Introduction
Fermentation is a chemical process in which enzymes break down organic substances into smaller compounds (Schlesinger and Bernhardt, 2013). A result of fermentation is more digestible, stable, and flavored foods, with enhanced nutritional values (Bernaert et al., 2013; El-Abbadi et al., 2014). Yogurt is a fermented milk product obtained from the fermentation of Streptococcus thermophilus (ST) and Lactobacillus delbrueckii ssp. bulgaricus (Wasilewska et al., 2019). It was first produced in the Middle East but has spread worldwide (Wang et al., 2013); it contains higher levels of protein, vitamin B, and minerals including calcium, magnesium, potassium, and zinc than milk (El-Abbadi et al., 2014). Furthermore, yogurt is a suitable dairy product for people who are lactose intolerant, as the lactose in milk is converted to lactic acid during fermentation (Savaiano, 2014).
Paprika is the ground spice made from the botanical fruit, pepper, consumed as a vegetable; it belongs to the Solanaceae family, genus Capsicum, and the species C. annuum L, and is one of the most consumed vegetables globally (Di Cagno et al., 2013). It has six subspecies and is referred to as sweet pepper, bell pepper, and paprika, depending on the country (Di Cagno et al., 2013). Capsicum annuum species contain a high concentration of carotenoid pigments, responsible for the yellow, orange, and red colors of the vegetables (Halah and Nayra, 2011). Paprika pigments are composed of capsanthin and capsorubin, which are xanthophyll carotenoids (Arimboor et al., 2015). Paprika is known as a health food because it is rich in vitamin A, C, E, and flavonoids (Hassan et al., 2019; Kantar et al., 2016). The phytochemical content and physiological activities of paprika differ depending on the color of the paprika (Kim et al., 2016). Kim et al. (2016) reported that 100 g of dried red and green paprika contained 70 and 10 mg of carotenoids, 50 and 100 mg of tocopherol, and 750 and 700 mg of polyphenols, respectively. Moreover, green paprika contains high levels of chlorophyll, yellow and orange paprika contain lutein, zepaxanthin, and β-carotene, while red paprika contains carotenoid pigments, such as capsanthin and capsorubin (Arimboor et al., 2015; Marin et al., 2004).
Paprika has received attention as a functional food and as a food additive, with various studies reporting that the phytochemicals in paprika exert antioxidant (Halah and Nayra, 2011), anti-cancer (Saponjac et al., 2014), anti-inflammatory (Chen and Kang, 2013), anti-obesity (Maeda et al., 2013), and anti-arteriosclerotic activities (Tsui et al., 2018). However, to date, there has been no active study on the colors of paprika, especially with yogurt and green paprika juice combined, and no research has been reported on the manufacturing of paprika color-specific yogurts.
Therefore, this study investigated the biochemical and active antioxidants characteristics of yogurt with red, yellow, and orange paprika juice; while observing the consequent quality changes under storage, this study presents basic information on the role of these food additives in enhancing food functionality.
Materials and Methods
Red, orange, and yellow paprika were purchased from a local market in Seoul, Korea at winter season. For preparing the paprika juice, the paprika were washed with tap water several times and were dehydrated. The paprika juice for each color was prepared by grinding in a juicer (DA 5000, Dasung Artron, Paju, Korea), and the solid particles were separated by filtering, using 4-layer sterile gauzes (JASCOR, Seoul, Korea) in the clean bench. Yogurt was prepared by adding skim milk powder (4%), pectin (0.2%), and white sugar (1%) to the milk and homogenizing using a Homogenizer T 25 (Janke and Kunkel type, Ika, Germany) for 15 min. The yogurt was inoculated with mixed strains of ST and L. delbrueckii ssp. bulgaricus (YO-MIXTM 496 LYO 250 DCU, Danisco, Copenhagen, Denmark) with 2.5 or 5% of each color paprika juice added. The yogurt was fermented at 43°C for 5 h and stored at 4°C.
The pH of the homogenized yogurt was measured using a digital pH meter (ISTEC 735P, Korea). The fermentation rate was determined using equation 1:
where, A was the pH before fermentation and B was the pH after fermentation.
The titratable acidity (TA) was measured by diluting the paprika yogurt sample to 1:9 with tertiary distilled water, as described previously (Kang et al., 2018). The sample was titrated with 0.1 N NaOH, with continuous stirring, to an end point of pH 8.3, recording the volume of NaOH (mL) used. The amount of acid produced during fermentation was calculated using equation 2:
where, VNaOH was the volume of NaOH required to neutralize the acid.
Lactic acid bacteria (LAB) were counted using ST agar plates composed of 10 g tryptone, 1 g sucrose, 5 g yeast extract, and 2 g K2HPO4 (Saccaro et al., 2011), after incubation for 48 h at 37°C, and expressed as CFU/g. Samples of the paprika yogurt were diluted; the diluted solutions from each step were spread on the ST agar plates.
The viscosity of the paprika yogurt was measured using a Bookfield-Viscometer (Model LVDV-E, Brookfield Engineering Lab., Middleboro, MA, USA) with spindle No. 63 at 5 rpm. The measurements were recorded three times, at intervals of 30 s after 3 min.
Syneresis measured the weight of the supernatant following centrifugation (640×g, 4°C, 20 min) of samples, and was calculated using equation 3:
The amino acid content was determined as described previously (Goodno et al., 1981). Samples (30 μL) were mixed with 1 mL of OPA reaction solution and reacted for 2 min at room temperature. The absorbance was measured at 340 nm with tryptone used as the reference.
Color analysis of the yogurt was performed using a Chroma meter (MINOLTA CHPOMA METER CR-210). The measurements were conducted under artificial light to minimize the effects of daylight. The color parameters L* (lightness), a* (red/greenness), and b* (yellow/blueness) of the yogurt samples were evaluated according to the International Commission on Illumination (CIE) L*a*b* system.
After dissolving the paprika yogurt in water and 95% methanol at a ratio of 1:9, samples were extracted at room temperature for 10 h. Folin-Ciocalteu reagent (0.2 mL, 1N) was added to each sample (0.2 mL) and thoroughly mixed. The solution was incubated at room temperature for 3 min, and 1 N Na2CO3 (400 μL) was added, mixed thoroughly, and further incubated at room temperature for 90 min in the dark. Absorbance of each sample was measured at 725 nm (UV-1601, Shimadzu, Kyoto, Japan) after mixing with 2 mL of distilled water (Wei, 2011). Known concentrations of gallic acid (Sigma-Aldrich, Schnelldorf, Germany; 5–60 μg/mL in ethanol) were treated as the yogurt extracts. Regression lines of the gallic acid standard were used to determine the total phenolic content of yogurt water extract using μg gallic acid equivalents (μg GAE)/mL.
The total flavonoid content was measured according to a previous method by Abeysinghe et al. (2007). The sample (0.42 mL) was mixed with 2.1 mL diethylene glycol and 0.21 mL 1 N NaOH. The mixture was incubated for 1 h in a water bath at 37°C; the absorbance was measured at 420 nm using quercetin (Sigma-Aldrich, St. Louis, MO, USA) as the standard.
The vitamin A and C levels were measured using high performance liquid chromatography (HPLC) at the Korea Testing and Research Institute, with retinol and ascorbic acid (Sigma-Aldrich, St. Louis, MO, USA) used as the standards. Vitamin A levels were analyzed using HPLC (Waters 616 system, Waters, Milford, MA, USA) equipped with SP column C18 (150×4.6 mm). Under the analysis conditions, column temperature was 35°C, flow rate 0.6 mL/min, run time 45 min, and injection volume 15 μL. The mobile phase comprised water:ethanol (20:80, v/v) and vitamin A was detected at 340 and 460 nm. Vitamin C levels were analyzed using HPLC (Waters 2695 system, Waters, Milford, MA, USA) equipped with SP column C18 (250×3.0 mm). Under the analysis conditions, column temperature was 40°C, flow rate 0.7 mL/min, run time 45 min, injection volume 20 μL, and detection wavelength 254 nm. Mobile phase A comprised water:methanol (97.5:2.5, v/v) and H solution 20 mL (heptanesulfonic acid (1 g)+water (10 mL)+acetic acid (10 mL)). Mobile phase B comprised water:methanol (50:50, v/v) and H solution (20 mL).
Free radical scavenging activities were measured using 2-diphenyl-2-picrylhydrazyl (DPPH) photometric assay, as described previously (Apostolidis et al., 2007), with some modifications. One milliliter of a sample was mixed with 0.25 mL of 0.15 mM DPPH (Sigma-Aldrich, St. Louis, MO, USA). The mixture was kept at room temperature in the dark for 30 min. The absorbance was measured at 517 nm using a spectrometer (UV-1601, Shimadzu, Kyoto, Japan). Inhibition of DPPH oxidation (%) was calculated using equation 4 (Apostolidis et al., 2007):
where, A was the absorbance at 517 nm.
The samples were stored in a 4°C refrigerator for 15 days and changes in pH, TA, LAB count, viscosity, yield, amino acid concentration, antioxidant activity, and color were assessed at 5-day intervals.
Data are expressed as means±SD of at least three replicates. Statistical analysis of the data was performed using one-way analysis of variance (ANOVA; SPSS 20) followed by Duncan’s multiple range test and Student t-test for mean comparison. Statistical significance was considered at p<0.05.
Results and Discussion
As shown in Table 1, the pH of the paprika yogurt decreased to 4.44–4.53 by increasing the content of paprika juice. The pH of the paprika yogurt with 5% yellow paprika juice was significantly lower than that of the yogurt without paprika (p<0.05). The pH of the paprika yogurt with 5% paprika orange paprika was significantly higher than that of the other yogurts (p<0.05). The pH of the yogurt with 2.5% red paprika juice was significantly higher than that of the other yogurts with 2.5% paprika juice (p<0.05). The pH of the paprika juice used in this study was 4.9–5.2 (data not shown), indicating weak acidity. This indicated that the addition of paprika juice resulted in an increase in the acidity of the paprika-containing yogurt. The TA value was 0.72% in the yogurt without the paprika juice but was significantly higher for the red, orange, and yellow paprika yogurts (each p<0.05) with TA values of 0.88%–0.95%. These results were similar to previous reports of the pH and TA of stirred yogurt with added fermented pepper powder as 4.3–4.61 and 0.8%–0.95%. respectively (Kang et al., 2018; Yu et al., 2014). According to Kim et al. (2011), the total organic acid content was 12,941.9 and 15,746.56 mg/100 g dry weight in red and green paprika, respectively. Thus, our results might be attributed to an increase in the organic acid content because of the addition of the paprika juice.
The LAB count in yogurts without paprika juice was 10.3–10.4 log10 (CFU/g). As the content of red and orange paprika juice increased, LAB increased to 10.45–10.85 log10 (CFU/g), a count significantly higher than that of control yogurt (p<0.05). The LAB counts were greater than 9.09–9.35 log10 (CFU/g) when red and green peppers were added to yogurt (Kang et al., 2018) and were over 100 times higher than the standard value prescribed by the Jigu Publisher Editing Department (2001). In this study, there was little change in LAB between the red, orange, and yellow paprika yogurts. These results demonstrate that all three paprika juices had positive effects on the growth of probiotic bacteria. According to the USDA (2019), raw red paprika has 6 g of carbohydrates and 4 g sugar per 100 g, while Jovanovic-Malinovska et al. (2014) reported that the carbohydrates in paprika are mostly sugars, such as glucose and fructose, which are sources of nutrients for probiotic bacterial growth.
The protein digestion of paprika yogurt without paprika juice was 0.11 mg/mL (Fig. 1A). However, as the amount of paprika juice increased, the peptide concentrations significantly increased from 0.11 to 0.21 mg/mL. Lorusso et al. (2018) reported that LAB increase the concentration of free amino acids. Syneresis in paprika yogurt without paprika juice averaged 28.3%, a value significantly lower than that found in the yogurt with 5% orange (34.98%) and yellow paprika juice (35.35%) (Fig. 1B). The gel network structure in yogurt is a relatively dynamic system, composed of casein, denatured whey proteins, and calcium phosphate crosslinks and is affected by many chemical and technological factors (i.e. dry matter content, enzyme activity, heat treatment, incubation temperature, and pH) (Lee and Lucey, 2010). Furthermore, Ziarno and Zareba reported that lower pH values result in higher syneresis (2019).
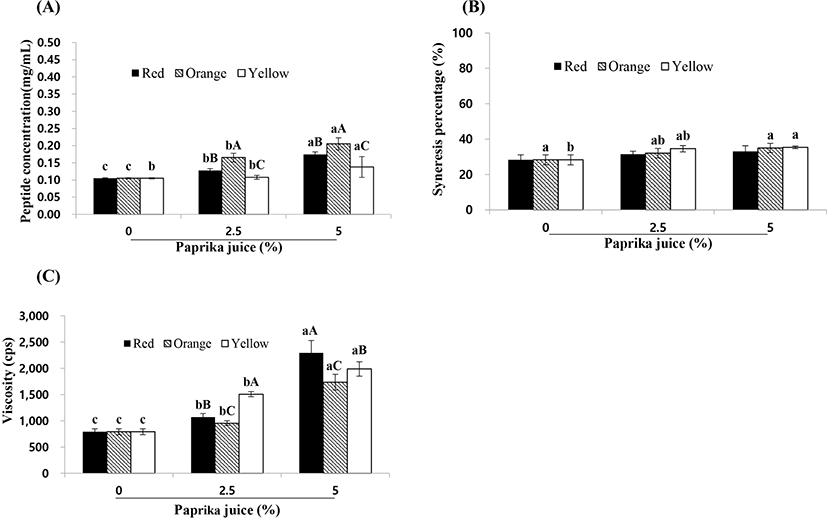
The viscosity of the paprika yogurt with 2.5% and 5% paprika juice was 954 and 2,296 cp, respectively, values that were significantly higher than the viscosity of the yogurt without paprika juice (p<0.05) (Fig. 1C). These results were inconsistent with reports of Kang et al. (2018) that the viscosity of yogurt decreased upon addition of fermented red or green pepper juice. Our results indicated that by increasing the levels of paprika juice, the pH value of the yogurt dropped. Similarly, Arioui et al. (2017) who observed that the viscosity of yogurt increased by lowering the pH. Sinaga et al. (2017) reported that changes in pH affect the casein micelle size and the gelation properties, as micelles swell in alkaline pH. These conditions weaken the cohesive interactions between the micelles, disrupting the hydrophobic bonds among the caseins, and eventually dissociating the casein micelles (Madadlou et al., 2009).
Therefore, we believe that the addition of paprika juice increases LAB count, free amino acid level, and syneresis, but decreases the pH, leading to an increase in viscosity.
Table 2 shows the changes in the color of yogurt containing 2.5% and 5% of paprika of different colors. An increase in the content of red paprika juice significantly decreased the lightness (CIE L*) of the yogurt, while the redness (CIE a*) increased significantly (p<0.05). In particular, the redness of the yogurt with 2.5% and 5% red paprika juice was 6.89 and 11.19, respectively, values that were significantly higher than those for yogurt containing orange and yellow paprika (p<0.05). The yellowness of the yogurt with 5% red paprika juice was similar to that of the yogurt without paprika juice. The redness and yellowness of the orange paprika yogurt were higher than those of the yogurt without the orange paprika juice but lightness was lower. In most yogurts containing paprika, the redness and yellowness increased and the lightness decreased as the amount of paprika juice was increased. According to Kim et al. (2016), the total carotenoid content of non-soil-cultivated paprika depends on its color: 55.80 mg/100 g dry weight for red, 62.57 mg/100 g dry weight for orange, and 35.32 mg/100 g dry weight for yellow paprika.
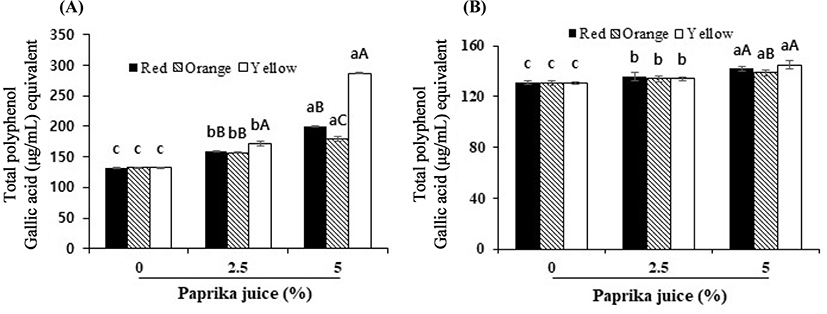
The TPC of the control yogurt was 132 μg/mL and 131 μg/mL (water extract and methanol extract, respectively) (Fig. 2). The TPC of the yogurt with the added paprika juice was higher in the water extract (159–287 μg/mL) than in the methanol extract (134–145 μg/mL). In contrast, Kang et al. (2018) found that the TPC of the methanol extracts was higher than that of the water extracts of yogurts containing red and green paprika. Addition of paprika significantly increased the TPC of the yogurt because paprika contains abundant polyphenols (Kang et al., 2018). Yogurt with added berries or aronia juice, known to be good sources of polyphenolic compounds, had higher TPC levels than plain yogurt (Nguyen and Hwang, 2016; Raikos et al., 2019).
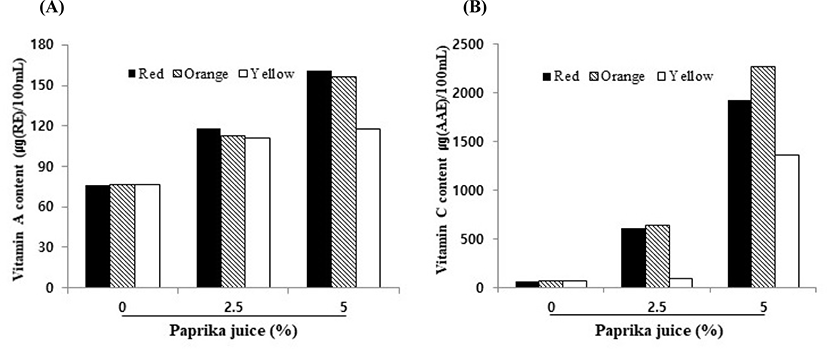
The vitamin A content of the yogurt without paprika juice was 76.54 μg/100 mL, while that of the yogurt with paprika juice was 111.15–161.06 μg/100 mL (Fig. 3). Vitamin A content in the yogurts containing 5% red and orange paprika was significantly higher than that in the yogurt containing 5% yellow paprika (p<0.05). Similarly, vitamin C levels were 610–1,920 μg/100 mL for the red paprika yogurt, 640–2,270 μg/100 mL for the orange paprika yogurt, and 100–1,360 μg/100 mL for the yellow paprika yogurt. The yellow paprika yogurt had the lowest vitamin C levels. In agreement, Chávez-Mendoza et al. (2015) observed a lower vitamin C level in yellow paprika than red, orange, or green paprika. In contrast, Nerdy (2018) observed that the vitamin C content in the yellow, orange, and red paprika was 159.61, 121.38, and 81.19 mg/100 g, respectively. Thus, it can be inferred that paprika-containing yogurt has abundant levels of vitamins A and C, which are good antioxidants.
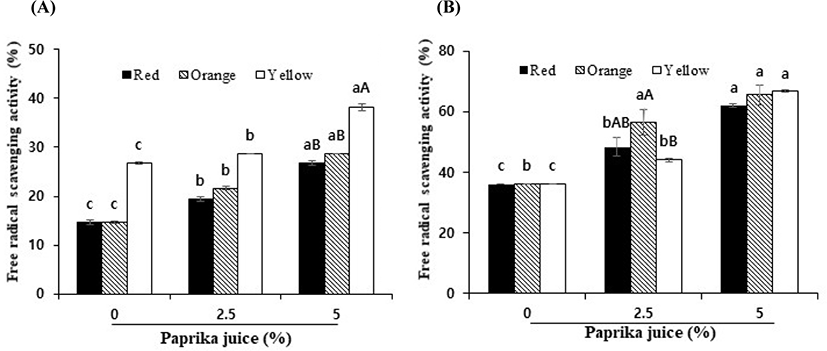
The antioxidant activities of both the water and methanol extracts of the yogurts were significantly increased with the addition of paprika of different colors (p<0.05) (Fig. 4). Interestingly, the antioxidant activities of the methanol extracts of the yogurts with paprika of different colors were 2 to 4-fold higher than those of the water extracts, although the TPC values of the water extracts were higher than those of the methanol extracts. This is probably because other bioactive compounds in the methanol extracts may mainly act on the antioxidant activities. Fresh peppers contain large amounts of phenolic compounds and vitamin C, and their various colors are because of the different carotenoid pigments, including β-carotene, with pro-vitamin A activity, and carotenoids such as capsanthin, capsorubin, and cryptocapsin, which are effective at scavenging free radicals (Chávez-Mendoza et al., 2015; Deepa et al., 2006).
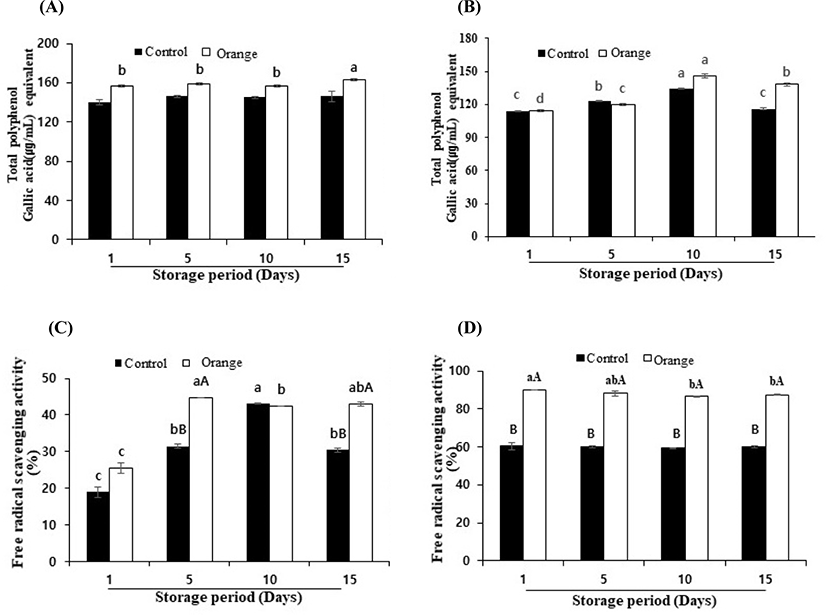
Since the antioxidant activity and peptide content of yogurt with orange paprika juice were shown to be higher than the yogurts with other color paprika and there was little difference between the added levels, yogurt with 2.5% orange paprika juice was selected to further evaluate its physiochemical characteristics during a storage period of 15 d at 4°C (Table 3; Fig. 5).
At the end of the storage period, the pH of the control and paprika yogurt decreased significantly (4.47 and 4.46, respectively; p<0.05). The pH of the paprika yogurt was 4.46–4.64, values that were lower than the pH of the control (4.47–4.76). During the storage period, the pH of yogurt was 4.46–4.76, while that of the semisolid type yogurt sold in Korea is 4.18–4.60 (Won et al., 2018). The pH of the yogurt is determined by the organic acid produced during fermentation. The pH range for improving the functionality and flavor is 4.1–4.7 (Lee et al., 2017; Won et al., 2018). Therefore, the paprika addition to yogurt helps maintain a suitable pH during storage.
In addition, TA, a parameter that relates to yogurt quality, increased in both the control and paprika yogurts with the length of the storage. The TA of the control was 0.77% on day 1 and 0.97% on day 15, while that of the paprika yogurt was 0.83% on day 1 and 1.01% on day 15. The TA of the control and paprika yogurt on day 15 of storage was significantly higher than that on day 1 of storage (p<0.05). A TA for yogurt of 0.85%–1.20% is suitable for Korean consumers (Lee et al., 2006). The best quality yogurt is when the TA is 1.0%–1.1% (Kim et al., 1993). Therefore, the results obtained in this study indicate that the paprika yogurt was well preserved over 15 days of storage.
The LAB count of the control and paprika yogurt did not change during storage. As the storage time increased, the viscosity of control and paprika yogurt increased significantly (p<0.05). However, there was little change in the amino acid content; this may be because of poor hydrolysis of proteins and unchanged LAB counts during storage.
Syneresis of the paprika yogurt increased with storage time. Layer separation occurred during the storage period even though 0.2% pectin was added to help prevent layer separation of the yogurt. This increased syneresis is believed to have resulted in an increased viscosity of the yogurt during the storage period.
The CIE L*, a*, and b* values of the control and the paprika yogurt did not change over the 15 days, but the CIE b* value was significantly higher in the paprika yogurt than in the control, believed to be because of the addition of orange paprika juice.
The TPC of the paprika yogurt (157–163 μg/mL for water extracts and 114–146 μg/mL for methanol extracts) was slightly higher than that of the control yogurt (140–146 μg/mL for water extracts and 114–134 μg/mL for methanol extracts) (Fig. 5A and B). For both the control and paprika yogurts, the antioxidant activities of the methanol extracts did not change but those of the water extracts increased significantly (p<0.05 Fig. 5C and D). In addition, antioxidant activities of the water and methanol extracts of the paprika yogurt were higher than those of the control yogurt. These results show that the addition of paprika juice increased TPC and antioxidant activities. A significant positive correlation between TPC and antioxidant activities in bell peppers has been reported (Chávez-Mendoza et al., 2015; Kim et al., 2011; Medina-Juarez et al., 2012; Zhuang et al., 2012).
Conclusion
LAB growth in yogurt was not affected by addition of different colored paprika juices. Furthermore, the addition of paprika juices increased TPC, levels of vitamin A and C, and antioxidant activity of yogurt. Thus, this study suggests that paprika can be a good natural food additive for the development of functional yogurts that can have an enhanced antioxidant effect and can be used as a natural pigment to enhance the visual effects of yogurt using a variety of paprika colors.













