical absorbance capacity.
Fig. 2C shows that ·OH radical scavenging activity of protein fractions. As shown in Fig. 2C, ·OH radical scavenging rates of F1, F2, F3, and F4 were 22.27±1.89%, 16.38±0.86%, 26.82±1.45% and 35.63±1.11%, respectively. Overall, ·OH radical scavenging rates of each fraction were F4>F3>F1>F2 in descending order. These data suggested that the fraction F4 protected cells effectively against damage and oxidative lipid peroxidation.
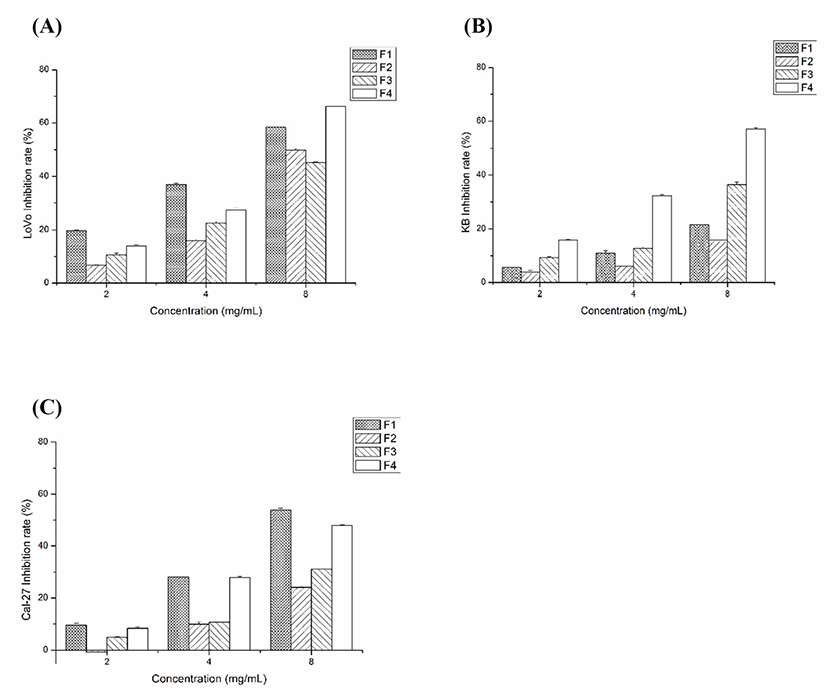
On Figs. 3A, B, and C, the effect of various concentrations on the inhibition of LoVo cells (A), KB cells (B), and Cal-27 cells (C) can be seen. With the increase of concentration, the inhibitory effect of F1 and F4 on tumor cells was significantly enhanced, while F2 and F3 were less effective than F1 and F4. At 8 mg/mL, F1 inhibited the growth of LoVo cells, KB cells, and Cal-27 cells by 58.28±0.89%, 21.71±0.53%, and 53.86±2.43%, respectively. At the same concentration, the inhibition rate of F4 was 66.23±0.61%, 57.14±1.67%, and 47.91±1.05%, respectively. These results confirmed that F1 and F4 inhibited cancer cell growth at low concentrations.
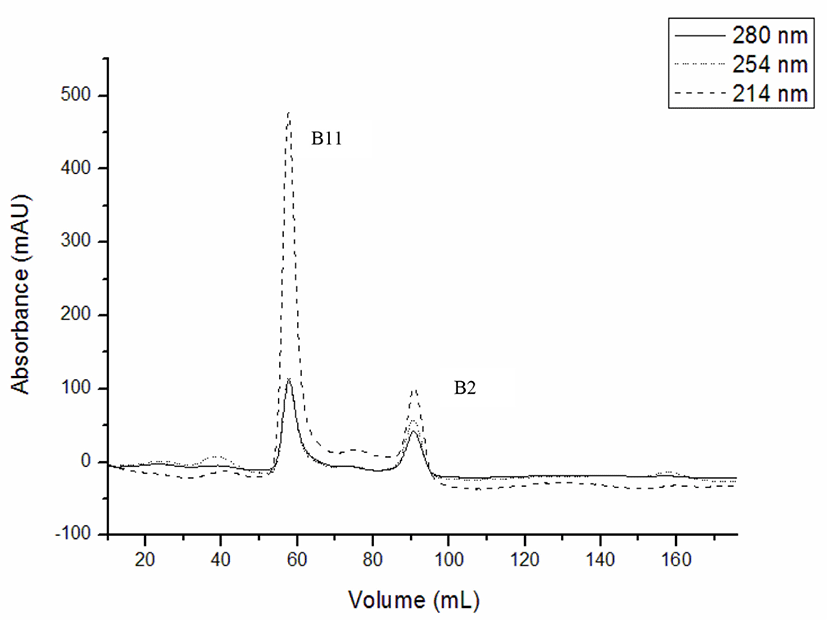
The fraction of F4 from macroporous resin D101 column with the highest antitumor and antioxidation effects was separated by gel filtration chromatography (Sephadex G-15). As shown in Fig. 4, two fractions (B11 and B2) were separated from F4. Due to the excessive conductivity of fraction B2, which contained more salt and less polypeptides, B11 was only used as the study material.
The results of the amino acids composition are presented in Table 1. The amino acid content of B11 is 51.421%, nearly three times that of F4 (18.949%). Acidic amino acids Glu and Asp, which are capable of scavenging radicals via proton transfer, were the primary amino acids contained in the peptide, accounting for 11.180% and 6.013%, respectively. There were seven kinds of essential amino acids (Thr, Val, Met, Ile, Leu, Phe, and Lys) in B11 accounting for 21.871% of total amino acids. Therefore, B11 not only had a high antioxidant activity, but also had higher nutritional value.
Fig. 5A shows that ABTS radical scavenging activity of B11. The ABTS radical clearance rates of B11 increased with the increase of sample concentration after purification, and when the concentration was 1.0 mg/mL, the ABTS radical scavenging rate was the highest, and the antioxidant activity was the strongest, reached 70.36±1.89%. In the control group, the ABTS radical scavenging rate gradually increased with increasing concentration, reaching 98% at the maximum concentration.
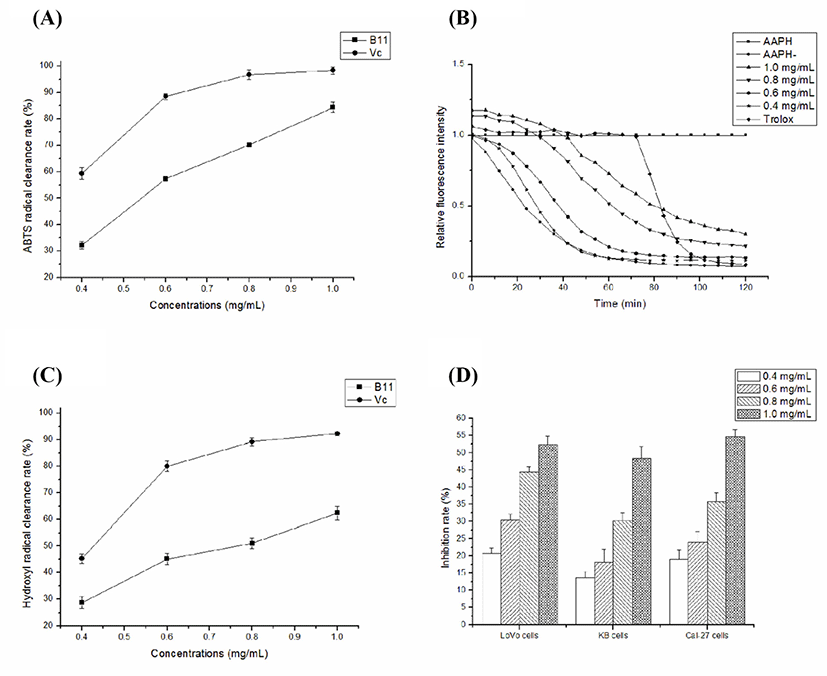
Fig. 5B shows that B11 has strong antioxidant capacity in a dose-dependent manner. The B11 (1.0 mg/mL) showed a high oxygen radical absorbance activity of 1,726.44±2.76 μM Trolox equivalent/g. The ORAC values for the remaining concentrations (0.4, 0.6, and 0.8 mg/mL) of B11 were 465.66±3.05 μM Trolox equivalent/g, 982.84±1.65 μM Trolox equivalent/g and 1,614.07±2.41 μM Trolox equivalent/g, respectively.
Fig. 5C shows that ·OH radical scavenging activity of B11. As shown in Fig. 5C, B11 demonstrated significant ·OH radical scavenging activity, in which the activity increased significantly with increasing concentration, but all concentrations were significantly weaker than Vc. At 1.0 mg/mL, B11 demonstrated scavenging activity of 62.43±2.64% against ·OH radicals. At other concentrations, B11 displayed scavenging activity of 51.04±1.95%, 46.33±2.07%, 28.63±2.33%, respectively.
As shown in Fig. 5D, the inhibition rate of cell proliferation gradually increased with increasing concentrations of B11 from 0.4 to 1.0 mg/mL, which demonstrates a dose-dependent relationship. For LoVo cells, the inhibition rate of B11 from 0.4 to 1.0 mg/mL was 20.78±1.65%, 30.47±1.63%, 44.41±1.56%, 52.36±2.36%, respectively. At this concentration, the cell activities of KB cells were 13.67±1.78%, 18.14±3.87%, 30.21±2.34%, and 48.42±3.42%, respectively, while that of Cal-27 cells were 18.97±2.87%, 24.10±2.96%, 35.74±2.78%, and 54.57±2.14%, respectively. Based on the above data, it can be can conclude that compared with the other tumor cells, B11 has a significant inhibitory effect on the proliferation of LoVo cells.
Discussion
Antioxidant peptides can be prepared through fermentation using microbial enzyme systems, to date, most studies have primarily concentrated on obtaining antioxidant peptides by fermenting whey protein with potential probiotics. Cui et al. (2022) found that the polypeptides of whey protein generated by Lactobacillus reuteri WQ-Y1 had the potential antioxidant activity. Virtanen et al. (2007) investigated the production of antioxidant activity during fermentation with 25 lactic acid bacterial strains, and all strains showed the antioxidant activity. In the present study, L. rhamnosus B2-1 isolated from the soaking liquid of corn was selected as the fermentation strain, and peptides with antioxidant activity were also obtained. It might mean that the antioxidant activity of peptides depends on the fermenting strains and the development of antioxidant activity is strain-specific.
The polypeptides produced by fermentation are generally a mixture, so it is particularly important to separate the purified parts. Macroporous resin adsorption technology and gel filtration chromatography have been widely used for natural products purification (Puchalska et al., 2015; Yan et al., 2015). Macroporous resin has the characteristics of distributed apertures, easy desorption regeneration ability, selectivity on the adsorbate and other features (Xu et al., 2010). As different volume fractions of ethanol have different polarity and affinity for antioxidant peptides with different hydrophobicity, gradient elution may produce antioxidant peptides with different hydrophobicity, and the physiological activity of these antioxidant peptides is closely related to their hydrophobicity. Therefore, in this study, the four fractions obtained by gradient elution with different volume fractions of ethanol had different antioxidant activities. The results showed that the most hydrophobic elution fraction F4 was indeed the best antioxidant activity among the four fractions, so it was completely feasible to use macroporous resin for preliminary separation and purification. Some researchers have reported that macroporous resin D101 can be used to separate and obtain hydrophobic active ingredients (Gao et al., 2018; Gu et al., 2019).
With the rise and in-depth study of antioxidant peptides, a variety of methods have been established to evaluate the activity of antioxidant peptides in vitro. The antioxidant activity of peptides may be classified into two groups, depending on the chemical reaction involved: 1) those peptides are based on the hydrogen atom transfer reaction, which are mainly analyzed by kinetic curves, and 2) those peptides are based on electron transfer reactions, which are mainly reflected by color changes (Moayedi et al., 2017; Ohata et al., 2016; Yang et al., 2017b). In the first case, it consists of ORAC assay and ABTS radical scavenging assay, while in the second case it consists of ·OH and DPPH radical scavenging assays (Shen et al., 2010; Tang et al., 2010). In this study, three in vitro assays were used to determine the antioxidant activity of protein fractions. The results showed that the antioxidant activity of the target peptide B11 gradually increased as the fermented solution was separated by different separation methods.
Researchers reported that most antioxidant peptides contained one or more hydrophobic amino acids since hydrophobic amino acid residues can act as hydrogen donors to break off free radical peroxide chain reactions with their aromatic residues (Xing et al., 2016). In the fraction B11, 23.349% of the amino acids were hydrophobic, which would lead to better oxidation inhibition than in F4. In this study, the content of Met and Tyr in B11 was higher than that of other fractions (F1, F2, F3, and F4). Polypeptides with Met and Tyr could effectively scavenge free radicals (Yang et al., 2017a), which was also one of the reasons for the improved ABTS free radical scavenging ability of B11. Wattanasiritham et al. (2016) reported that peptides containing Pro, Gly, Ala, Val, and Leu had intrinsic antioxidant activity. Amino acids such as His, Leu, Gly, and Pro were suggested to play an important role in radical caging (Scholljegerdes et al., 2005). Moreover, peptides containing Tyr, Pro, Phe, and His, displayed strong antioxidant properties (Ren et al., 2008; Shen et al., 2010). The results of the present study supported the above statement by the strong antioxidant properties of B11 with the presence of intrinsic antioxidant amino acids. As a whole, the strong antioxidant properties of B11 may be attributed to their constituent amino acids, which can provide protons or act as electron donors to the free radicals and react with them to convert them to more stable products and terminate radical chain reaction (Zielinski et al., 2009). All of these reasons may explain the strong antioxidant activity of B11. Results from this study indicated that it was feasible to produce natural antioxidants from the whey protein fermented by L. rhamnosus.
Cells have an innate ability to modulate apoptosis using various redox systems, in which cancer cells are more dependent on antioxidant systems than other non-transformed cells, so they are especially vulnerable to increased oxidative stress as this redox balance is frequently deregulated in cancer cells (Eliassen et al., 2002; Trachootham et al., 2009). It was identified that antioxidants had been proposed as potential candidates in the prevention and treatment of diseases associated with active oxygen species, particularly cancer diseases (Leng et al., 2005). In this study, the results showed that B11 could significantly inhibit the proliferation of cancer cells, indicating that that the antioxidant and antiproliferative activities of the peptide were interrelated. Therefore, it might be proposed that higher antioxidant activity leads to better antitumor activity, and the possible reason was the initial antitumor effect by regulating the level of oxidative stress in cancer cells (Kannan et al., 2008). Similar results were observed previously, Sun et al. (2017) demonstrated that the polypeptide from Pleurotus eryngii mycelium exerted significant antioxidant effects and could inhibit the growth of cervical, breast, and gastric cancer cells, which was also a bifunctional BAP.
Conclusion
In summary, our study reports the antioxidant activity of peptides derived from the whey protein fermented by L. rhamnosus B2-1 by macroporous resin and gel chromatography purification for the first time. After whey protein fermented from L. rhamnosus B2-1, the obtained fermented solution was separated by macroporous resin D101 to four fractions (F1, F2, F3, and F4). Fraction F4 showed significantly greater antioxidant activity than the other three fractions. F4 was re-separated using the Sephadex G-15 chromatography, and the final antioxidant fraction, B11, was obtained. Fraction B11 exhibited potent in vitro antioxidant activity in ABTS, ·OH radical scavenging activity and ORAC assay, and antitumor activity on LoVo, KB and Cal-27 cancer cells proliferation. The high antioxidant activity of B11 can be attributed to the presence of intrinsic antioxidant amino acids. Thus, B11 might be a good source of antioxidant peptide that may act as a food additive and bioactive material, and open up new possibilities for the utilization of whey by-products.













