Introduction
Probiotics are live microorganisms that impart various beneficial effects on the host, when consumed in an appropriate concentration (de Melo Pereira et al., 2018). The genus, Lactobacillus is the representative heterogeneous group of the lactic acid bacteria (LAB). The functional benefits of this genus are widely explored due to its physiological characteristics and genetic diversity (Montoro et al., 2016). Several Lactobacillus have been used as probiotics in functional foods and biotherapeutic agents for rendering multifactorial benefits to humans and other animals over the past decades (Ren et al., 2014). Although there are many commercial probiotic strains available in the market, novel probiotics have been identified with specific properties in past few years, especially those isolated from the traditional fermented foods (Martins et al., 2013).
Jeotgal or jeot is a salted and fermented Korean seafood that used as Korean cuisine to enhance the flavor or the taste of the food (Koo et al., 2016). Jeotgal is prepared from various types of seafood including oyster, shellfish, shrimp, and fish and also adding about 20%–30% (w/w) salt and subsequently undergo fermentation process which improves the palatability and the shelf life of the dish (Guan et al., 2011). Previous studies have reported the probiotic potential of the bacterium isolated from jeotgal, particularly adapt to resist acidic and high concentrations of bile salts that body imposes (Koo et al., 2016).
The synergistic combination of probiotic and prebiotic found in products such as foods and supplements is known as “Synbiotic” (Adebola et al., 2014). Various combinations of synbiotic show therapeutic effects against diseases such as respiratory infections, diarrhea, allergy, and diabetes and some prebiotics showed anti-adhesive activities against enteropathogens (Shoaf et al., 2006). Further, the synergistic action of probiotics and prebiotics exhibited great efficacy compared to the use of either probiotic or prebiotic individually (Mohanty et al., 2018). Dairy products can be effectively used as a probiotic and prebiotic carrier due to their cohesive structure, pH, and fat content (Speranza et al., 2018). In addition, prebiotic may also incorporate to the food to enhance the quality, safety and functionality of the food. Some authors have reported the enhanced anti-mutagenic, antioxidant, antibacterial, and anti-proliferative effect of synbiotic yoghurt (Sah et al., 2015, 2016).
This study was aimed to investigate the probiotic potential of L. brevis KU200019, an isolate from Korean jeotgal and to evaluate the synergistic interaction with various commercial prebiotics to enhance the antagonistic activity against foodborne pathogens, as well as to evaluate the potential use of novel strain in a fermented-synbiotic dairy product in order to enhance its functionality.
Materials and Methods
The probiotic strain used in this study was the Korean jeotgal isolate, L. brevis KU200019. The organism was identified by 16S rRNA sequencing performed by Bionics Inc. (Seoul, Korea). The sequencing results were analyzed by GenBank database from the website http://blast.ncbi.nlm.nih.gov by using the Basic Local Alignment Search Tool (BLAST). The commercial strain, L. brevis ATCC 14869 used for comparative analysis. The L. brevis strains were propagated and maintained in MRS (de Man, Rogosa, and Shape, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) broth at 37°C.
The tolerance in simulated gastric conditions of the L. brevis strains were evaluated as method of Son et al. (2017b). The L. brevis strains were cultured in 10 mL MRS broth at 37°C for 18 h. The MRS broth was gastric conditioned by adding 0.3% (w/v) of pepsin (Sigma-Aldrich, St. Louis, MO, USA) and changing the pH of the media to 2.5 by using 0.1 M HCl. One milliliter of the bacterial culture was suspended in 9 mL gastric conditioned MRS broth and incubated at 37°C for 3 h.
To evaluate bile salt tolerance, L. brevis strains were cultured in 10 mL of MRS broth at 37°C for 18 h. One milliliter of the bacterial culture was suspended in 9 mL of MRS broth supplemented with 0.3% (w/v) oxgall (Difco, Detroit, MI, USA) followed by incubation at 37°C for 24 h.
The survival rate was calculated using the following formula:
where, N and N0 represents the viable cell count after and before incubation, respectively.
The enzyme activity of L. brevis strains was measured using the API ZYM kit (BioMerieux, Lyon, France). The bacterial cell suspension (65 μL) was inoculated to each cupule of the API ZYM strips and incubated at 37°C for 4 h. The ZYM A and B reagents were added to each of the cupules and the enzyme activity was determined based on the color intensity; 0 (no activity) to 5 (≥40 nM).
The antagonistic activity of L. brevis strains against Listeria monocytogenes ATCC 15313, Escherichia coli O157:H4 FRIK 125, Staphylococcus aureus KCCM 40511, and Salmonella Enteritidis ATCC 13076 was evaluated following the methods of Son et al. (2017a). The LAB suspension (10 μL) was spotted on the MRS agar plates and incubated at 37°C for 18 h. The MRS agar was overlaid with 9 mL of soft nutrient agar inoculated with 1 mL of pathogenic strain (1×106 CFU/mL). The plates were incubated at 37°C for 18 h and the inhibition zone diameter was measured.
Autoaggregation and coaggregation assays were modified slightly from Jeon et al. (2017). The overnight culture of bacterial cells was harvested by centrifugation at 6,000×g for 10 min at 4°C. The harvested cells were suspended in phosphate buffered saline (PBS; Gibco, Grand Island, NY, USA) to achieve an optical density (OD) of 0.3±0.02 at 600 nm (OD0). The LAB suspension (4 mL) was infcubated at 37°C for 24 h. The absorbance of the samples was measured at 600 nm after 4 h and 24 h (ODt). The autoaggregation value was calculated using the following formula:
For coaggregation assay, 2 mL LAB strain was mixed with 2 mL pathogenic strain and incubated at 37°C for 24 h. The absorbance of LAB (ODL), pathogenic strain (ODP), and LAB-pathogen mixture (ODmix) was measured at 600 nm after 4 h and 24 h. The coaggregation value was calculated using the following formula:
The cell surface hydrophobicity was assessed by measuring the bacterial adhesion to the hydrocarbons following the method described by Lee et al. (2015). Briefly, the bacterial cells were suspended in PBS to achieve an OD of 0.5±0.02 at 600 nm (OD0). Then 3 mL of bacterial suspension was mixed with 1 mL xylene and vortexed. The vortexed samples were incubated at 37°C for 20 min till the two phases separated. The absorbance of the aqueous phase was measured at 600 nm (ODt) and the cell surface hydrophobicity was calculated using the following formula:
Six commercial prebiotics used in this study, lactulose (4-O-β-D-galactopyranosyl-D-fructose (C12H22O11), fructooligosaccharide [FOS, (C6H10O5)n (n>10)], xylitol (C5H12O5), inulin (C6H10O5)n (n~36), and dextran (C6H10O5)n (Shinbhi International, Seoul, Korea). The effect of prebiotics on the growth of L. brevis was evaluated as described previously (Bevilacqua et al., 2016). Briefly, MRS medium without glucose (MB cell, Seoul, Korea) was supplemented with 2% (w/w) prebiotic compounds, 2% (w/w) glucose (positive control), or without carbon source (negative control). Following, inoculated with 6 Log CFU/mL of each L. brevis strain and incubated for 48 h. L. brevis growth was evaluated through the measurement of absorbance at 600 nm. Data were interpreted as growth index (GI), through the equation proposed by Bevilacqua et al. (2016).
Where, ODs is the absorbance of the samples with different prebiotics, and ODc is the absorbance of the positive control. In addition, pH of the sample was evaluated periodically by a pH meter (inoLab pH 7110, Xylem Analytics, Weilheim, Germany). Prebiotics was selected based on their synergistic effect on GI for further study.
The human colon adenocarcinoma cell lines (HT-29, KCLB 30038) was supplied from the Korean Cell Line Bank (KCLB, Seoul, Korea). The HT-29 cells were cultured in RPMI 1640 medium (Hyclone, Logan, UT, USA) supplemented with 10% (v/v) fetal bovine serum (Hyclone), and 1% (v/v) penicillin-streptomycin (Hyclone) at 37°C in a humidified atmosphere containing 5% CO2. The adherence ability of L. brevis strains to HT-29 cell lines was performed as described previously (Yang et al., 2019). Aliquot of bacterial suspension 1×108 CFU/mL was added to confluent HT-29 monolayers and subsequently, supplemented with prebiotics (20 mg/mL) and incubated at 37°C for 2 h. Samples either added with L. brevis strains or prebiotics were considered as control samples. To determine the number (CFU/mL) of L. brevis strains that adhered to the HT-29 cells, the bacterial cells were harvested with 1% Triton X-100 (Sigma-Aldrich), and serially diluted in PBS and plated on the MRS agar plates.
The ability of L. brevis strains and prebiotic to inhibit the adhesion of the pathogen to the HT-29 cell line was evaluated following the method of Jang et al. (2019). The confluent HT-29 monolayers were inoculated with 1:1 mixture of pathogenic bacteria and L. brevis (1×108 CFU/mL) and incubated at 37°C for 2 h in a CO2 incubator. The prebiotics (final concentration, 20 mg/mL) were added to each well immediately before incubation. Samples without LAB or prebiotics were considered as control. To determine the number (CFU/mL) of pathogens that adhered to the HT-29 cells, the bacterial cells were harvested with 1% Triton X-100 and serially diluted in PBS and plated on the Listeria selective agar and eosin methylene blue agar (Becton-Dickinson, Franklin Lakes, NJ, USA) for detecting L. monocytogenes and E. coli, respectively. The plates were incubated at 37°C for 24 h.
The probiotics were evaluated for potential application in synbiotic dairy products by cultivating in skim milk. Strains were activated in MRS broth at 37°C for 24 h and harvested by centrifugation at 6,000×g for 10 min at 4°C. The L. brevis strains (8–8.5 Log CFU/mL) were inoculated into sterilized skim milk powder (12%) supplemented with either FOS, lactulose or xylitol (2%). Skim milk without prebiotic addition was used as control. Samples were stored at 4°C for 28 days and cell cultivability was evaluated in 0, 7, 14, 21, and 28 days by spread plating in MRS agar and incubating at 37°C for 48 h.
Five types of fermented skim milk samples were prepared by co-culturing with commercial starter culture (Culture Systems, Mishawaka, IN, USA) and L. brevis KU200019 or L. brevis ATCC 14689 (8–8.5 Log CFU/mL). The samples were incubated at 42°C till the pH drops to 4.0–4.5. Samples were labeled as, fermented skim milk added with; starter culture (C), starter culture and L. brevis ATCC 14689 (S1), starter culture and L. brevis KU200019 (S2), starter culture, L. brevis ATCC 14689, and FOS (S3), and starter culture, L. brevis KU200019, and FOS (S4). Samples were stored at 4°C for further analysis.
After 7-day water soluble extracts (WSE) of fermented skim milk samples were prepared as described previously with slight modifications (Sah et al., 2014). Fermented skim milk samples were centrifuged at 22,600×g at 4°C for 30 min. The supernatant was filtered using a 0.45-μm membrane filter and freeze dried in a freeze drier and all the lyophilized samples were kept at –80°C for further analysis. The protein content (mg/mL) of each WSE was estimated using a Bradford assay with a BSA (0.1–2.0 mg/mL) standard.
The antioxidant activity of WSE (0.5 mg of protein/mL) was analyzed by DPPH (2,2-diphenyl-2-picrylhydrazyl radical) and ABTS [2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) di-ammonium salt] radical scavenging assays as described previously (Kariyawasam et al., 2019). Radical scavenging activity was modulated as below formula,
ODc and ODs is the absorbance of control (distilled water) and samples, respectively.
The experiments were performed in triplicates and the data are presented as mean±SD. Statistical analyses were conducted using IBM SPSS statistics 20 (SPSS/IBM Corp., Chicago, IL, USA). The data were analyzed using one-way analysis of variance (ANOVA) or independent samples t-test. A difference was considered significant at p≤0.05.
Results and Discussion
The probiotics must adapt and maintain their functions even under stress conditions. The survival rate of L. brevis ATCC 14869 and L. brevis KU200019 in acidic pH (pH 2.5 and 0.3% pepsin) condition was 94.20±0.50% and 99.38±0.21%, respectively (data not shown). Similarly, in previous studies, probiotics isolated from jeotgal showed 98.76%–99.37% acid tolerance values (Akther et al., 2017).
The survival rate of L. brevis ATCC 14869 and L. brevis KU200019 in the presence of bile salt (0.3% oxgall) was 102.45±0.53% and 115.10±0.13%, respectively (data not shown). The survival rate of the L. brevis KU15006 strain isolated from Korean kimchi was 105.24% in the presence of bile salt (0.3% oxgall), which was lower than that of L. brevis KU200019 (Son et al., 2017a). Thus, the high survival rate of L. brevis KU200019 in the presence of acids and bile salts indicates its ability to survive and colonize in the gut to be considered as a potential probiotic.
The toxicity of the probiotics was assessed by measuring the production of enterotoxin enzymes, such as α-chymotrypsin, β-glucuronidase, or N-acetyl-β-glucosaminidase that are associated with intestinal diseases (de Melo Pereira et al., 2018). The results revealed that both the strains did not produce the enterotoxin enzymes (Table 1).
The selective antagonistic activity of probiotic strains against foodborne pathogens can have applications in the food industry to prevent food spoilage. In this study, L. brevis strains inhibited the growth of L. monocytogenes ATCC 15313, E. coli O157:H4 FRIK 125, S. aureus KCCM 40511, and S. Enteritidis ATCC 13076. The antibacterial activity of L. brevis KU200019 was higher than that of commercial strain. The highest antagonistic activity of L. brevis KU200019 was observed against L. monocytogenes ATCC 15313 (34.5±1.5 mm) followed by S. aureus KCCM 40511 (33.5±3.54 mm), S. Enteritidis (30±3.54 mm), and E. coli O157:H4 FRIK 125 (29±0.60 mm). However, L. brevis ATCC 14869 exhibited the highest antagonistic activity against S. Enteritidis (29±2.60 mm), followed by L. monocytogenes ATCC 15313 (27.5±0.71 mm), S. aureus KCCM 40511 (26±1.41 mm), and E. coli O157:H4 FRIK 125 (25±1.41 mm). The antibacterial activity of LAB may be due to the production of organic acids, diacetyl compounds, hydrogen peroxide, and bacteriocin-like peptides (Kariyawasam et al., 2019). Furthermore, the difference in antagonistic activity can be explained by the level and type of antimicrobial agents produced during the fermentation process by the LAB strains (Gad et al., 2016). Thus, novel strain showed high antimicrobial activity against various foodborne pathogens. Particularly, L. brevis KU200019 was shown high antagonistic activity against L. monocytogenes and the value was significantly higher than the that of the commercial strain, L. rhamnosus GG showed 22.33±0.58 mm (Jang et al., 2019). This indicated the potential use of this organism as an adjunct culture in food industry. Particularly, the products such as fresh cheese and yoghurt those are highly vulnerable to contamination by L. monocytagenes.
The autoaggregation and coaggregation properties of the probiotics with the potential enteric pathogens can be used as a preliminary screening marker for the selection of the probiotic strain for administration to humans and for colonizing property in intestinal tract (de Melo Pereira et al., 2018). The autoaggregation property ensures that the probiotic reaches a high cell density in the GIT and subsequently contribute to the adhesion mechanisms (Ogunremi et al., 2015). The autoaggregation values are presented in Table 2. The autoaggregation values of novel strain was distinguished from the L. brevis strains isolated from other fermented fish product like Hentak (Aarti et al., 2017). Furthermore, autoaggregation value of L. brevis KU200019 was higher than L. brevis ATCC 14869 which indicates the high colonization activity of novel probiotic in gut (p<0.05; Table 2).
Coaggregation is also a probiotic property that enables the pathogen agglomeration with the probiotic cells, which results in the elimination of pathogen from GIT through feces. The strongest coaggregation was observed between L. brevis KU200019 and L. monocytogenes ATCC 15313 (41.21±2.61%) followed by L. brevis KU200019 and E. coli O157:H4 FRIK 125 (32.70±4.03%; Table 2). The coaggregation value for L. brevis ATCC 14869 was lower than that for L. brevis KU200019.
General bacteria with high hydrophobic capacity have high adherence ability to intestinal mucosa. The correlation between the adhesion ability and hydrophobicity of bacterial surface was reported by Murtini et al. (2016). The hydrophobicity of the L. brevis KU200019 was higher than that of the L. brevis ATCC 14869 (Table 2). This indicated that the high adherence of L. brevis KU200019 to the host epithelial cells. Hydrophobicity value of L. brevis KU200019 was distinguished from hydrophobicity values of L. brevis strains isolated from jeotgal by other authors. The hydrophobicity value for L. brevis G1 and L. brevis KU15006 were varied from 47%–48% (Son et al., 2017a).
Growth of L. brevis strains in laboratory medium supplemented with prebiotics (2%) was evaluated and lowest growth was observed in xylitol and negative control (8%–9%). Partial growth of L. brevis strains was observed in presence of inulin and dextran (18.8%–21.3%). Lactulose was positively influenced growth of L. brevis strains, showed GI values 70.07% and 75.32% for L. brevis ATCC 14869 and L. brevis KU200019, respectively. Moreover, presence of FOS showed highest GI value (~100%; Fig. 1). Nevertheless, concerning the interpretation of GI values it can be concluded that FOS can be provided the optimal growth conditions for LAB growth (Bevilacqua et al., 2016). In addition, pH of inulin, dextran, and xylitol supplemented laboratory media were reduced slightly after 48 h (0.5–0.8), whereas positive control and FOS supplemented samples showed pH 3–4 reduction after 48 h (data not shown). Effective utilization of FOS and glucose by L. brevis strains may cause to produce microbial metabolic products including short chain fatty acids (SCFA) and lactose and these organic acids production might have caused for declining pH (Cremon et al., 2018). These findings are concurred with the fact that selective utilization of prebiotics by LAB.
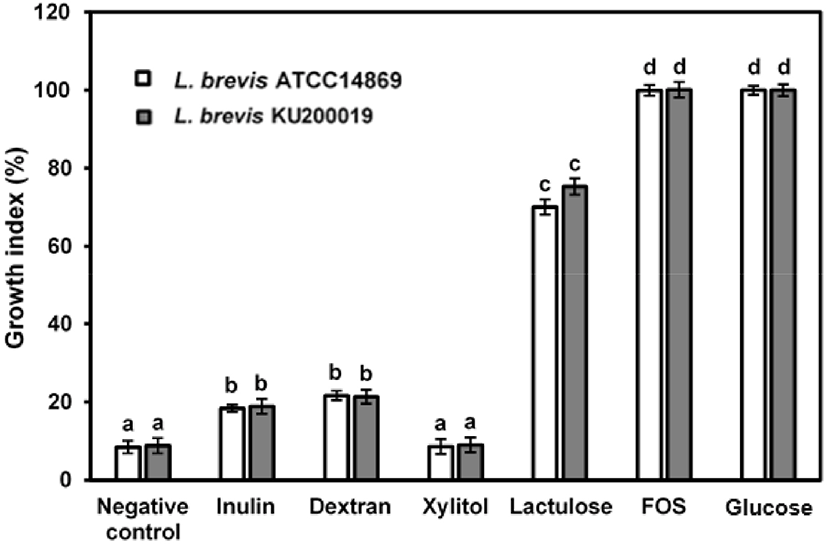
Concerning the GI results, FOS and lactulose showed the better features for future synbiotic application. Although xylitol did not have positive influence for L. brevis growth, xylitol was also used in further experiments for comparative analysis.
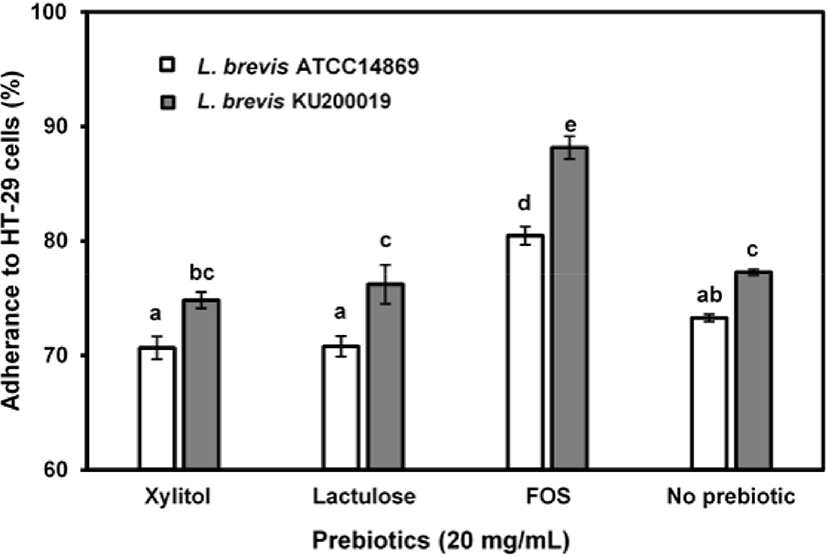
The adhesion of the potential probiotic candidates to the intestinal mucus and to the enterocytes is important for the colonization of the host intestinal tract (de Melo Pereira et al., 2018). The adhesion of L. brevis ATCC 14869 and L. brevis KU200019 to HT-29 cells was 73.26±0.33% and 77.25±0.22%, respectively. Difference in the adhesion values can be explained by variations in the physicochemical properties of the probiotic cell surface such as autoaggregation and hydrophobic capacities. FOS supplementation significantly enhanced the adhesion of L. brevis ATCC 14869 (80.45±0.79%) and L. brevis KU200019 (88.14±0.98%) to the HT-29 cells (p<0.05). In contrast, there was no significant difference between control sample and lactulose or xylitol supplemented samples (p>0.05; Fig. 2). The enhanced adherence in FOS supplemented samples can be explained by the fact that, fermentation of oligosaccharides could produce the SCFAs, supply the required energy for the proliferation of probiotics and modulate the colonic microbial population and activity (Mohanty et al., 2018).
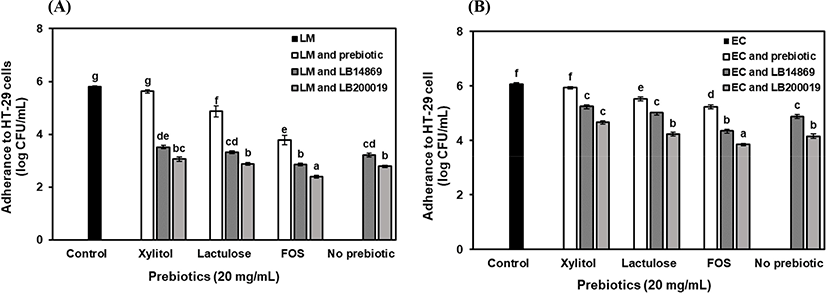
The cell count of L. monocytogenes ATCC 15313 and E. coli O157:H4 FRIK 125 that adhere to HT-29 cells was 5.80±0.07 Log CFU/mL and 6.06±0.12 Log CFU/mL, respectively (Fig. 3). The number of pathogens adhere to HT-29 cells was reduced when the pathogens were co-incubated with the L. brevis strains (p<0.05). The cell count of L. monocytogenes ATCC 15313 that adhere to HT-29 cells reduced when co-incubated with L. brevis ATCC 14869 (3.22 Log CFU/mL) or L. brevis KU200019 (2.79 CFU/mL). Similarly, the cell count of E. coli O157:H4 FRIK 125 that adhere to HT-29 cells reduced to 4.89 Log CFU/mL and 4.16 Log CFU/mL, when co-incubated with L. brevis ATCC 14869 or L. brevis KU200019, respectively. However, L. monocytogenes ATCC 15313 and E. coli O157:H4 FRIK 125 adhesion inhibition ability of novel strain was higher than that of the previous authors. Jang et al. (2019) reported 0.12 CFU/mL and 0.59 CFU/mL reduction of adhesion inhibition for L. monocytogenes ATCC 15313 and E. coli O157:H4 FRIK 125, respectively when co-incubated with L. brevis KU15153. This anti-adhesion property may be due to the competition for the binding sites on the epithelial cells and available nutrients between the probiotics and pathogens (de Melo Pereira et al., 2018). When prebiotic tested alone for adherence inhibition, treatment with FOS resulted in an adherence inhibition of 3.78 Log CFU/mL and 5.24 Log CFU/mL for L. monocytogenes and E. coli, respectively. The adherence inhibition of FOS was higher than the other prebiotics (p<0.05). The adherence inhibition of pathogens by prebiotic oligosaccharides are mediated by various mechanisms. First, metabolism of prebiotic oligosaccharide by LAB results in the production of antagonistic agents that inhibit the growth of the pathogens (Shoaf et al., 2006). Second, prebiotic oligosaccharides exhibited anti-adhesive activity by mimicking the host cell receptor sites. The intestinal pathogens recognize and bind to these mimicked receptor sites and subsequently prevent the binding of pathogenic bacteria to the intestinal cells (Kunz et al., 2000). Moreover, prebiotic oligosaccharide treated along with L. brevis stains and subsequently, adherence inhibition was measured. The cell count of L. monocytogenes ATCC 15313 that adhere to HT-29 cells was reduced to 2.86 Log CFU/mL and 2.40 Log CFU/mL, when cultured in a medium supplement with FOS and co-incubated with L. brevis ATCC 14869 or L. brevis KU200019, respectively. Similarly, the cell count of E. coli O157:H4 FRIK 125 that adhere to HT-29 cells was reduced to 4.65 Log CFU/mL and 3.85 Log CFU/mL, when cultured in a medium supplement with FOS and co-incubated with L. brevis ATCC 14869 and L. brevis KU200019, respectively. Therefore, the results demonstrated that synergetic interactions among prebiotic oligosaccharide (FOS) and L. brevis KU200019 has highest adherence inhibition of pathogens rather administration them individually (p<0.05).
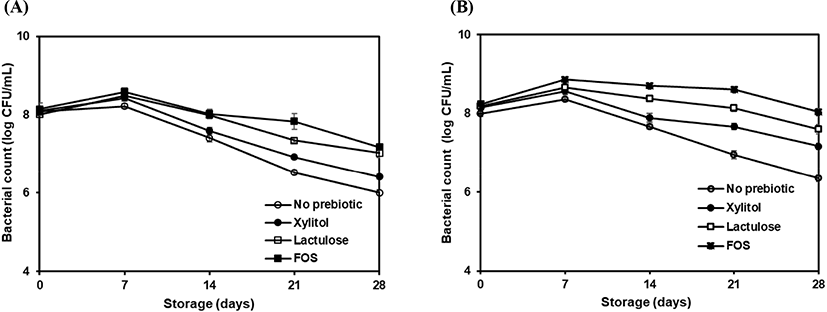
The strains were evaluated for their ability to use as a probiotic in dairy products and effect of prebiotics on strains cultivability by culturing L. brevis ATCC 14869 and L. brevis KU200019 in skim milk supplemented with or without prebiotics during 28 days of storage at 4°C (Fig. 4). The initial count of L. brevis ATCC 14869 and L. brevis KU200019 were 8.0 to 8.3 Log CFU/mL in all samples. The bacterial counts were increased until 7 days of storage in all samples while highest viability was observed in FOS added skim milk samples. The values for L. brevis ATCC 14869 and L. brevis KU200019 were 8.59±0.17 Log CFU/mL and 8.86±0.07 Log CFU/mL, respectively. However, LAB count continued to decrease after 14 days. The steep decline of L. brevis ATCC 14869 and L. brevis KU200019 were observed in control sample and skim milk added with xylitol, whereas the steady decrease was observed in skim milk added with lactulose and FOS. Moreover, the skim milk with FOS showed lowest reduction of bacterial count and L. brevis KU200019 count was above 8 Log CFU/mL throughout the storage period. At the end of the shelf-life (28 days) L. brevis ATCC 14869 and L. brevis KU200019 in FOS added skim milk were 7.18±0.15 Log CFU/mL and 8.04±0.16 Log CFU/mL, respectively. Concerning the skim milk, prebiotics showed a positive effect on maintaining probiotic viability. Noteworthy, L. brevis ATCC 14869 and L. brevis KU200019 cultivability was positively influenced by lactulose and FOS. Some authors suggested that protective effect of prebiotics to maintain the probiotic viability (Speranza et al., 2018). Furthermore, high viability in skim milk added with FOS can be explained by the protective role of the fructans by interactions with membrane phospholipids to maintain the stability of the cytoplasmic membrane of probiotics (Vereyken et al., 2003).
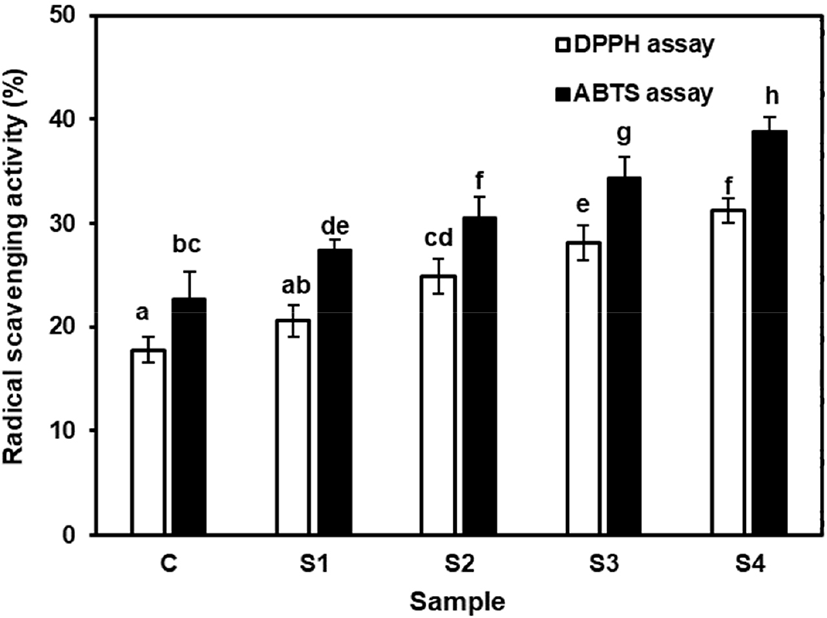
Antioxidant activity of the fermented skim milk samples were evaluated after 7 days. All samples showed varying degrees of radical scavenging capacities for DPPH and ABTS assays (Fig. 5), indicating differences in polarity, hydrolysis of proteins, and ability to donate atoms and electrons of antioxidant bio-factors (Yilmaz-Ersan et al., 2018). Co-culturing of probiotics with starter cultures resulted in enhanced antioxidant activity (Fig. 5). The antioxidant activity of S2 and S3 were differ in both assays (p<0.05). This indicates that antioxidant capacity of hydrolysates for the same substrate, depends on the types of enzyme from LAB. This may be due to specific proteases are involved in the hydrolysis of specific peptide bonds (Sah et al., 2014). Nevertheless, supplementation of FOS has enhanced the antioxidant activity of fermented skim milk samples. The values for S3 and S4 was (1) DPPH assay: 28.14±1.64% and 31.23±1.14% and (2) ABTS assay: 34.36±2.04% and 38.82±1.46%, respectively. The strong scavenging activity in prebiotic supplemented samples may be due to enhanced viability and activity of probiotics. This finding is in agreement with Sah et al. (2015) who observed enhanced antioxidant activities in inulin or pineapple peel supplemented yoghurt samples.
Conclusion
The present study revealed that L. brevis KU200019 isolated from jeotgal showed dominant probiotic properties compared to commercial strain. L. brevis KU200019 exhibited higher survival rate in gastric conditions and antimicrobial activity against various foodborne pathogens including L. monocytogenes ATCC 15313, E. coli O157:H4 FRIK 125, S. aureus KCCM 40511, and S. Enteritidis ATCC 13076 compared to L. brevis ATCC 14869. Moreover, synergistic interactions between L. brevis KU200019 and FOS markedly enhanced the adherence inhibition of foodborne pathogens to HT-29 cells and confirming the potential use in modulate the gut microbiota and prevention of pathogen-associated diarrhea. Furthermore, high survival rate over 8 Log CFU/mL in skim milk and high antioxidant activity in fermented skim milk confirmed the potential use of L. brevis KU200019 as an adjunct culture in synbiotic-fermented dairy products to enhance the safety and quality.













