Introduction
Kefir, an acidic-alcoholic fermented milk product with an acidic taste and a creamy consistency, is produced by fermenting milk with a complex mixture of microorganisms, consisting of acetic acid bacteria, lactic acid bacteria, and yeast, at 25°C–28°C. Among the complex microbial composition, Lactobacillus spp., is the dominant species in the kefir microbial population, accounting for up to 80% of all microorganisms, while the rest is represented by Bifidobacterium sp., Lactococcus sp., and yeast (Miguel et al., 2010; Witthuhn et al., 2004). Blooming reports on the health-promoting effects of kefir resulting in an emerging trend for the use of kefir as a healthy and rehydrating beverage (de LeBlanc et al., 2007; Hertzler & Clancy, 2003; Huseini et al., 2012; Liu et al., 2005, 2006; Lopitz-Otsoa et al., 2006; Matsuu et al., 2003).
Yeast in kefir can play a double role. The production of carbon dioxide (CO2) and ethanol by yeast during alcoholic fermentation are responsible for the unique flavor in kefir (Adriana and Socaciu, 2008). However, uncontrolled growth and the excess production of CO2 and ethanol through secondary alcoholic fermentation in yeast could lead to off-flavor, accumulation of CO2, leading to swollen containers during storage and packaging, eventually blowing off the package (Kwak et al., 1996; O’Brien et al., 2016). As food safety has become a primary global concern, food spoilage as in the accumulation of CO2 at the headspace of kefir has become an obstacle for the rapid economic growth and industry development (Danilović et al., 2018).
Hence, strain selection is essential to the production of a unique starter culture that could maintain the traditional attributes of the product while improving its aroma, safety, shelf-life, and functional benefits of the product. In this study, a new functional starter was developed by replacing yeast in a commercial starter with two functional probiotic strains, the bacteriocin-producing L. acidophilus KCNU and the colitis-ameliorating L. brevis Bmb6, for the production of kefir-like fermented milk with additional health benefits.
Materials and Methods
Twelve starter microorganisms (Bifidobacterium longum, Lactobacillus casei, Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus fermentum, Lactobacillus reuteri, Streptococcus thermophilus, three Lactococcus lactis spp., and Saccharomyces cerevisiae) were provided by Samik Dairy & Food Co. Ltd, Gangnam-gu, Seoul, Korea. The bacteriocin-producing Lactobacillus acidophilus KCNU was obtained from the Korean Culture Center of Microorganisms, Seodaemun-gu, Seoul, Korea. Lactobacillus brevis Bmb6 with prominent anti-inflammatory effects was isolated from kimchi (Shin, 2017). All Lactobacillus strains were cultivated and maintained in MRS broth at 37°C; Lactococcus strains were cultivated and maintained in M17-lactose broth at 32°C; Streptococcus strains were cultivated and maintained in M17 broth 43°C; Bifidobacterium longum was cultivated and maintained in BL broth at 37°C under anaerobic condition; Yeast (S. cerevisiae) was cultivated and maintained in YGC broth at 28°C.
The crude bacteriocin was extracted from L. acidophilus KCNU, as described previously (Oh et al., 2008). L. acidophilus KCNU was cultured in MRS broth at 37°C for 18 h, followed by centrifugation at 6,000×g for 30 min at 4°C. Cell-free supernatant was collected as crude bacteriocin, and the pH was adjusted to 7.0 using 10 N NaOH. The crude protein was subjected to filtration through a 0.45 μm filter, heat-treated, and vacuum-dried or freeze-dried before being evaluated for its antimicrobial activity by the spot-on-lawn method (Ahn and Stiles, 1990). The antimicrobial activity of the crude bacteriocin was expressed as arbitrary units (AU) per mL of crude bacteriocin.
The microbial composition of the control starter and the non-yeast functional starter were tabulated in Table 1. All strains were cultured for 1–2 days to reach 1010 CFU/mL. Cell pellets were collected via centrifugation at 3,000×g for 30 min. Cell pellet from each strain was mixed and resuspended in 40% (v/v) glycerol in reconstituted skim milk as cryoprotectant. The mixture was then subjected to freeze-drying for 72 h. The freeze-dried starter powder was vacuum packed in an aluminum-coated vinyl pack. The starter was either stored at −20°C (frozen storage) or 5°C (cold storage) for 12 weeks, and the total number of viable cells was determined by at every 4 weeks interval. The total number of viable cells was the average of the viable count of Lactobacillus, Lactococcus, Bifidobacterium, Streptococcus, and yeast. Lactobacillus strains were cultivated in MRS-lactose agar at 37°C under anaerobic condition for 48 h; Lactococcus strains were cultivated in M17-lactose agar at 32°C under anaerobic condition for 24 h; Bifidobacterium longum was cultivated in BL agar at 37°C under anaerobic condition for 48 h; Saccharomyces cerevisiae was cultivated in PDA agar at 28°C under anaerobic condition for 72 h.
2) Lactobacillus acidophilus KCNU was obtained from Korean Culture Center of Microorganisms, KCCM10753P.
3) Lactobacillus brevis Bmb6 was obtained from Chonnam National University (Shin, 2017).
Sterilized milk was inoculated using 0.02% (w/w) of the functional starter pack (1010 CFU/g) and fermented at 25°C under normal atmospheric conditions for approximately 24 h. The control consisted of sterilized milk inoculated with 2% (w/w) of original commercial starter (107 CFU/g) and fermented at 25°C under normal atmospheric conditions until the pH 4.5 was reached. The pH and the total number of viable cells of fermented milk were evaluated and recorded at every 4 h interval. The total number of viable cells was the average of the viable count of Lactobacillus, Lactococcus, Bifidobacterium, Streptococcus, and yeast cultivated in different media and culture conditions. The final products were evaluated by 50 regular fermented milk consumers, consisting of university students and staff, to determine the general public acceptability. The fermented milk was scored 1 (worst) to 7 (best) according to taste, texture, and sourness of the beverages.
Seven-weeks old female ICR mice were obtained from the Daehan Lab (Daejeon, Korea) and acclimated for one week in the Animal Housing Unit (room temperature of 22-25°C, 50%–60% humidity, and 12 h light/dark cycle; standard mouse chow-diet and water were provided ad libitum), according to the guidelines provided by the Institutional Animal Care and Use Committee of the Chonnam National University (CNU-IACUC-YB-2016-47; Chonnam National University, Gwangju, Korea).
The control and treatment groups were administered with PBS for the first seven days. Drinking water was then replaced with 4% (w/v) dextran sulfate sodium (DSS) water from day-7 to day-14 to induce colitis in mice. The control group was administered with PBS through oral gavage from the beginning until the end day-14, while the samples group was administered with 0.1 g of functional starter-fermented milk through oral gavage from day-7 to day-14. Bodyweight, fecal condition, and disease activity index (DAI) was assessed daily based on a scoring system, as shown in Table 2 (Herías et al., 2005).
| Score | Weight loss (%) | Stool consistency2) | Gross bleeding |
|---|---|---|---|
| 0 | None | Normal | Negative |
| 1 | 1–5 | Loose | Negative |
| 2 | 5–10 | Loose | Hemoccult positive |
| 3 | 11–15 | Diarrhoea | Hemoccult positive |
| 4 | >15 | Diarrhoea | Bleeding |
Results
The antimicrobial activity of crude bacteriocin produced by L. acidophilus KCNU was not affected by heat treatment, vacuum concentration, or freeze-drying process was maintained at 6,400 AU/mL, regardless of the treatment process (Table 3), indicating the ability of crude bacteriocin to withstand downstream processes for industrial application.
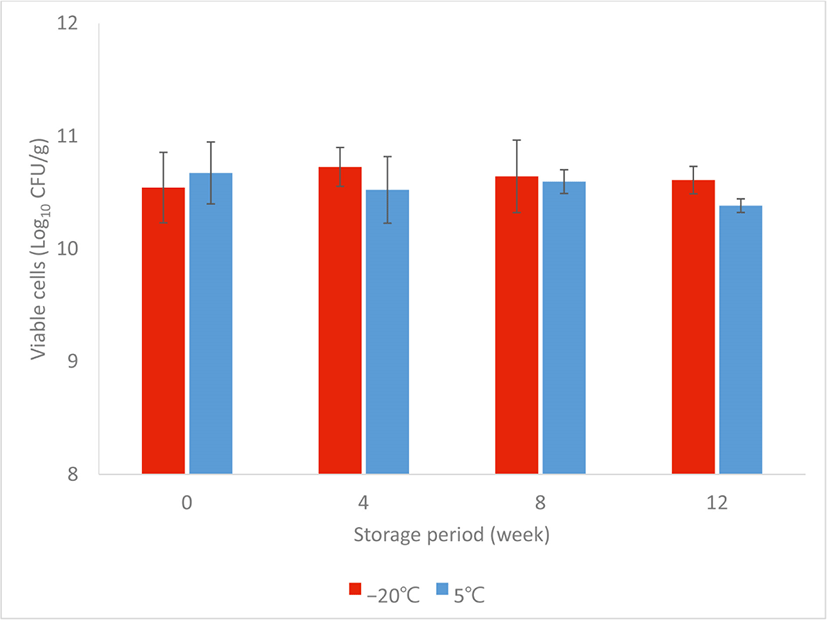
Upon the freeze-drying process, the functional starter achieved total viability at 1010 CFU/g, and the number fluctuated within the range of 1010 CFU/g throughout the study (Fig. 1). At the end of 12 weeks storage period, functional starter stored at –20°C contained 10.66±0.1110 CFU/g of total viable cells and 10.38±0.0610 CFU/g of total viable cells for functional starter stored at 5°C. indicating their stability over long-term storage at low temperatures.
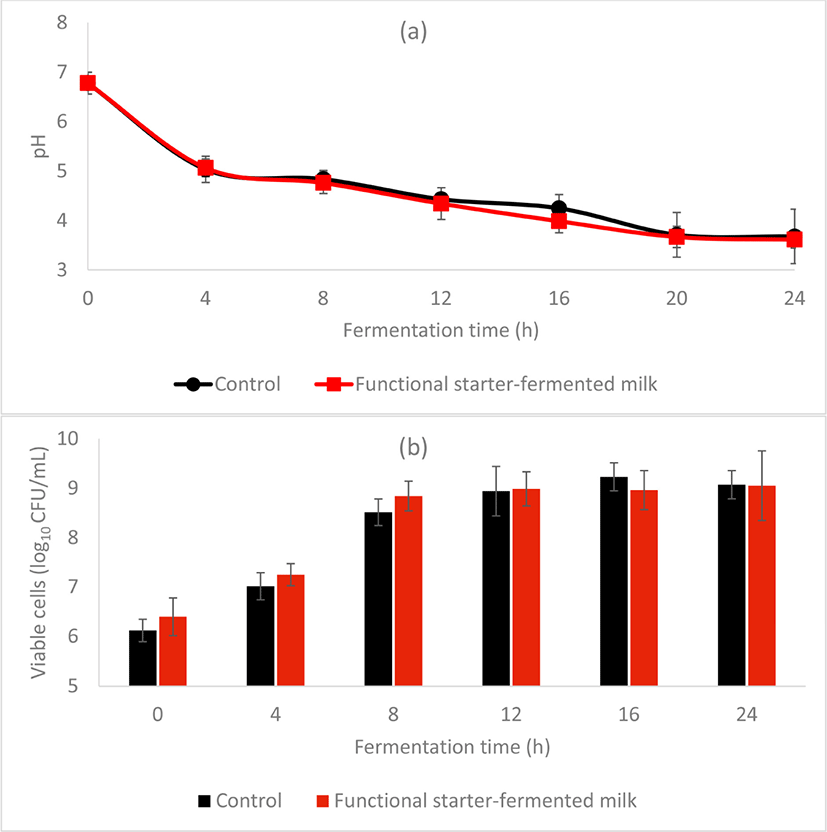
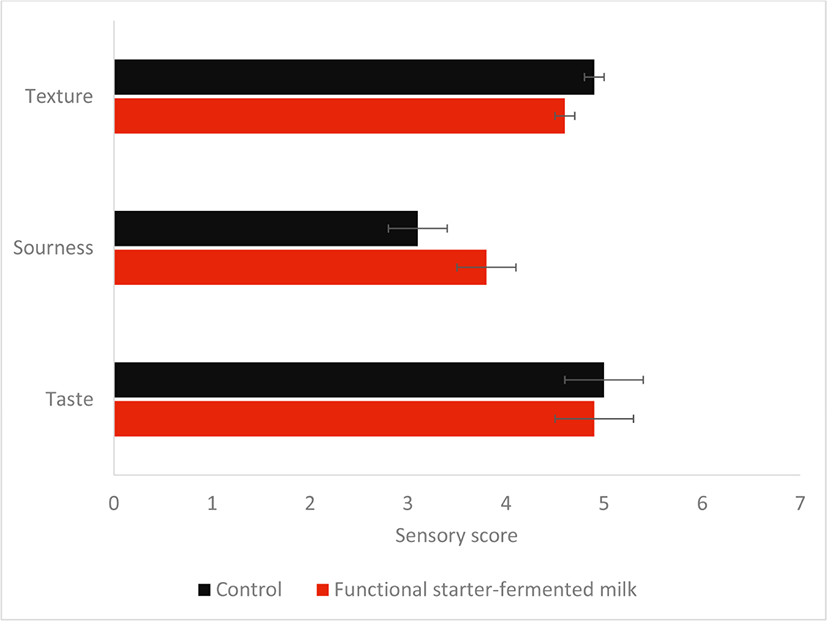
The fermentation efficacy of the functional starter was comparable to the control starter, both achieved pH 4.5, the optimal pH of kefir at a time near 12 h (Fig. 2a). Moreover, at 12 h, both fermented milks have a similar number of viable Lactobacillus count, with 8.94±0.5010 CFU/mL in control and 8.99±0.3510 CFU/mL in functional starter-fermented milk (Fig. 2b). Also, the sensory evaluation revealed the acceptance of the functional starter-fermented milk by the panels, with a similar texture, sourness, and taste as the control kefir beverage (Fig. 3).
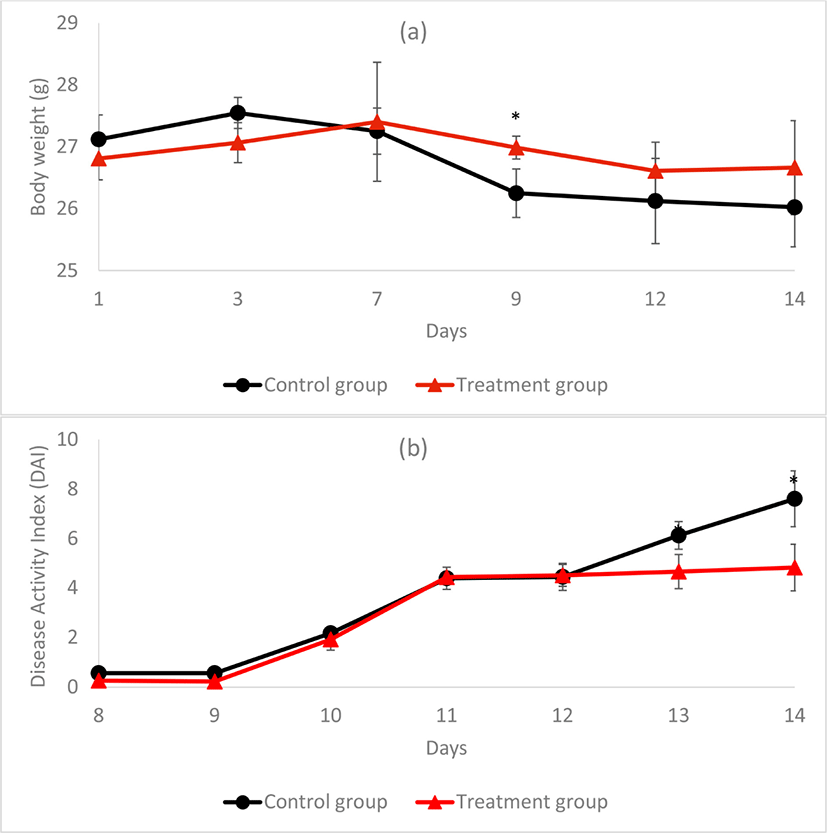
In this experiment, treatment group mice were administered with functional starter-fermented milk, which contained the bacteriocin-producing L. acidophilus KCNU and the anti-inflammatory L. brevis Bmb6 strains. Upon DSS induction, the control group mice exhibited a drastic decrease in body weight on day-9 and continued until the end of the day-14 (Fig. 4a). In contrast, the body weight of the treatment group mice was decreased gradually throughout the study, but the reduction rate was lower, as compared to the control group. The control group had a DAI score of 0.4 on day-8, which showed a gradual increase in the DAI score to 7.6 at day-14 (Fig. 4b). Meanwhile, the DAI score of treatment group mice begun to increase from 2.0 on day-10 and fluctuated between 4.3 to 4.6 until day-14. Whereas, the DAI score of control group mice continued to increase with DAI score of 7.6 at the end of the study. These results were showing that the ability of functional starter-fermented milk to prevent further deterioration of colitis-symptoms in treatment group mice, as compared to the control.
Discussion
The microbial composition of a starter greatly affects the flavor, nutritional value, health-promoting effects, and shelf life of a fermented dairy product. The use of yeast as a member of the starter culture plays a double role. Yeast could either positively or negatively affect the quality of dairy products, depending on their interaction with other starter strains and the fermentation conditions (Viljoen, 2001). For instance, formation and accumulation of CO2 in the containers, leading to swollen and bloated containers during storage, had shortened the shelf-life of the products and causing economic loss to the food manufacturer as well (Danilović et al., 2018; Foschino et al., 1993; Kwak et al., 1996; O’Brien et al., 2016; Sarkar, 2008). Hence, in the present study, the use of yeast in starter was replaced by two functional Lactobacillus strains, L. acidophilus KCNU and L. brevis Bmb6, for production of a health-promoting kefir-like fermented milk.
L. acidophilus KCNU, a bacteriocin-producing strain, isolated from the porcine small intestine, has been reported to be effective against various pathogens, including Bacillus cereus, Enterococcus aerogenes, Escherichia coli, Listeria monocytogenes, Staphylococcus aureus, Pseudomonas aeruginosa, and Yersinia enterocolitica. In addition, L. acidophilus KCNU also reported exerting prominent acid and bile tolerance and cholesterol-lowering ability, which suggests its use for food fermentation or pharmaceutical (Oh et al., 2008). Hence, the use of L. acidophilus KCNU as a member of the starter could greatly improve the safety by reducing the risk of contamination of the starter culture and spoilage on the end products, while assisting host to a maintain balance gut homeostasis through suppressing the growth of gut pathogens. It is crucial for the bacteriocin from L. acidophilus to resist the harsh downstream dehydration processes, including heat-treatment, vacuum drying, or freeze-drying process. In this study, upon the dehydration treatments, the crude bacteriocin from L. acidophilus KCNU able to maintain a stable antimicrobial activity at 6,400 AU/mL, similar to the antimicrobial activity of the unprocessed crude bacteriocin, suggesting its suitability to be used as a starter culture.
The development of starter culture is crucial for the production of fermented foods as it allows a consistent production of fermented foods. It is of utmost importance to maintain the viability of the bacteria and prolong the shelf-life of commercial starter culture (Taskila, 2017). The newly developed L. acidophilus KCNU and L. brevis Bmb6 containing functional starter exhibited excellent stability by maintaining the total number of viable cells at 1010 CFU/g throughout the 12 weeks storage period, at −20°C and 5°C. Freeze-drying is one of the most commonly practiced preservation techniques for commercial starter culture production, owing to its capability to maintain a high number of viable bacterial cells and to prolong the shelf-life of the product (Taskila, 2017). Based on our results, frozen storage is preferable to cold storage when considering storing the product for a more extended period (>12 weeks).
Ideally, industrial kefir fermentation should be completed within 8 h, with the final pH of 4.5, to meet the smooth and continuous downstream procedures such as the cooling and packaging process (Lee et al., 2018). Certain yeast strains were capable of assimilating lactate, producing alkaline end products that could neutralize the acids, thereby prolong the fermentation period (Potter and Hotchkiss, 1995; Soulides, 1955). However, the replacement of yeast with L. acidophilus KCNU and L. brevis Bmb6 did not improve the fermentation efficacy of the functional starter. Also, the absence of yeast in the functional starter did not alter the total number of the viable count, with 108 CFU/mL upon completion of the milk fermentation, indicating the use of L. acidophilus KCNU and L. brevis Bmb6 did not affect the total number of viable cells in the fermented milk as in the control kefir. Meanwhile, sensory evaluation by regular fermented milk consumers revealed that the functional starter-fermented high resembled the control yeast-kefir in terms of the taste and texture of the fermented milk, with enhanced sourness taste.
Previously, the administration of L. brevis Bmb6 has been reported to effectively improve the symptoms of bowel inflammation in DSS-induced colitis mice (Shin, 2017). In this study, the colitis-ameliorating property of L. brevis Bmb6 remained in the end product, fermented milk after a series of industrial manufacturing processes. Administration of the functional starter-fermented milk prevented a drastic decrease in body weight of DSS-induced mice, thereby rendering further deterioration of colitis-symptoms in DSS-induced mice at the later stages (Day-13 and -14), suggesting that L. brevis Bmb6-containing fermented milk can mitigate the symptom of gastrointestinal disorders. This colitis ameliorating effects of L. brevis Bmb6 was attributed to enhance gut epithelium integrity, promote the recovery of epithelial cells, and suppress the pro-inflammatory cytokines, TNF-α, and IFN-γ (Shin, 2017).
In summary, a non-yeast functional starter has been developed by substituting yeast in a commercial starter with L. acidophilus KCNU and L. brevis Bmb6. The new functional starter demonstrated excellent storage stability and the functional starter-fermented milk high resembling the control yeast-kefir in terms of fermentation efficacy, total viable cells, taste, and texture of the fermented milk, with enhanced sourness. Moreover, the functional starter-fermented milk retained the ability L. brevis Bmb6 in relieving intestinal inflammation. The outcome of this study provides an insight into the development of non-yeast starter for fermented dairy products through the use of other functional lactic acid bacteria.













