Introduction
Scientific publications, especially population-based epidemiologic studies have always emphasized the role of diet in the prevention and control of disease and premature death caused by non-communicable diseases. Nowadays, many people prefer to consume functional food products especially probiotic products. Dairy products such as fermented milks can be good carriers of probiotic bacteria (Delavari et al., 2014). Lactobacillus plantarum is one of the probiotic bacteria which regarded as safe. L. plantarum has good acidification ability and produces plantaricin bacteriocin in order to increase the safety of food products. So this bacterium can be used as starter for production of fermented milks due to technological and safety properties (Essid et al., 2009).
Omega -3 fatty acids are a group of polyunsaturated fatty acids (PUFA) which are important in keeping the cardiovascular system healthy. Linoleic acid, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are the most important omega fatty acids whose major resources include seafood and vegetable products such as walnut, rapeseed and linseed (Lavie et al., 2009; Lopez-Huertas, 2010). Omega-3 fatty acids can be used to enrich low-fat foods and to produce functional foods. However, since these compounds are hydrophobic and do not dissolve in water, enrichment of food products and aqueous drinks with them is very difficult. As they possess unsaturated bonds, these components are extremely sensitive to oxidation whereby they undergo off-odor and off-flavor changes because of oxidation, and their health-promoting effects diminish. Application of nanocarriers to transport and protect fat-soluble micronutrients, or in other words, nanoencapsulation of such substances is a suitable way to overcome these problems (Liu et al., 2008).
Nanoencapsulation is a technique in which carriers, less than 100 nm in size, are produced and utilized to enrich food products and transport nutraceutical and bioactive compounds (essential fatty acids, antioxidants, carotenoids, etc.) to target special sections (Ye et al., 2006). Nanocapsules differ from on another depending on their applications in food matrices. They are divided into two types of biopolymeric and polymeric. Proteins, polysaccharides or their complexes are used in the production of biopolymeric nanocarriers (Zimet and Livney, 2009). In nanoencapsulation, various types of carbohydrates are often used as wall material or carrier (McName et al., 1998). Carbohydrates including gums, different types of starch, maltodextrin and solid corn syrup are abundantly applied for encapsulation, owing to their diversity and low price as well as their properties such as low viscosity at high concentrations and desirable solubility (Mutka et al., 1988).
Gum arabic (GA) is the most important gum used as wall material in the nanoencapsulation of flavor compounds. Desirable solubility, low viscosity, emulsifying properties and the high holding capacity of volatile compounds and oil droplets have caused this gum to be widely noticed for nanoencapsulation (Shiga et al., 2001).
Some researchers have examined the encapsulation efficiency (EE) of fish oil as a core material. The nano-particles were prepared by spray drying and or emulsion preparation via high energy emulsifying techniques such as microfluidization or ultrasonication. Maltodextrin was utilized as wall material in combination with biopolymers (modified starch or WPC) at a ratio of 3:1. The results revealed that microfluidization was an efficient method for emulsification, resulting in a fish oil powder with the minimum amount of surface oil mostly due to its ability for emulsification within nano range (210–280 nm) (Jafari et al., 2008). Ghorbanzade et al. (2017) encapsulated fish oil in nanoliposoms and used it in yogurt fortification. The results showed that yogurt containing nanoencapsulated fish oil had higher DHA and EPA contents than yogurt containing free fish oil. Moreover, yogurt containing nano-encapsulated fish oil had closer characteristics to control sample in terms of sensory properties than yogurt containing free fish oil.
Given the increased tendency towards application of fish oil in food, pharmaceutical and sanitary products, it should be researched and the findings should be applied to the respective formulations. Accordingly, the aim of the present study was to encapsulate fish oil by preparing an O/W nanoemulsion using GA as wall material and to examine its effects on the properties of probiotic fermented milk.
Materials and Methods
Fish oil was purchased from Nooshdaru Darya Co. (Iran). GA (density=1.4 g/cm3), sorbitan mono-9-octadecenoate (tween 80), ethanol, n-hexane and 2-propanol were supplied from Merck Co. (Germany). Lactobacillus plantarum IBRC-M 10817 was purchased from Iranian Biological Resourco Center. Other high purity chemicals were purchased from Merck Co. (Germany).
In this study, the wall material was considered 20% and 25% (w/w). The solution of GA was prepared in deionized water. This was done using a magnetic stirrer at 500 rpm and 60°C for 1 h until the wall material (GA) completely dissolved in water. Next, the prepared solution was kept in a refrigerator overnight for complete hydration. Then, tween 80 was added to it at 2% and 4% (w/w). Subsequently, fish oil was incorporated into the mixtures dropwise at 6% (w/w). The resulting mixtures were agitated using a magnetic stirrer at 500 rpm for 30 min. Finally, ultrasonic waves were exploited for nanoemulsion preparation at 24 kHz for 120 s (Saberi et al., 2014).
The prepared emulsions were frozen at −20°C for 24 h. Next, they were placed in a freeze drier at −50°C for 48 h. The dried emulsions were powdered using a pestle and mortar for further experiments (Kaushik and Ross, 2007; Klinkeson et al., 2005).
The particle size distribution was determined by dynamic light scattering (DLS, Malvern, UK) to measure the emulsion droplets diameter.
For this extraction, 8 mL of hexane was added to 1 g of sample powder and mixed for 2 min, then centrifuged at 5,724×g for 20 min. The supernatant was filtered through filter paper and the solvent was evaporated at 70°C. The extracted oil was dried at 50°C until a constant weight was obtained (Klinkesorn et al., 2006). The total oil was extracted as follows: solvent (3:1 isopropanol:hexane) was added to 0.5 g of powder, stirred for 5 min and the clear organic solution phase was evaporated at 70°C until a constant weight was obtained (Jimenez et al., 2004). The EE was measured as follows (Wang et al., 2006):
Analysis of the methyl esters of fatty acids was performed using a gas chromatograph (Plus 2010, Germany) equipped with mass spectrometry detector and a sol-gel 1 ms column with a length of 30 m and a diameter of 0.32 m. Omega-3 fatty acids were extracted from the complex powders with acetone derived by 1 mL of Boron tri-fluoride methanol, 1 mL of NaOH 2 N and 1 mL of n-hexane. It was then injected into GC whereby the fatty acid composition was examined (Ghorbanzade et al., 2017).
The 24-h MRS broth cultures of L. plantarum were separately centrifuged at 5,000 ×g for 15 min. The supernatant was discarded and the cells were rinsed twice with distilled water. Then, a suspension of the cells was prepared in distilled water at a concentration of 109 CFU/mL. The suspension was subsequently diluted to 108 CFU/mL. After that, 1% (v/v) of the suspension was inoculated into the pasteurized milk containing 1.5% fat. Next, the inoculated milk samples were incubated at 37°C for 10 h, the nanocapsules were added to them so that 0.3 g of the omega-3 fatty acids existed in each serving size (240 mL) of the low fat probiotic fermented milk. The samples were finally stored at 4°C.
MRS agar was used for enumeration of L. plantarum. Incubation was performed at 37°C for 72 h under anaerobic condition (Golestani and Pourahmad, 2017).
Peroxide value (PV) was determined based on AOCS method (AOCS, 2007). The oil sample (3 g) was dissolved in acetic acid (30 mL) and chloroform (20 mL) at a ratio of 2:3 (v/v). The mixture was kept in the dark for 1 min and then blended with 50 mL distilled water. It was eventually titrated with sodium thiosulfate 0.01 N. PV was calculated by the following equation:
Where S is the volume of sodium thiosulfate (mL), N stands for the normality of sodium thiosulfate and W denotes the oil sample weight (g).
The sensory attributes (flavor, color, texture and overall acceptance) of the low fat probiotic fermented milk containing fish oil nanocapsules, were assessed by 10 trained panelists through 5-point hedonic scale (Ilyasoglu and Nehir El, 2014).
Results and Discussion
According to Table 1, there was a significant difference (p<0.05) between the diameter of emulsion droplet of the samples.
| Sample | Composition (% emulsifier-wall material) | Diameter of emulsion droplet |
|---|---|---|
| 1 | 2–20 | 82±0.58a |
| 2 | 2–25 | 68±0.58b |
| 3 | 4–20 | 45±1.15c |
| 4 | 4–25 | 36.67±1.33d |
The sample with 25% wall material and 4% emulsifier had the lowest diameter of emulsion droplet. The highest diameter of emulsion droplet was related to the sample with 20% wall material and 2% emulsifier. Many factors affect the droplet size. They can include the amount and type of emulsifier, phase type, pH and preparation method of emulsion, the ratio of components, speed of stirrer, sequence and rate of adding ingredients (Izquierdo et al., 2002; Klinkesorn et al., 2005). The wall composition, gum concentration, mixing conditions and emulsifier can influence the nanoparticle size in emulsions containing gum arabic (Ghayempour and Mortazavi, 2015). The results showed that the particle size decreased as the wall material (gum arabic) increased. The highly branched structure of gum arabic acts as an emulsifier and decreases the particle size through covalent bonding of the oil droplets and the branches. As the emulsification capacity of the wall increased, the migration of ingredients to the capsule surface reduced. In a similar study, Najaf-Najafi et al. (2011) investigated the encapsulation of castor oil and reported that the type and concentration of the wall affected the particle size and the modified starch produced larger particles than skim milk.
According to Table 2, there was a significant difference (p<0.05) between surface oil content of the samples. The highest surface oil content was related to the sample with 20% wall material and 2% emulsifier. The lowest surface oil content was recorded for the sample with 25% wall material and 4% emulsifier. The size of the emulsion can influence the surface oil. Jafari et al. (2008) reported that the surface oil increases significantly with an increase in the size of the emulsion droplets due to destabilization of the emulsion by bigger droplets, which is in agreement with the findings of this study. Davidov-Parado et al. (2008) showed that the particle size of emulsions influenced the surface oil content. They found that smaller oil droplets were effectively encapsulated in the matrix of the wall component.
| Sample | Composition (% emulsifier-wall material) | Surface oil |
|---|---|---|
| 1 | 2–20 | 39±1.95a |
| 2 | 2–25 | 35±0.58c |
| 3 | 4–20 | 37±0.57b |
| 4 | 4–25 | 31.66±0.54d |
According to Table 3, sample 4 (the capsule with 25% gum arabic and 2% emulsifier) had the highest EE. The lowest EE was related to samples 1 (the capsule with 20% gum arabic and 2% emulsifier) and 3 (the capsule with 20% gum arabic and 4% emulsifier). EE represents the degree of surface oil on particles and the degree to which the wall can prevent encapsulated oil extraction. It has been observed that larger emulsion droplets allow increased expulsion of the surface oil content. Particle encapsulated with 25% gum arabic and 4% emulsifier had the highest EE value and the lowest surface oil because of the smaller diameter. The difference of the molecular weight of the wall materials may be a reason for difference in oil holding capacity. An increase in molecular weight increased the material holding capacity, effective oil droplet distribution in solid materials and the low percentage of surface oil (Klaypradit and Huang, 2008). An increase in viscosity in the continuous phase reduced the mobility of the droplets and created enough time for adsorption of the emulsifier onto the surface of the water and oil droplets, which reinforced droplets against sedimentation and stabilized the emulsion, creating capsules with higher EE values after drying (Makri and Doxastakis, 2006). In a similar study, Ilyasoglu and Nehir El (2014) encapsulated EPA/DHA with gum arabic and sodium caseinate and recorded an EE for the encapsulated oil of 78.88±2.98% for the protein-polysaccharose complex. Moreover, Badee et al. (2012) encapsulated peppermint oil with gum arabic, maltodextrin and their combination by spray-drying. They reported that the highest oil retention of 81% and 80%, respectively, was during drying for gum arabic and the gum arabic: maltodextrin 1:1 combination. In another study, Krishnan et al. (2005) encapsulated cardamom oleoresin in maltodextrin-modified starch-gum arabic. They found that an increase in gum arabic increased EE.
| Sample | Composition (% emulsifier-wall material) | Encapsulation efficiency |
|---|---|---|
| 1 | 2–20 | 81.77±0.68c |
| 2 | 2–25 | 85.29±0.35b |
| 3 | 4–20 | 81.79±0.35c |
| 4 | 4–25 | 87.17±0.28a |
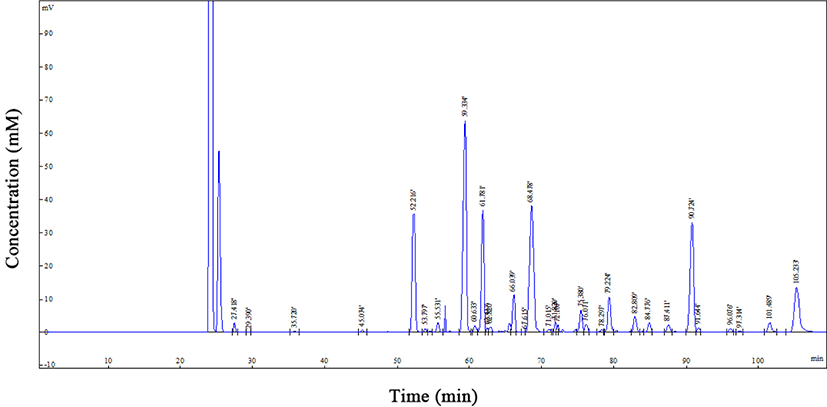
Fig. 1 illustrates the fatty acid composition of the fish oil. In the present study, palmitic acid was the major fatty acid accounting for 18.31 mg/g fish oil. Of the unique properties of fish oil is containing large amounts of omega-3 fatty acids, particularly EPA, DHA, alpha linolenic acid (ALA) compared with other oils. The contents of EPA and DHA were determined 15.18 and 11.32 mg/g fish oil, respectively. The content and composition of fish oil fatty acids vary depending on the fish species, fishing season, fish nutrition and many other factors (Ackman et al., 2005). Fish oil, like most animal fats, is so variable in terms of natural antioxidants and has small amounts of tocopherol which is minimized during refining. Moreover, owing to its considerable PUFA content, making up 23.89 mg/g fish oil in the present study, fish oil is prone to oxidation. Therefore, encapsulation could protect this substance from oxidation.
According to Table 4, on the first day of storage, the highest PV was related to samples A, B and C. Samples A and B had the highest PV on the 7th day. On the 14th day, the highest PV was related to sample A. On the first, 7th and 14th days, sample D and control sample had the lowest PV. Encapsulation efficiency indicates the presence of surface oil on the particle surfaces and the ability of the wall material to prevent the oil from diffusing out of the capsule. In general, the larger the emulsion droplets, the higher the surface oil content (Klaypradit and Huang, 2008). As a result, the capsule in which 25% wall material and 4% emulsifier was used, was smaller in diameter; hence, it had smaller amounts of surface oil and lower PV. The fermented milk sample containing fish oil nanoencapsulated with 4% emulsifier and 25% wall material had the lowest PV. The reason behind this is that this sample had the smallest particle sizes thus, having the highest EE and the lowest surface oil. The lower the surface oil content, the lesser it is exposed to oxygen and as a result, the smaller the PV. The highest PV was related to fermented milk sample containing fish oil nanoencapsulated with 20% wall material and 2% emulsifier which had the lowest EE and the highest surface oil content. This led to a larger extent of oxidation and a higher PV. During storage, PV of the fermented milk samples significantly (p<0.05) increased but the sample containing fish oil nanoencapsulated with 25% wall material and 4% emulsifier had the lowest PV, because this nanocapsule had the highest EE and as mentioned earlier, this parameter reflects the presence of oil on the particle surfaces and the ability of the wall to prevent the oil from being released (Vahid Moghadam et al., 2019). The higher the EE, the lower the surface oil content and hence, the lower the oxidation rate and the smaller the PV so that the PV of the sample with 25% wall material and 4% emulsifier was equal to that of the control on the first day of storage. The control sample had the lowest PV because of being free of the fish oil. Since a decrease in particle size raises the particle surface, this may give rise to PV. Therefore, it is necessary that the size reduction process to be carried out under controlled atmosphere and the process conditions reduce the impact of the factors affecting oxidation, otherwise, the process itself may elevate the oxidation rate. Some researchers found that the PV and anisidine value (AV) of bread and the milk enriched with omega-3 PUFA significantly raised during storage (Rasti et al., 2017). Tamjidi et al. (2012) incorporated encapsulated fish oil into yogurt and come to conclusion that the PV of the controlled sample was equal to zero during the 22 days of storage. Nonetheless, the samples containing the encapsulated and surface oil increased up to 72 and 260% respectively during storage. Ghorbanzade et al. (2017) reported similar results. They found out that the PV of the fish oil extracted from the yogurt samples containing free fish oil was 0.92 on the first day and increased up to 1.61 after 21 days, while the PV of the yogurt samples containing the nanoencapsulated fish oil was nearly constant during storage. Smet et al. (2008) compared the oxidation of various types of milk with different fatty acid compositions and suggested that the milk with a more unsaturated fatty acid composition was more sensitive to oxidation. Chee et al. (2005) accomplished similar results and reported that the PV of the yogurt enriched with omega-3 PUFA and flavored with strawberry was elevated up to 200% during the 22 days of storage, whereas the PV of the control sample remained equal to zero.
According to Table 5, on the first day of storage, the fermented milk sample containing fish oil nanoencapsulated with 4% emulsifier and 25% wall material had the highest EPA content. On the other hand, the fermented milk sample containing fish oil nanoencapsulated with 2% emulsifier and 20% wall material had the lowest EPA content. The same results were observed on the 7th and 14th days of storage, too. Regarding Table 6, the fermented milk sample containing fish oil nanoencapsulated with 4% emulsifier and 25% wall material had the highest DHA content on the first, 7th and 14th days. On the first and 7th days, the lowest DHA content was related to samples A, B and C. Sample A (the fermented milk sample containing fish oil nanoencapsulated with 2% emulsifier and 20% wall material) had the lowest DHA content on the 14th day. Encapsulation efficiency indicates the presence of surface oil on the powder particles and the wall capability for inhibiting the oil from being extracted (Klaypradit and Huang, 2008). Our results also demonstrated that among the test samples, the highest EPA and DHA contents was related to the fermented milk sample containing fish oil nanoencapsulated with 4% emulsifier and 25% wall material, as this sample had the highest EE. This, in turn, led to the prevention of oil release thereby yielding the highest EPA and DHA contents. Borneo et al. (2007) reported that the addition of microencapsulated fish oil to food products was one of the best ways of retaining and increasing the stability of omega-3 fatty acids in food formulations. The results of the present study showed that EPA and DHA contents of the samples decreased significantly (p<0.05) during storage. In a similar study, Lee et al. (2007) observed that the content of EPA and DHA decreased during the four-week storage of the strawberry yogurt enriched with fish oil. Moreover, Ghorbanzade et al. (2017) also found that the nanoencapsulation of fish oil and yogurt enrichment with it had a significant effect on the residue of omega-3 fatty acids. In the samples containing the encapsulated fish oil, the maximum residues of EPA and DHA were equal to 57% and 12% respectively during 21 days of storage. On the other hand, the sample containing the free oil had EPA and DHA contents of 6% and 27%, respectively. This could be a result of nanoencapsulation which has a protective effect on omega-3 fatty acids against environmental conditions.
According to Table 7, on the first day of storage, the highest viability of probiotics was related to the fermented milk sample containing fish oil nanoencapsulated with 25% wall material and 4% emulsifier. In contrast, the lowest viability of probiotics was associated with the control sample. The same results were obtained on the 7th and 14th days of storage, too. The population of L. plantarum significantly (p<0.05) decreased during storage. Numerous factors including pH, the presence of other microorganisms, incubation temperature and the presence of oxygen have been identified in fermented milk which can influence the viability of probiotic bacteria. The decrease in the number of lactobacilli in probiotic products is probably due to the damage caused by acid to the organisms (Damin et al., 2008). Georgieva et al. (2009) performed a study on the milk fermented with L. plantarum. They inoculated 106 CFU/mL of the bacterium to skim milk. During fermentation, the number of the bacteria was raised up to 2 logarithmic cycles. During refrigerated storage until the 28th day, the viability of the bacteria was retained to a desirable extent and reached approximately 107 CFU/mL. As a result, fermented milk is a suitable carrier for L. plantarum strains, which comprise a large number of viable cells in the final product when being consumed. The findings of the present study revealed that the addition of the encapsulated fish oil to the probiotic fermented milk loaded with L. plantarum brought about a significant (p<0.05) enhancement in the viability of the probiotic bacteria, compared with the control sample (the probiotic fermented milk free of the encapsulated fish oil). This could be ascribed to the unsaturated fatty acids which considerably stimulate the growth of probiotic bacteria. Because of stimulating the growth and activity of probiotics, the presence of prebiotic compounds is one of the major reasons behind the viability of most bacteria. Prebiotics could provide some of the nutrients necessary for the growth of microorganisms (Saad et al., 2013). It should also be noted that the population of L. plantarum significantly (p<0.05) decreased in all the samples at the end of the storage period which could be caused by the aggregation of acid as a result of the production of lactic acid and other organic acids including acetic acid, formic acid and acetaldehyde by the yogurt starter culture. This led to a reduction in pH and an increase in acidity (Joung et al., 2016). In addition, the rise in oxidation-reduction potential (Eh) and in the concentration of hydrogen peroxide resulting from the metabolic activity of bacteria (Dave and Shah, 1997) are of the factors reducing the population of probiotic bacteria during yogurt fermentation. However, the final population of L. plantarum was higher than the minimum required for imparting health effects. Sendra et al. (2008) claimed that the incorporation of citrus fiber into the fermented milk enriched with probiotics improved the viability of bacteria. Donkor et al. (2007) reported that the addition of inulin to yogurt also improved the survival of Lactobacillus casei during storage.
According to Table 8, the highest flavor score was associated with the control sample on the first, 7th and 14th days. Samples A, B, and C had the lowest score of flavor. During storage, the flavor score of the samples did not change significantly. The results indicated that the control sample had the largest amount of taste and aroma owing to not having the nanoencapsulated fish-oil. As mentioned earlier, EPA and DHA are prone to oxidation and undergo numerous changes during processing because of being unsaturated. When exposed to light, proxidants and high temperatures, these fatty acids are oxidized rapidly and thus, primary oxidation products are produced. The sensory changes caused by the decomposition of hydroperoxides to secondary oxidation products such as aldehydes, ketones, acids and alcohols, are proportional to the chain length and degree of unsaturation of fatty acids. Some of these compounds have a very low olfactory threshold, thus influencing the sensory quality of a product at low concentrations, leading to off-odor and off-flavor (Bibi et al., 2011). Oxidation of fish, fish oil and the fish oil-enriched emulsions consisting of omega-3 PUFA, indicates the presence of volatile components which are recommended as appropriate factors for detecting the degradation omega-3 fatty acids and play an important role in off-flavor (Rasti et al., 2017). In a similar study, Ghorbanzade et al. (2017) used nanoencapsulated fish oil in yogurt and reported that the control sample attained the highest taste score.
Regarding Table 9, samples A and B had the lowest color score on the first and 7th days. On the 14th day, there was no significant difference between color score of the samples. During storage, the color score of the samples did not change significantly. Among the test samples, the fermented milk samples containing nanoencapsulated fish oil with 20% and 25% wall material and 2% emulsifier had the lowest score, because of its low EE and more release of the oil from the nanocapsule wall into the milk during storage which led to color changes in the milk. In a similar study, Ghorbanzade et al. (2017) encapsulated fish oil and used it in yogurt fortification. They reported that the control sample acquired the highest color score.
According to Table 10, there was no significant difference between texture score of the samples. The results demonstrated that adding nanoencapsulated fish oil did not affect the texture of the samples. During storage, the overall acceptance score of the samples did not change significantly.
Based on Table 11, the control sample had the highest overall acceptance score on the first, 7th and 14th days. The lowest overall acceptance score was related to samples A, B and C. During storage, the overall acceptance score of the samples did not change significantly. The results demonstrated that the control sample was the most acceptable one due to being free of the nanoencapsulated fish oil. Among the test samples, the highest overall acceptance belonged to the fermented milk sample containing fish oil nanoencapsulated with 4% emulsifier and 25% wall material which was caused by the highest EE and the lowest amount of surface oil, resulting in a lesser extent of oxidation and release of the oil from the capsule walls during the storage of the probiotic low fat fermented milk. Consequently, the off-flavor of the milk was reduced and its overall acceptance increased.
Conclusion
This study indicated that use of nanoencapsulated fish oil significantly (p<0.05) increased the survival of probiotic bacteria in low fat fermented milk. At the end of 14 days of storage, the sample containing fish oil nanoencapsulated with 25% wall material and 4% emulsifier had the highest survival of probiotic bacteria (8.41 Log CFU/mL) and the lowest peroxide value (0.57 mEq/kg). This sample had high EPA and DHA contents. Utilizing fish oil nanoencapsulated with 25% wall material and 4% emulsifier did not adversely affect fermented milk overall acceptability. Therefore, this nanoencapsulated fish oil can be used for fortification of low fat probiotic fermented milk and it is possible to produce functional low fat fermented milk in favor of consumers.