Introduction
Ginseng (Panax ginseng C.A. Meyer), one of the most popular herbal plants and healthy food for vitality, is known for increasing energy and providing refreshment (Ramesh et al., 2012). Many studies have found that ginseng has several a pharmacological properties and anti-inflammatory, anti-aging, anti-diabetes, and anti-obesity effects, which make it suitable for treatment of many diseases, and it has been used for such purpose in Asia for thousands of years (Lee et al., 2014; Lee et al., 2015).
Lactic acid bacteria (LAB) are important microorganisms commonly used for the fermentation of foods (Liu et al., 2011). In terms of human health, probiotic LAB increase lactose tolerance and digestion, reduce cholesterol and intestinal pH, stimulate the immune response, and beneficially influence the intestinal microflora. Lactobacillus plantarum NK181 was isolated from Korean fermented fish, jeotgal, and exhibited desirable traits such as high resistance to artificial juice and bile acid, strong adherence to Caco-2 cells, antioxidant properties, and cholesterol-reducing effect (Lee et al., 2006).
Yogurt is the most widespread dairy products, having originated in the Middle East and Asia (Desai et al., 2013). Yogurt is manufactured from milk by adding yogurt starter cultures and a range of substances including flavoring, sweeteners, and fruits as desired. Specifically, Lactobacillus species and Streptococcus thermophilus are commonly used as starter cultures. Today, it is very easy to find yogurt containing Lactobacillus acidophilus, Lactobacillus casei, Bifidobacteria, or combinations of these bacteria in the market (Mani-López et al., 2014).
Yogurt fortified with red ginseng extract has enhanced antioxidant activity (Jung et al., 2016). Although many studies regarding red ginseng and yogurt have been reported, probiotic yogurt with ginseng is still scarce. Therefore, the aim of this study was to manufacture yogurt with ginseng and probiotic L. plantarum NK181 and investigate the composition, pH, titratable acidity, viable cell number, and antioxidant effects of the yogurt.
Materials and Methods
Milk and skim milk powder were purchased from Seoul Milk Co. (Korea), and ginseng extract was obtained in Fine Korea Co. (Korea). L. plantarum NK181 (Lee et al., 2006) and S. thermophilus were used as the starter culture. In addition, pectin was purchased from Daejung Chemicals & Metals Co., Ltd. (Korea). The ginseng extract powder (GEP) was obtained by freeze-drying.
The yogurt samples were prepared with different concentrations of GEP (0%, 0.5%, 1%, 1.5%, and 2%). Milk and 2% skim milk powder were mixed with GEP and 0.1% pectin then pasteurized at 90℃ for 10 min. Pasteurized milk was cooled and inoculated with 1% starter culture containing L. plantarum NK181 and S. thermophilus (1:1). The inoculated mixture was incubated at 40℃ until a pH of 4.4–4.5 was achieved, and then, the mixture was stored in a refrigerator overnight. Yogurt without GEP was used as control.
The composition, protein, fat, moisture, total solids, and ash of the yogurt were measured following the protocol laid out by the Association of Official Analytical Chemists (AOAC, 2000). In addition, pH, titratable acidity, and viable cell count of the yogurt samples were measured during fermented periods. The pH and titratable acidity were indicated by using a pH meter following the modified method of Jung et al. (2016). The cell counts were determined by cultivation on selected medium, MRS agar (Difco Laboratories, USA) at 37℃ for 48 h and M17 agar (Difco Laboratories, USA) containing 10% lactose at 42℃ for 48 h, respectively.
To analyze antioxidant activity, the supernatants of yogurt samples were obtained by centrifugation at 12,000 rpm at 4℃ for 30 min and filtered through a 0.45 μm filter (cellulose acetate, Toyo Roshi Kaisha Ltd., Japan). The supernatants were stored at –20℃ prior to measurement of antioxidant activity.
2,2-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity was measured according to a modified method (Do et al., 2014). The sample (200 μL) was mixed with 1 mL of 100 μM DPPH solution. The mixture was shaken and left for 15 min in a dark space for the reaction to occur. The absorbance of mixture was measured at 517 nm using the spectrophotometer. The percentage of inhibition of free radicals was calculated using the following formula:
The antioxidant activity was measured following the method of Lage et al. (2013) with modification. β-Carotene (2 mg), linoleic acid (44 µL), and Tween 80 (200 µL) were dissolved in 10 mL of chloroform, respectively. Then, 5 mL of the β-carotene solution was pipetted into a round-bottom flask. Chloroform was removed using a rotary evaporator at 50℃. Removed β-carotene solution was added to 100 mL of distilled water immediately. Aliquots (4.5 mL) of β-carotene were transferred to test tubes containing 0.5 mL of samples. Each tube was placed in a water bath at 50℃. The absorbance of each sample was measured at 470 nm using a spectrophotometer at 8 h. The percentage of inhibition of free radicals was calculated using the following formula:
The antioxidant activity was measured by modifying the ferric reducing antioxidant power (FRAP) assay (Park et al., 2015). The cocktail solution was prepared using 300 mM acetate buffer (pH 3.6), 10 mM of tripyridyltriazine solution, and 20 mM of ferric chloride solution in a 10:1:1 ratio, and the solution was pre-heated at 37℃. The sample (50 μL) and cocktail solution (950 μL) were mixed and incubated at room temperature in the dark for 30 min. The absorbance was read at 593 nm, using from 50 μM to 1 mM FeSO4 solution for the standard curve. FRAP value represented μM FeSO4 equivalents (eq.) produced in the samples.
Results and Discussion
The composition and physicochemical properties of yogurt with added GEP (0%, 0.5%, 1%, 1.5%, and 2%) were measured. Used GEP primarily contained diverse pharmacological ginsenoside such as Rb1, Rb2, Rc, Rd, Rg1, Rg3, and Rh2 by TLC (data not shown). Yogurt with ginseng concentrations of 1.5%–2.0% had previously been reported as unacceptable due to the bitter taste of ginseng (Eom et al., 2017; Jung et al., 2016). The protein, fat, moisture, total solids, and ash content of yogurt fermented with 0%, 0.5%, and 1% GEP are represented in Table 1. Contents of yogurt supplemented with GEP were significantly affected (p<0.05). The control yogurt contained 4.08% protein, 3.25% fat, 85.55% moisture, 14.44% total solids, and 0.90% ash. Among yogurts fermented with GEP, the moisture and fat content decreased with an increase in GEP content, whereas protein, ash, and total solids content increased. Previous research showed that fat content decreased during fermentation because of conversion of fat to flavor compounds (Jung et al., 2016; Ye et al., 2013). In addition, the protein, total solid, and ash content was dependent on the concentration of added red ginseng extract in fortified yogurt (Jung et al., 2016). The components of yogurt with added GEP were slightly higher than those of control yogurt (p<0.05). Thus, yogurt composition was influenced by the addition of GEP. However, the composition of yogurt with 1.5% and 2% ginseng did not change significantly (p>0.05) (data not shown).
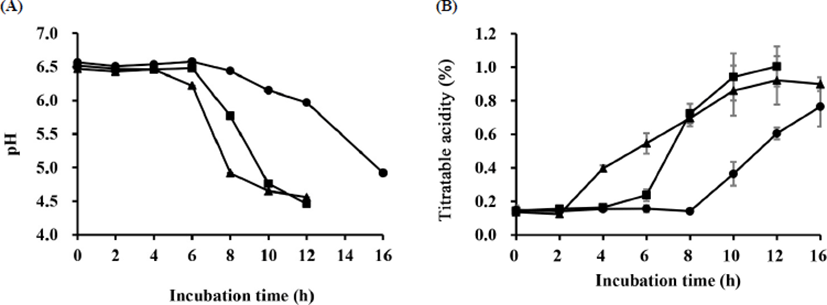
The pH and titratable acidity are expressed in Fig. 1. The pH value and titratable acidity were correlated with each other (p<0.05). The pH ranged from about 4.5 to 6.4, whereas the titratable acidity ranged from 0.15 to 0.9 during fermentation. Decreases in pH value confirmed increases in titratable acidity. These results were similar to those of previous studies (Jaster et al., 2018; Jung et al., 2016). In addition, it has been reported that pH value and titratable acidity are correlated with the quality of the yogurt (Gao et al., 2018).
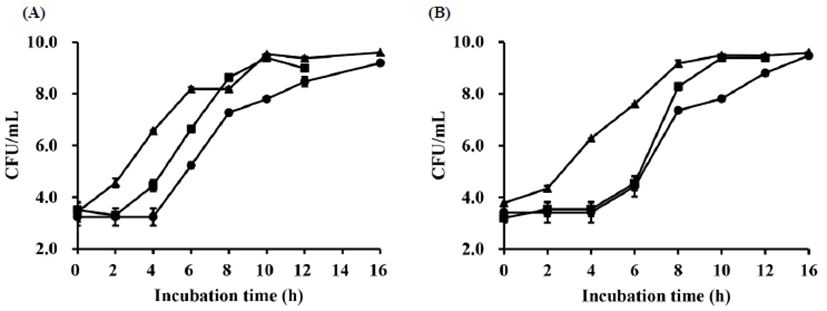
The viable cell counts of L. plantarum NK181 and S. thermophilus in yogurt during fermentation are shown in Fig. 2; they ranged from 3.43 to 9.60 Log CFU/mL and 3.42 to 9.64 Log CFU/mL, respectively. The viable cell counts of yogurt with added GEP were higher than that of the control. In particular, L. plantarum NK181 yogurt with a viable cell count of greater than 9 Log CFU/mL is notable from the point of view to developing probiotic yogurt. As the concentration of GEP was increased from 0% to 1%, the viable cell count of yogurt increased. However, viable cell number of lactic acid bacteria was not elevated further in 1.5% and 2% GEP, and it slightly decreased with higher concentrations of GEP (data not shown). These results might be caused by the antimicrobial effect of ginseng extract. Previous research reported a similar pattern to what appears in these results (Jung et al., 2016; Park et al., 2006). Many researchers have suggested that the viability of lactic acid bacteria is influenced by the concentration of ginseng and red ginseng. Despite diminishing returns at higher concentrations, a small amount of red ginseng extract promoted the growth of lactic acid bacteria (Bae et al., 2005). In addition, probiotic cultures may influence the pharmacological properties of ginseng by modulating the intestinal microflora (Cimo et al., 2013).
The antioxidant activity of yogurt with GEP was assessed using three methods of DPPH radical-scavenging, inhibition of β-carotene-linoleic acid oxidation, and FRAP. The results of the antioxidant activities of yogurt with GEP are presented in Table 2. DPPH radical scavenging activity has generally been measured using the antioxidant activity method. A previous study measured the DPPH radical scavenging activity of supernatant of L. plantarum NK181 showed at 30% (Lee et al., 2006). However, in the present study, the DPPH radical scavenging activity of yogurt supplemented with 0.5% to 2.0% GEP was 71.62% to 89.60% (data not shown), whereas that of control yogurt was 65.25%. The DPPH radical scavenging activity of the yogurt increased with increasing GEP concentration (p<0.05). In addition, Panax ginseng (PG)-supplemented yogurt was reported to have DPPH activity of that increased depending on the dose of PG (Lee et al., 2013), and that of yogurt supplemented with red ginseng extract showed around 94% DPPH radical scavenging activity (Jung et al., 2016).
The inhibition of β-carotene and linoleic acid oxidation is widely used to assess antioxidant activity. This method is based on oxidation and discoloration of products by lipid oxidation (Jung et al., 2016; Kato et al., 2009). Yogurts with GEP had higher antioxidant activity than the control yogurt (p<0.05). The inhibition of β-carotene and linoleic acid oxidation of yogurt supplemented with GEP (0.5%, 1.0%, 1.5%, and 2%) was 42.28% to 80.12% (data not shown), whereas that of the control was 21.18%. In addition, the inhibition of β-carotene and linoleic acid oxidation of yogurt with red ginseng extract (0.5%, 1.0%, 1.5%, and 2%) was 50.25%–53.48% (Jung et al., 2016). The inhibition measured for yogurt with GEP (<1.0%) was higher than for yogurt with additional red ginseng extract over 1.0% concentration.
Using the FRAP method, the antioxidant activity of yogurt supplemented with GEP depending on concentration was estimated by the ability of the samples to reduce Fe3+-TPTZ to Fe2+-TPTZ. Results from FRAP showed significant differences in yogurt with GEP (p<0.05). The FRAP value for control yogurt was 827.13 μM. However, the value increased from 827.54 to 1,328.54 μM in GEP yogurt samples (0.5%, 1.0%, 1.5%, and 2%) (data not shown). These measurements indicated that the FRAP value also increased in proportion to increasing GEP concentration.
Research has demonstrated that the antioxidant effects of whey and casein proteins in yogurt could be related to their ability to chelate metals (Perna et al., 2014). Yogurt fermentation is influenced by the production of bioactive peptides (Gómez-Ruiz et al., 2008; Virtanen et al., 2007), addition of natural products such as red ginseng extract (Jung et al., 2016), and characteristics of the fermentation starter (Gupta et al., 2009; Hernández-Ledesma et al., 2005; Virtanen et al., 2007). Therefore, antioxidant activity in this study was influenced by addition of GEP.
Conclusion
Despite the functional benefits of ginseng in foods, its bitter taste has been a disadvantage in its application as a food supplement, and its bioavailability depends on intestinal microflora. In the present study, the yogurt fermented with GEP and L. plantarum NK181 was similar to control yogurt in physicochemical properties. In addition, increased GEP concentration affected the viability and antioxidant activities of probiotic L. plantarum NK181. We demonstrated that the probiotic characteristics and antioxidant activities of dairy products improve by using L. plantarum NK181 and GEP.













