Introduction
Cheese is considered a relatively safe food, but 0.4% of all foodborne illnesses reported in Europe in 2006 were caused by cheeses (EFSA, 2008). In particular, Gormley et al. (2011) reported that 2.6% of all the foodborne illnesses that occurred in England and Wales from 1992 to 2008 by dairy products, were caused by Escherichiacoli O157 and Campylobacter spp.
According to Rossi et al. (2008), foodborne bacteria originating in the dairy farm environment may not be completely eliminated from dairy products after pasteurization. The possible sources of microbial contamination of cheeses during the manufacturing process are the starter culture, salt, floor, packaging materials, cheese vat, work clothes, curd cooking knife, and ripening room (Temelli et al., 2005). Of these possible factors, workers are known to be the main cause of Staphylococcusaureus contamination in cheese (Callon et al., 2008). Moreover, contamination of raw milk with pathogenic bacteria such as fecal Listeria spp., Salmonella spp., and E. coli has been found to occur in the dairy farm environment (Hancock et al., 1998). B. cereus spores have been detected at a high rate in hay, silage, and feed, the primary sources of contamination for cattle that could be an indirect cause of raw milk contamination; most B. cereus contamination levels ranged from 101 to 106 CFU/g (Desmarchelier, 2001).
S. aureus is gram-positive, toxic bacterium, causing diarrhea and abdominal pain with a very short incubation period (Shelin et al., 2011). This pathogen is also known to be resistant to antibiotics, particularly to methicillin (Livermore, 2000; Zapun et al., 2008). B. cereus causes foodborne illnesses. The diarrheal type has an incubation period of 8-16 h and is characterized by symptoms such as diarrhea, dizziness, and abdominal pain. The emetic type has a short incubation period of several hours.
Farmstead cheeses are produced in small-scale operations, and a lack of food safety knowledge and mechanization has led to foodborne pathogens being isolated from these cheeses in Korea. However, the pathogenic characteristics of these isolates are not yet well-known. Therefore, the objective of this study was to determine the pathogenic characteristics and antibiotic resistances of foodborne pathogens from farmstead cheese.
Materials and Methods
Twenty-seven isolates from farmstead cheeses, including two E. coli isolates from mozzarella cheese, 18 B. cereus isolates from string, mozzarella, cottage, berg, colby, and gouda cheeses, and seven S. aureus in string, mozzarella, cottage, quark, and gouda cheeses, were obtained from our previous study. To extract the chromosomal DNA of the isolates, colonies of B. cereus, E. coli, and S. aureus was suspended using a sterilized loop in separate microtubes containing 30 μL of sterile distilled water, followed by placement of the tubes in a heating block at 99°C for 10 min and cooling them for 2 min at room temperature. After cooling, centrifugation (14,000 rpm, 3 min) was performed, and the supernatants were further used for PCR. To determine the pathotypes (EHEC, EPEC, ETEC, EAEC, and EIEC) of E. coli isolates, PowerChekTM Diarrheal E. coli 8-plex Detection Kit (Kogen Biotech, Seoul, Korea) was used according to the manufacturer’s protocol. To distinguish diarrheal and emetic type B. cereus isolates, the diarrheal type (hbIC, nheA, cytK, bceT, and entFM) genes and emetic type (CER) genes were investigated by PowerChek TMBacilluscereus Toxin 6-plex Detection Kit (Kogen Biotech) according to the manufacturer’s instruction. To determine if S. aureus isolates were methicillin-resistant S. aureus (MRSA), Fastmix Kit (iNtRON Biotechnology Inc., Korea) was used for the S. aureus isolates with mecA primers, and the thermal condition suggested by the manufacturer was slightly modified as follows; denaturation at 94°C for 2 min, followed by 35 cycles of denaturation at 94°C for 20 s, annealing at 63°C for 10 s and extension at 72°C for 20 s, and final 72°C for 3 min.
A colony for each B. cereus isolate was inoculated into 10 mL tryptic soy broth (TSB; Becton, Dickinson and Company, USA), and incubated at 30°C for 24 h. Then, 0.1 mL of the culture was transferred into 10 mL fresh TSB and incubated at 30°C for 24 h. The culture was centrifuged (1,912 × g, 15 min, and 4°C), washed twice with phosphate buffered saline (PBS, pH 7.4; 0.2 g of KH2PO4, 1.5 g of Na2HPO4·7H2O, 8.0 g of NaCl, and 0.2 g of KCl in 1 L of distilled water), and resuspended in PBS. The suspensions were then adjusted to OD600=0.1.
A disc diffusion assay was performed according to the protocol described by Bauer et al. (1966). The bacterial inoculum was smeared on the surface of Muller Hinton Agar (MHA; Becton, Dickinson and Company) using a sterile cotton swab. After drying for 10 min, six different antibiotic discs (Oxoid Ltd.) such as ampicillin (10 μg), oxacillin (1 μ g), erythromycin (15 μg), penicillin G (10 units), tetracycline (30 μg) and vancomycin (30 μg), were placed on the medium, followed by incubation at 30°C for 24 h, and the sizes of their inhibition zones (mm) were measured. The antibiotic resistance of B. cereus isolates was determined as described by Bauer et al. (1966) and Brown (2001).
Results and Discussion
Pathotype analysis of two E. coli isolates revealed neither of them to be pathogenic (Fig. 1A). Of the 18 B. cereus isolates, 17 isolates (94.4%) were diarrheal type and one isolate (5.6%) was emetic type, suggesting that the diarrheal type B. cereus is more prevalent in farmstead cheeses in Korea. In contrast, Kim et al. (2010) previously reported that the foodborne illnesses related to B. cereus reported in Korea were primarily caused by the emetic type. Among the diarrheal type toxin genes, nheA (100%), entFM (88.9%), cytK (72.2%), hbIC (66.7%), bceT (66.7%), and CER (5.6%) were detected (Fig. 1B). In a study by Owusu-Kwarteng et al. (2017), HBL complex genes (hbIA, hbIC, and hbID) of hemolytic enterotoxin and NHE complex genes (nheA, nheB, and nheD) of non-hemolytic enterotoxin were analyzed to identify the toxin type of B. cereus isolates from dairy farms and traditional dairy products. The results showed that more NHE complex genes (60%) were detected than HBL complex genes (13%), which is similar to the results of our study. This result indicates that most of the diarrheal type B. cereus produce hemolytic and non-hemolytic enterotoxin. B. cereus foodborne outbreaks caused by cheese consumption have not yet been reported in Korea, possibly because of the very short history of farmstead cheese production. Since this industry is relatively new in Korea, attention from the government and consumers may not be intensive. Hence, they may fail to detect outbreaks of diseases caused by B. cereus. B. cereus can be isolated easily from dairy farm environments. In particular, B. cereus spores can be cross-contaminated from dairy farms to the farmstead cheese production process, and the spores survive the process because they are very resistant to harsh environments. Once the spores germinate, they may cause foodborne illnesses. In addition, all B. cereus isolated from our previous study possessed toxin genes. Thus, B. cereus is a very critical foodborne pathogen to be controlled in farmstead cheese.
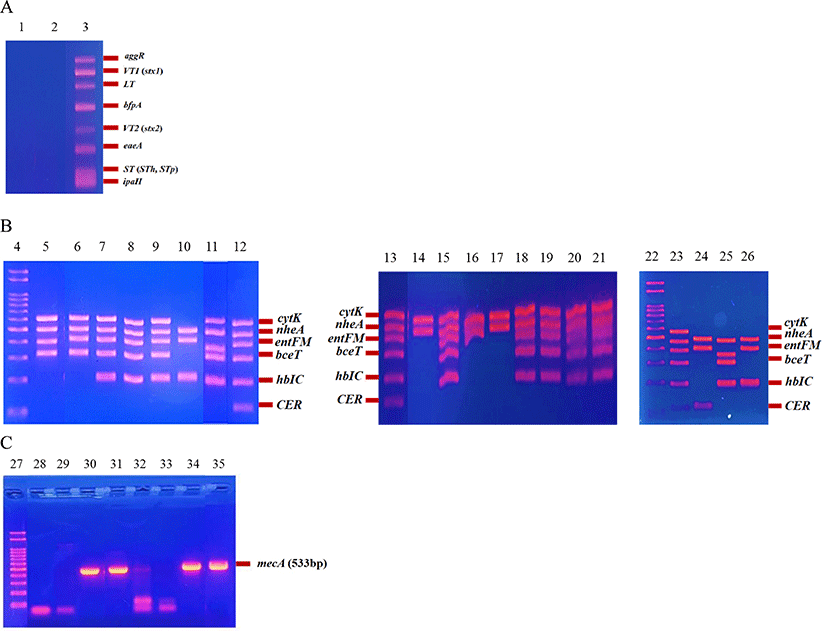
For detection of MRSA-associated mecA, three (42.9%) S. aureus isolates were positive, which had been isolated from string, gouda, and cottage cheeses (Fig. 1C). Haran et al. (2012) investigated the prevalence of MRSA in dairy herds, and the positive rate was only 4%. Cortimiglia et al. (2015) reported that the detection rate of MRSA in bulk tank milk from dairy goat farms in Northern Italy was 2%. Compared to these studies, our detection rate of MRSA 42.9%) is very high. This is a very concerning result for farmstead cheese in Korea. MRSA contamination in farmstead cheese could be cross-contaminated by workers and from equipment during the process. Although even contamination of S. aureus in farmstead cheese is a problem, the high MRSA rate of S. aureus isolates makes this problem worse. Thus, food safety approaches for farmstead cheese must be extended.
Among the bacterial isolates, including E. coli, B. cereus and S. aureus, E. coli isolates were determined by PCR to be non-pathogenic, and PCR assays were performed to identify MRSA strains among the S. aureus isolates, as well as the typical pathogenic characteristics of S. aureus. Only 18 B. cereus isolates were subjected to the antibiotic resistance assay. Five antibiotics except for oxacillin were also examined in a study by Bauer et al. (1966), and the antibiotics are considered typical antibiotics for the antibiotic disc diffusion assay, and oxacillin is also used for determination of antibiotic resistance in Gram-positive bacteria (Brown, 2001). Most B. cereus isolates showed similar antibiotic resistance patterns (Table 1). Specifically, most isolates of B. cereus exhibited resistance to oxacillin (94.7%), penicillin G (94.7%), and ampicillin (26.3%), which are β-lactam antibiotics. For erythromycin, tetracycline, and vancomycin, B. cereus presented lower resistant rates of 5.3%, 10.5%, and 5.3%, respectively, compared to those for the former three antibiotics. These results are consistent with previous studies (Jensen et al., 2001; Park et al., 2009).
1)AMP 10, Ampicillin 10. 2)OX 1, Oxacillin 1 mcg. 3)E 15, Erythromycin 15. 4)P 10, Penicillin G 10 unit. 5)TE 30, Tetracycline 30. 6)VA 30, Vancomycin 30. 7)R, Resistant. 8)S, Suceptible. 9)I, Intermediate.
Zone diameter of AMP 10: R≤11, I=12-13, S≥14; Zone diameter of OX 1: R≤14, S≥15; Zone diameter of E 15: R≤13, I=14-17, S≥18; Zone diameter of P 10: R≤20, I=21-28, S≥29; Zone diameter of TE 30: R≤14, I=15-18, S≥19; Zone diameter of VA 30: R≤9, I=10-11, S≥12; Zone diameter of B. cereus isolates was interpreted according to the studies by Bauer et al. (1966) and Brown (2001).
Contamination of foodborne pathogens in farmstead cheese can be a serious food safety problem by itself, but the S. aureus and B. cereus isolates from farmstead cheeses also had obvious pathogenic characteristics, as well as antibiotic resistance. Therefore, dramatic improvements in the processing of farmstead cheese are required to make it safe for consumption.













