Introduction
Periodontitis is a chronic inflammatory disease caused by infections, and the tooth support tissue is destroyed. It is one of the major causes of tooth loss that characterized by the rapid destruction of periodontal attachment (Riep et al., 2009). Common treatments for periodontitis include scaling and root planning with systemic antibiotics. However, the overuse of antibiotics has led to the emergence of drug-resistant microorganisms and disturbs the beneficial bacteria, including lactobacilli in the oral cavity.
Probiotics are defined as live microorganisms that confer health benefits to the host when administered in adequate amounts (Hill et al., 2014). The use of probiotics is not restricted to the gut and intestine but also to the oral cavity and respiratory tract. Probiotics should be selected for their potential nutritional benefits (Gibson et al., 2017). Recently, research on fermented products showing better efficacy by fermenting various additives, including probiotics and prebiotics, has been actively proposed. Prebiotics, plant extracts, or other additives pass through the gastrointestinal (GI) tract undigested. Consequently, these additives act as substrates for advantageous microorganisms and enhance their growth and biological activity (Gibson et al., 2017).
In this review, recent studies on the relationship between probiotic-mediated bioconversion (PMB) and periodontitis have been summarized. The correlation between oral microorganisms and periodontitis was reviewed, the mechanisms used by probiotics to control periodontitis were analyzed, and various studies on PMB have been summarized. In addition, correlations between various metabolites in PMB and periodontitis were considered.
Oral Microbiome Characteristics
The microbiome includes both the microbiota, the microbial community, and the “theater of activity” (structural elements, metabolites/signal molecules, and ambient conditions; Berg et al., 2020). Microbiota refers to bacteria, archaea, fungi, protists, algae, and the microbiome includes the “theater of activity” of theses microbial communities. “Theater of activity” is described as a characteristic microbial community in a reasonably well-defined habitat with unique physiochemical properties. The characteristic consists of microbial structural elements and internal/external structural elements. Microbial structural elements contain proteins/peptides, lipids, polysaccharides, and nucleic acids. Internal/external structural elements contain environmental conditions and microbial metabolites such as signaling molecules, toxins, and organic molecules (Berg et al., 2020). The oral microbiome is the microbiota and “theater of activity” that resides in the oral cavity (Dewhirst et al., 2010; Kolenbrander et al., 2002; Turnbaugh et al., 2007). More than 700 species of oral microbiota have been detected in the human mouth, and one individual’s resident microbiota may consist of 30–100 species. Research on the oral microbiome continues because the oral microbiome has low biological variation, which provides an ideal source for biomarker discovery. The normal temperature of the oral cavity is 37°C, and it is suitable for bacterial growth (Aas et al., 2005; Takahashi et al., 2005). In addition, as saliva maintains a pH of 6.5–7.5 and keeps the mouth hydrated, it is suitable for the growth of most bacteria (Aas et al., 2005; Takahashi et al., 2005). The bacteria in the oral cavity maintain homeostasis by coevolving with both pathogenic and mutualistic bacteria. These bacteria develop communities to form a multispecies organization known as dental plaque (Kolenbrander et al., 2002). The oral cavity includes several distinct microbial habitats, such as periodontal pockets and the surfaces of teeth and cheeks (Danser et al., 2003). Microorganisms in the tongue often move around the oral cavity to colonize other areas, facilitated by saliva (Danser et al., 2003). Representative microbes in the tongue include Aggregatibacter actinomycetemcomitans, Capnocytophaga spp., Porphyromonas gingivalis, Prevotella intermedia, Selenomonas spp., and Veillonella atypica (Danser et al., 2003). Oral microbiome is known to be associated with periodontal diseases and systemic diseases (Zhang et al., 2018). Periodontal diseases often include A. actinomycetemcomitans, P. gingivalis, Tannerella forsythia, and Treponema denticola, in the oral microbiome (Kumar et al., 2003; Socransky et al., 1998). Listgarten (1987) reported that oral microbiome directly or indirectly mediates the inflammatory response to develop periodontal disease. According to Scannapieco (2013) study, high proportion of oral pathogen in the oral microbiome tended to have a high rate of C-reactive protein, which is a marker of cardiovascular disease. Also, periodontal disease patients had a high incidence of type 1 or type 2 diabetes (Borgnakke et al., 2013). The recent studies also suggested an association of poor oral health with cancers, Alzeheimer's disease, dermentia, and Rheumatoid arthritis (Ahn et al., 2012; Kamer et al., 2008; Kaur et al., 2013). Thus, oral microbiome has a key role in not only periodontitis but also other systemic diseases.
Periodontitis
Periodontitis is loss of connective tissue attachment leading to the resorption of alveolar bone and subsequent tooth loss that showed gingival inflammation (Armitage, 1995; Sanz et al., 2018; Tonetti et al, 2013). The disease encompasses hard and soft tissues, inflammatory responses, microbial colonization, and adaptive immune responses. In particular, microbial biofilms are the primary etiological factor in chronic periodontitis (Page, 1986; Sanz et al., 2017). The main pathogenic bacteria associated with periodontitis are A. actinomycetemcomitans, P. gingivalis, Treponema denticola, and Tannerella forsythia. These bacteria have a range of virulent characteristics that allow them to colonize the subgingival sites, escape the defense system of the host, and thereby cause tissue damage. The persistence of the immune response of the host also contributes to disease progression (Gupta, 2011; Houle and Grenier, 2003). There is an increase in plaque mass and a shift toward obligatory anaerobic and proteolytic bacteria, many of which are Gram-negative in periodontal diseases.
Conventional treatment modalities for periodontal disease include nonsurgical and surgical management, which emphasizes mechanical debridement, often accompanied by antibiotics. The ideal treatment of chronic periodontitis results in a reduction in periodontal pocket depth, with gains in clinical attachment levels (Goodson et al., 2012). Conventionally, nonsurgical periodontal therapy, including oral hygiene instructions and scaling and root planning, is considered the main treatment modality for chronic periodontitis (Claffey et al., 2004). Systemic antibiotic therapy has been used to reinforce mechanical therapy and support host defense by killing the subgingival microbial pathogens that remain after scaling and root planning (van Winkelhoff et al., 1996). In conjunction with scaling and root planning, systemic antibiotics may offer additional benefits. However, antibiotics are not innocuous drugs because they are accompanied with side effects and the potential emergence of antibiotic-resistant bacterial strains (Kapoor et al., 2012). In addition, drug therapy including nonsteroidal anti-inflammatory drugs (anti-cytokine substances, COX-2 inhibitors, and nitric oxide synthetase inhibitors), antiprotease, and anti-bone resorption agents have been used. Despite the widely discussed clinical benefits of nonsurgical periodontal therapies, including antibiotic therapy, and scaling and root planning therapy, they do not always result in improvements, especially for sites with deep probing depths, or when patients suffer from comorbidities (diabetes mellitus, obesity, and cardiovascular disease; D’Aiuto et al., 2018; Teeuw et al., 2014; Tomasi et al., 2007). In addition, there is no commercialized treatment for fundamental periodontitis that can prevent the destruction of gums and alveolar bone. As a result of these limitations, efforts have been made to explore the use of probiotics as an alternative method to modulate the microbial composition of pathogenic biofilms in conjunction with scaling and root planning (Teughels et al., 2008).
Probiotics and Periodontitis
Probiotics are defined as live microorganisms that confer health benefits to the host when administered in adequate amounts (Hill et al., 2014). Probiotics can be used in a variety of internal organs, including the gut, intestine, oral cavity, female urogenital tract, and respiratory tract (Gibson et al., 2017). Recently, probiotics have been used as substitutes for antibiotics to treat various oral diseases, including periodontitis, dental caries, and halitosis.
The main mechanism of probiotics in alleviating periodontitis is to maintain microbial balance in the oral cavity by competing with oral pathogens. Probiotics produce antimicrobial agents, such as lactic acid, acetic acid, diacetyl, and hydrogen peroxide, which inhibit the growth of periodontal pathogens. In addition, probiotics directly interact with dental plaque formation by the intervention of bacterial attachment to each other, thereby competing with other organisms for attachment to the teeth. Probiotics also modulate the host response to oral pathogens. Probiotics not only affect the local immune response but also modulate the systemic immune response in favor of the host. The immunomodulatory mechanisms of probiotics include an inhibition of periodontal pathogens through secretion of metabolites with antimicrobial activity, a stimulation of specific and non-specific immune responses by T lymphocyte activation, and a stimulation of producing cytokines. In this way, probiotics can be effectively used for periodontal disease (Kaźmierczyk-Winciorek et al., 2021). Probiotics are also known to produce antioxidants that neutralize the free electrons required for the mineral action of plaque and prevent the plaque formation. In addition, probiotics reduce the production of oral pathogen-associated pro-inflammatory cytokines. Plaque causes periodontal disease, and probiotics have been proven to inhibit plaque formation. Their mode of action lowers the pH of the saliva so that the bacteria involved in plaque formation cannot form it, and probiotics compete with other organisms for attachment to the teeth. Probiotics can break down the putrescent odor by fixing volatile sulfur compounds and changing them to the gases needed for metabolism.
Ishikawa et al. (2003) observed in vitro inhibition of P. gingivalis, P. intermedia, and Prevotella nigrescens by daily ingestion of Lactobacillus salivarius in tablet form. The inhibitory activity of homofermentative lactobacilli against periodontal pathogens is principally related to the production of acid, not hydrogen peroxide or bacteriocin (Kõll-Klais et al., 2005). Grudianov et al. (2002) analyzed the effect of probiotics on different grades of periodontitis and reported that probiotic treatment group resulted in better microbiota normalization than the control group (Grudianov et al., 2002; Gupta, 2011). Gupta (2011) reported that the prevalence of Lactobacillus gasseri and Lactobacillus fermentum in the oral cavity was greater in healthy participants than chronic periodontitis patients. In addition, lactobacilli inhibited the growth of periodontopathogens, including A. actinomycetemcomitans, P. gingivalis, and P. intermedia suggesting that lactobacilli residing in the oral cavity play a role in the oral ecological balance. Kõll-Klais et al. (2005) observed that L. gasseri strains isolated from periodontally healthy subjects inhibited the growth of A. actinomycetemcomitans more efficiently than that from periodontally diseased subjects. Randomized controlled clinical trials using Bifidobacterium animalis subsp. lactis HN019-containing probiotic lozenges for chronic periodontitis patients reported the test group significantly decreased probing pocket depth and clinical attachment gain higher than control group (Invernici et al., 2018). Also, periodontal pathogens and expression level of proinflammatory cytokines were significantly decreased in the test group. The randomized controlled clinical trials using probiotics (Lactobacillus reuteri DSM17938 and L. reuteri ATCCPTA5289) for an adjunct to periodontitis reported a significant strengthening of pocket closure and a reduction of periodontal tissue inflammation in probiotics intake group compared to placebo group (Grusovin et al., 2020; İnce et al., 2015; Laleman et al., 2020; Pelekos et al., 2020; Schlagenhauf et al., 2020; Tekce et al., 2015; Teughels et al., 2013; Vicario et al., 2013; Vivekananda et al., 2010).
Probiotics-Mediated Bioconversion (PMB)
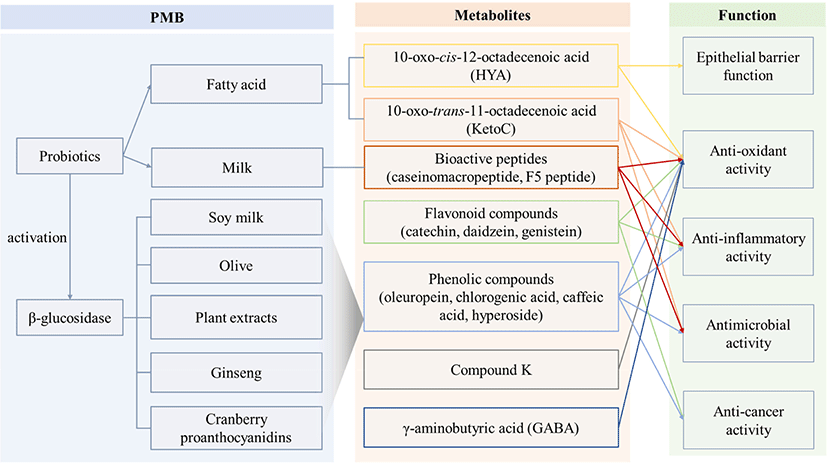
Microbial bioconversion is the process of converting organic compounds into structurally related compounds through enzymatic reactions using microorganisms (Perkins et al., 2016). This strategy has great potential to produce novel bioactive metabolites (Fig. 1). Furthermore, Pervaiz et al. (2013) suggested that microbial bioconversion is a cost-effective and environmentally protective tool for drug design. Probiotics are suitable for the conversion of biomass into value-added products because of their potential nutritional benefits (Aarnikunnas et al., 2003; Berezina et al., 2010; John et al., 2007).
Dairy products, medicinal plants, or plant compounds contain bioactive compounds, which can be transformed by PMB process, expressing or enhancing biological activity (Table 1). Most PMB metabolites from fermented dairy products include proteins, peptides, oligosaccharides, fats, and organic acids (Ebringer et al., 2008). In particular, lactic acid bacteria (LAB) in fermented dairy products hydrolyze proteins and release specific peptides that have bioactive, immunomodulatory, antifungal, antimicrobial, antioxidant, and anticarcinogenic activities (Fernandez et al., 2017). Probiotics containing many Lactobacillus strains show high hydrolytic activity of milk protein that leads to release bioactive peptides. Bioactive peptides released from fermented milk by Lactobacillus spp. have been reported in various studies to have antioxidant activity and angiotensin I-converting enzyme (ACE) inhibitory activity (Elfahri, 2012; Gobbetti et al., 2004; Gonzalez-Gonzalez et al., 2011; Nejati et al., 2013; Solieri et al., 2015). Digestibility of fat is another bioactive ingredient in fermented milk. Conjugated linoleic acid (CLA) is one of the major ingredients produced by milk fermentation, and the CLA exhibits antidiabetic, antiatherogenic, and immune system modulator effects (Xu et al., 2005). In addition, polyphenol-rich foods have significant antioxidant, anti-inflammatory, and proapoptotic effects, suggesting their use as chemo-preventive agents (Stagos et al., 2012). However, most polyphenols cannot be absorbed in their native forms, and the polyphenols should be modified by microbial conversion (Selma et al., 2009). Rupasinghe et al. (2019) reported that PMB enhanced the bioactivities by producing additional metabolites of cranberry proanthocyanidins. Cranberry proanthocyanidin extract bioconverted by Lactobacillus rhamnosus completely inhibited HepG2 cell proliferation with IC50 values that indicates the amount of bioconversion to inhibit HepG2 cell proliferation by 50%. The major metabolites produced from PMB are 4-hydroxyphenylacetic acid, 3-(4-hydroxyphenyl) propionic acid, catechol, hydrocinnamic acid and pyrogallol (Rupasinhe et al., 2019). Liu et al. (2018a) reported that the major bioactive metabolites for the antioxidative activity of PMB of polyphenol compounds contained epicatechin, catechin, caffeic acid, chlorogenic acid, and hyperoxide. Moreover, L. reuteri and Enterococcus faecalis showed a high bioconversion rate and high anti-radical activity, which might have excellent potential for PMB (Liu et al., 2018a). The pharmacological effects of PMB are also well known. The main bioactive components in ginseng are ginsenosides Rb1, Rb2, and Rc, which are transformed to C-K by the human gut microbiome, which has anticancer effects in tumor cells (Bae et al., 2003). Jung et al. (2019) suggested that bioconversion of red ginseng by Lactobacillus plantarum KCCM11613P generates ginsenoside Rd, which may be converted to compound K to make the inhibitor material in oxidation. Leuconostoc mesenteroides LH1 also produces β-glucosidase to convert Rb1 to Rd, F2, and C-K (Quan et al., 2011). The PMB of Artemisia species also generates novel bioactive metabolites to enhance their functions. Specifically, PMB of Artemisia capillaris exhibited enhanced anti-inflammatory activity (Son et al., 2017), and the extracts of Artemisia argyi folium exhibited immunomodulatory activities, including inhibition of pro-inflammatory cytokines IL-6 and TNF-α production in macrophages (Han et al., 2008). In addition, PMB of Artemisia princeps Pampanini inhibited the degranulation of RBL-2H3 cells, which showed anti-allergic effects (Shin et al., 2006).
| Metabolites | Bioconverted substrates | Probiotics | Beneficial effects | References |
|---|---|---|---|---|
| Bioactive peptides | Milk | Lactobacillus fermentum | Antioxidant activity | Ebringer et al., 2008 |
| Catechin (flavonoid) | Grape seed polyphenols | Lactobacillus plantarum | Antioxidant activity | Tabasco et al., 2011 |
| Daidzein and genistein (flavonoid) | Black soymilk | Streptococcus thermophiles | Antioxidant activity | Lee et al., 2015 |
| Chlorogenic acid, caffeic acid, atechin, picatechin, and hyperoside (polyphenol compounds) | Lotus seed epicarp | Lactobacillus reuteri, Enterococcus faecalis | Antioxidant activity | Liu et al., 2018a |
| Gallic acid and protocatechuic acid (phenolic acid) | Wine (Pinot Noir) | L. plantarum | Antioxidant activity | Suthanthangjai et al., 2014 |
| Phenolic content | Inula britannica extract | Lactobacillus acidophilus, S. thermophilus | Antioxidant activity | Lee et al., 2016 |
| Ginsenoside | Ginseng | L. plantarum | Antioxidant activity | Jung et al., 2019 |
| Total polyphenol and flavonoid contents | Forsythiae Fructus | L. plantarum, L. acidophilus, Lactobacillus casei, Lactobacillus lactis, Leuconostoc mesenteroides | Antioxidant, anti-inflammatory activity | Yang and Choe, 2011 |
| F5 peptide | Milk | Lactobacillus helveticus | Anti-inflammatory activity | Tellez et al., 2010 |
| Isoflavone aglycones | Soy milks | L. plantarum, Lactobaciilus rhamnosus, L. fermentum, | Immunomodulatory effect on Caco-2/T27 cells | Di Cagno et al., 2010 |
| Oleuropein | Olive extract | L. plantarum, Lactobacillus paracasei | Anti-microbial activity | Omar, 2010 |
| Isoflavone aglycone | Biotin-supplemented soymilk | L. fermentum | Increased permeability of treated cell membranes | Ewe et al., 2012 |
| Total phenolic contents and total flavonoids | Magnolia flower petal extract | Pediococcus acidilactici | Antioxidant, anticancer activity | Park et al., 2015 |
| Crude, dihydrochalcone, anthocyanin, proanthocyanidin, and catechin | Cranberry proanthocyanidins | L. rhamnosus | Anticancer activity | Rupasinghe et al., 2019 |
The mechanism of PMB action differs depending on the substance and bioconversion, and the bioactive compounds are also diverse. Bioactive peptides are usually produced by LAB proteolysis in fermented milk. For example, fermented milk containing Lactobacillus lactis releases biologically active oligopeptides such as casomorphines, lactorphines, casokinines, and immunopeptides from α-casein, β-casein, and κ-casein (Ebringer et al., 2008). The mechanisms of PMB action using polyphenol-rich foods include deglycosylation, ring fission, dehydroxylation, decarboxylation, or reduction of carbon double bonds (Aura, 2008; Rice-Evans et al., 1997), which convert a few polyphenols into bioactive forms (Thilakarathna et al., 2018). β-Glucosidase catalyzes the hydrolysis of glycosidic bonds to remove glucopyranosyl residues from the nonreducing end of β-glucosides (Ketudat Cairns and Esen, 2010). It is present in bacteria, fungi, and yeast, and exists in some Lactobacillus species (Spano et al., 2005). β-Glucosidase hydrolyzes a wide range of substrates to produce specific aglycones that show bioactive effects (Grandits et al., 2013). These bioactive metabolites, including γ-aminobutyric acid (GABA), hydroxytyrosol, ginsenosides, isoflavones, and phenolic compounds have been known to have antioxidant activity as a major beneficial effect (Lee and Paik, 2017). In addition, the metabolites have anti-inflammatory effects (Yang and Choe, 2011), permeabilizing the membrane of treated cells to act as metabolites (Ewe et al., 2012), and show anticancer activity (Park et al., 2015). For example, Lactobacillus metabolizes polyphenols using glycosidase, and then converting them to secondary metabolites such as catechol, gallic acid, and pyrogallol (Rodríguez et al., 2009; Tabasco et al., 2011). In addition, L. plantarum metabolizes phenolic acids and their derivatives, esters, through the activities of feruloyl esterase, tannase, phenolic acid decarboxylase, and phenolic acid reductase (Curiel et al., 2009; Rodríguez et al., 2008; Wang et al., 2004). Lactobacilli contribute to the release of phenolic acids bound to protocatechuic and p-hydroxybenzoic acids that are insoluble plant cell wall materials. Therefore, probiotics play a major role in increasing the antioxidant activity of polyphenols through the PMB process (Jakesevic et al., 2011; Li et al., 2013).
The mechanism of antimicrobial activity by PMB has also been suggested. PMB using whey showed antimicrobial activity that reduced Escherichia coli and Listeria monocytogenes (Lee et al., 2020). This result suggested that quorum sensing and intercellular connections to form a biofilm structure composed of a bacterial community and extracellular polymeric matrix were inhibited by PMB with whey. According to Zokaityte et al. (2020), the antimicrobial activity of PMB using apple by-products might be due to the synthesis of galactobiose and galactotriose. PMB using apple by-products showed high antimicrobial activity, and high synthesis activity of galactobiose and galactotriose. Also, the PMB showed high production of galactooligosaccharides.
Probiotics-Mediated Bioconversion (PMB) and Periodontitis
Various studies have reported that PMB regulates periodontal disease. Recent research presented that an administration of fermented milk using Lactobacillus curvatus to periodontitis-induced mice leads to a significant decrease of the expression levels of inflammatory cytokines in the oral gingiva tissue and colon tissue compared to control group (Choi et al., 2021). Also, randomized clinical trial was performed to confirm the effect of PMB with fermented bovine milk in periodontal disease, and papillary-marginal-attached index, gingival index, and probing depth score of the experimental group showed greater tendency than those of placebo group (Oda et al., 2019). Liu et al. (2018b) studied the effect of an ethanol extract of PMB with fermented skim milk on periodontal inflammation in rats. According to this study, the PMB-treated rats showed decreases in the levels of alveolar bone loss and pro-inflammatory cytokines, and oxidative stresses in periodontal tissue. Vieira et al. (2021) reported that PMB with milk kefir reduced alveolar bone loss and pro-inflammatory cytokine expression level on periodontitis-induced rats. Several studies demonstrated that 10-hydroxy-cis-12-octadecenoic acid (HYA) and 10-oxo-trans-11-octadecenoic acid (KetoC), the main bioactive PMB metabolites generated by L. plantarum through saturation metabolism of polyunsaturated fatty acids, were effective in alleviating periodontal disease (Kishino et al., 2013; Sulijaya et al., 2018; Yamada et al., 2018).
Reactive oxygen species (ROS) induced by pathogenic bacteria play an important physiological role in intracellular signaling pathways and promote the production of pro-inflammatory cytokines in gingival epithelial cells (Wang et al., 2017). ROS production results in E-cadherin damage in the junctional epithelium of the periodontium (Lee et al., 2016a). Thus, periodontitis can be defined as a disease associated with oxidative stress (Varela-López et al., 2015). Enzymatic antioxidants include superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), and these antioxidants convert superoxide radical and hydrogen peroxide into harmless product water (Hyun et al., 2002; Weydert and Cullen, 2010). According to Novakovic et al. (2014), CAT, GPx, and SOD levels were significantly higher in healthy periodontal patients than in patients with chronic periodontitis. Fermented goat milk increased the antioxidant activity and reduced GPx1 expression, thus limiting biomolecular oxidative damage compared to unfermented milk (Moreno-Fernandez et al., 2017). Ebringer et al. (2008) suggested that the main antioxidant factors of fermented milk were peptides released from α-casein, α-lactalbumin, and β-lactoglobulin (Ebringer et al., 2008). KetoC, a PMB metabolite, stimulates antioxidant-related gene expression, and its treatment increased heme oxygenase-1 expression in gingival epithelial cells (Yokoji-Takeuchi et al., 2020). In addition, the metabolite activates Nrf2-ARE signaling by binding to the G protein-coupled receptor (GPR) efficiently (Yokoji-Takeuchi et al., 2020). Flavonoid compounds, such as catechin, daidzein, and genistein, which are major bioactive metabolites produced in the PMB process, increased the 1,1-diphenyl-2-picryl hydrazyl (DPPH) free radical scavenging activities (Lee et al., 2015). Phenolic compounds are major products of fermentation, also increase anti-radical activity (Lee et al., 2016b; Liu et al., 2018a; Suthanthangjai et al., 2014). Ginsenoside, an active compound in ginseng, is converted to smaller compound such as compound K during fermentation with probiotics, and this compound has enhanced inhibitory activity of β-carotene and linoleic acid oxidation (Jung et al., 2019). In addition, PMB using ginseng has bioactive metabolites such as flavonoids produced during the fermentation process of probiotics and ginseng, displaying higher SOD-like activity than general ginseng (Doh et al., 2010). Hence, flavonoid compounds derived from PMB, which have antioxidant and anti-radical activities, are thought to be effective against periodontitis.
Anti-inflammatory activity has become a treatment strategy for periodontitis (Sulijaya et al., 2019). Fermented milk with Lactobacillus helveticus LH-2 and its fraction peptide F5 increased TNF-α, IL-1β, and IL-6 through stimulation of macrophages with production of nitric oxide and phagocytic activity, which showed that the F5 peptide fraction could modulate macrophage functions (Tellez et al., 2010). KetoC exhibits anti-inflammatory efficacy through mitogen-activated protein kinase and NF-κB signaling in RAW 264.7 macrophages induced by bacterial lipopolysaccharide (Yang et al., 2017). In addition, KetoC binding to its receptor GPR 120 suppressed TNF-α, IL-1β, and IL-6 in macrophages stimulated with P. gingivalis LPS to partially inhibit NF-κB and p65, which could be an anti-inflammatory bioactive metabolite in periodontal disease (Sulijaya et al., 2018). HYA inhibits the loss of alveolar bone by reducing the mRNA levels of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) in gingival tissue in vivo (Yamada et al., 2018). HYA also decreased the expression of tumor necrosis factor receptor 2 (TNFR2) in colitis mice, thereby decreasing the production of pro-inflammatory cytokines (Miyamoto et al., 2015). The total polyphenol and flavonoid contents and bioactive metabolites of PMB of Forsythiae Fructus extracts inhibited nitric oxide synthesis (Yang and Choe, 2011). PMB of soymilk synthesized isoflavone aglycones (daidzein, genistein, and glycitein) and equol, and PMB inhibited the inflammatory status of Caco-2/TC7 cells (Di Cagno et al., 2010). It is believed that the anti-inflammatory effects of PMB, such as HYA, polyphenol, and flavonoid contents, daidzein, genistein, and glycitein, may also act on periodontitis.
Glycosylated caseinomacropeptide, produced by hydrolyzing the κ-casein phenylalanine-105 and methionine-106 peptide bonds by the action of chymosin in the process of milk fermentation, inhibited the entry of toxins, pathogenic adhesion to the cell wall, and infections by oral pathogens such as P. gingivalis, S. mutans, and Streptococcus sobrinus (Córdova-Dávalos et al., 2019; Malkoski et al., 2001). KetoC inhibited the growth of P. gingivalisin vitro and reduced alveolar bone destruction in a periodontitis mouse model (Sulijaya et al., 2019). Furthermore, fluorescence microscopy after LIVE/DEAD bacterial staining of KetoC-treated P. gingivalis cells showed that KetoC decreased the viability of the bacteria (Sulijaya et al., 2019). In addition, a comparative analysis of the inhibitory effects of KetoC and KetoB against P. gingivalis showed that only KetoC lowered the viability and proliferation rate of P. gingivalis (Sulijaya et al., 2019). According to Lee et al. (2020) study, PMB of whey showed anti-biofilm effect against pathogenic bacteria. The PMB of olive produces oleuropein, a type of phenolic compound, which exhibits antimicrobial activity (Omar, 2010). In other words, olive extract-derived oleuropein generated by probiotic-mediated fermentation hindered the growth of Bacillus cereus, Campylobacter jejuni, Escherichia coli, Helicobacter pylori, Klebsiella pneumoniae, Salmonella Enteritidis, and Staphylococcus aureus (Omar, 2010). Also, PMB of soy increased aglycone production by β-glucosidase activity of probiotics and inhibited the growth of oral pathogens (Enterococcus faecalis, Streptococcus pyogenes, and S. aureus; How et al., 2020).
Destruction of the gingival epithelial barrier by proteases and infiltration into the basal tissue causes periodontal tissue disruption in periodontitis (Brooke et al., 2012; DiRienzo, 2014). Various bacteria may interact with the epithelial cells, generating gingival barrier function (Takahashi et al., 2019). Probiotics in PMB induce antimicrobial peptides against barrier-disrupting microbial pathogens (Diamond et al., 2009; Ostaff et al., 2013; Sulijaya et al., 2016). LfcinB, known as a major antibacterial peptide in fermented milk, showed efficacy in the intestinal epithelial barrier function and was also suggested to be effective in improving intestinal tight junctions (Haiwen et al., 2019; Sibel Akalın, 2014). Bacteriocins derived from PMB such as salivaricin, reuterin, plantaricin, and nisin are also main antimicrobial peptides that relevant to oral cavity (Baca-Castanon et al., 2015; Heeney et al., 2019; Masdea et al., 2012). In addition, PMB metabolites, such as HYA and KetoC, stimulate tight junction-related gene expression to regulate the epithelial barrier. An in vivo study using a mouse experimental periodontitis model showed that HYA treatment of periodontitis-induced mice reduced inflammation and damage in mouse gingival tissues by inhibiting the breakdown of E-cadherin/-catenin in P. gingivalis, thereby strengthening the epithelial barrier junction (Yamada et al., 2018).
Conclusion
The aim of this review was to summarize the use of PMB and the major metabolites in the oral cavity. The microbial environment in the oral cavity is highly correlated with periodontitis, and various treatment methods have been proposed to control the disease. Studies on various mechanisms to control periodontitis by PMB and probiotics have been reported. Although there have not been many studies on PMB that control the oral cavity of periodontitis, there have been many studies on their antioxidant, anti-inflammatory, and antimicrobial effects in relation to periodontitis. The main components of these activities include KetoC and HYA biotransformed from phenols or flavonoid compounds, as a result of probiotic-mediated fermentation of dairy products, plants, or fruits. This suggests that PMB is effective against periodontitis, and studies are needed to confirm the treatment effect on periodontitis using bioactive compounds of PMB, especially to confirm the efficacy of KetoC and HYA on periodontitis.