Introduction
Meat products are valuable sources of protein, essential amino acids, crude fat and various nutrients such as minerals. And recently there has been a rapid increase in consumer demand for meat worldwide (Ursachi et al., 2020). Dried meat food products have the advantage of being conveniently consumed due to their small size compared to their rich protein content, and the salting and drying processes greatly extend the shelf life of products by inhibiting the growth of microorganisms and other bacteria thanks to their low water activity (Mediani et al., 2022). Additionally, dried meat products are manufactured by seasoning prepared meat with spices and additives, then preserving it through low temperature drying and smoking, which makes the production process relatively simple (Konieczny et al., 2007). Therefore, the consumption of these dried meat products has gained popularity due to the ever-changing preferences of consumers, their growing interest in high-protein foods, and the desire for convenience (Aykın Dinçer, 2023).
Among these options, 'Pemmican' is a dried meat product made by combining dried meat with animal fat (Ngapo et al., 2021). Throughout history, pemmican has been a popular choice for providing a convenient source of nutrition during arduous travels or extended periods of labor in harsh climates (Kark et al., 1945). It offers a concentrated dose of energy, thanks to its high fat and protein content, and can be further enriched with vitamins and minerals by incorporating berries (Merriam, 1955). Additionally, pemmican is easy to carry and has excellent storage stability at room temperature.
Compared to dried meat products obtained from other meat sources, products derived from beef are highly popular due to their rich flavor and versatility (Aung et al., 2023). In this study, Hanwoo, a premium Korean beef, was selected as the meat for making pemmican. Renowned for its excellent marbling, tenderness, and rich flavor, Hanwoo significantly enhances the quality of the final meat product (Joo et al., 2017). Its high-quality protein and favorable fat composition make it an ideal choice for meat products, contributing to both nutritional value and taste. As a result, many studies are being conducted to incorporate it into meat products.
However, excessive intake of saturated fats found in animal fats can increase low-density lipoprotein (LDL) cholesterol levels, leading to obesity, diabetes, hypertension, and various cardiovascular diseases (Maki et al., 2021). Therefore, the World Health Organization (WHO) and Food and Drug Administration (FDA) recommend reducing the content of saturated fatty acids (SFAs) by including unsaturated plant oils to prevent chronic diseases (De Vogli et al., 2014). Additionally, incorporating vegetable oils into meat products has been identified as an effective approach for lowering cholesterol and SFA content, while also enhancing the levels of natural antioxidants such as tocopherols, β-carotene, and various phenolic compounds (Rodríguez-Carpena et al., 2012). This, in turn, improves the nutritional value of these products. As a result, the food industry has been actively researching the substitution of animal fats with vegetable oils.
Canola oil (CA) is a vegetable oil derived from the genus Brassica in the Cruciferae family (Chew, 2020). It is known for having the lowest SFA content among commercially available edible oils, with 5%–8% SFAs, 30%–35% polyunsaturated fatty acids (PUFAs) and 60%–65% monounsaturated fatty acids (MUFAs; Goyal et al., 2021). The high concentration of unsaturated fatty acids in CA, such as oleic acid and linoleic acid, has been shown to reduce levels of LDL cholesterol, thus contributing to a lower risk of diseases like heart disease and diabetes (Okuyama et al., 2016). In addition to its appropriate fatty acid composition, CA also contains natural antioxidants such as various phenolic compounds and tocopherols, specifically the γ-isomer. These antioxidants have the ability to inhibit spoilage (Przybylski et al., 2005). However, effectively incorporating plant-based oils with a high proportion of PUFA, such as CA, into products presents challenges. These challenges include the oxidative instability caused by their high unsaturation and the fluidity of vegetable oils causes physical instability (Jiang and Xiong, 2015). As a result, researchers face the significant challenge of providing oxidative stability while utilizing vegetable fats that contain a high amount of unsaturated fatty acids. Many studies are being conducted to propose the most suitable fatty acid composition and ratio from both health and quality perspectives.
The objective of this study is to enhance the fatty acid composition of pemmican by substituting beef tallow with CA. Furthermore, we aim to determine the optimal concentration of CA that maintains the physicochemical properties and storage stability of the product. The findings of this research will serve as valuable foundational data for future studies on pemmican.
Materials and Methods
Hanwoo top round meat and beef tallow were obtained from a butcher shop located in Chungcheongbuk-do. Raisins (Raisin, Nutree, Paju, Korea), dried blueberries (Songrim Food, Gimpo, Korea), dried cranberries (Dried Cranberries, Nuts Farm, Gwangju, Korea), and CA (CJ, Seoul, Korea) were used.
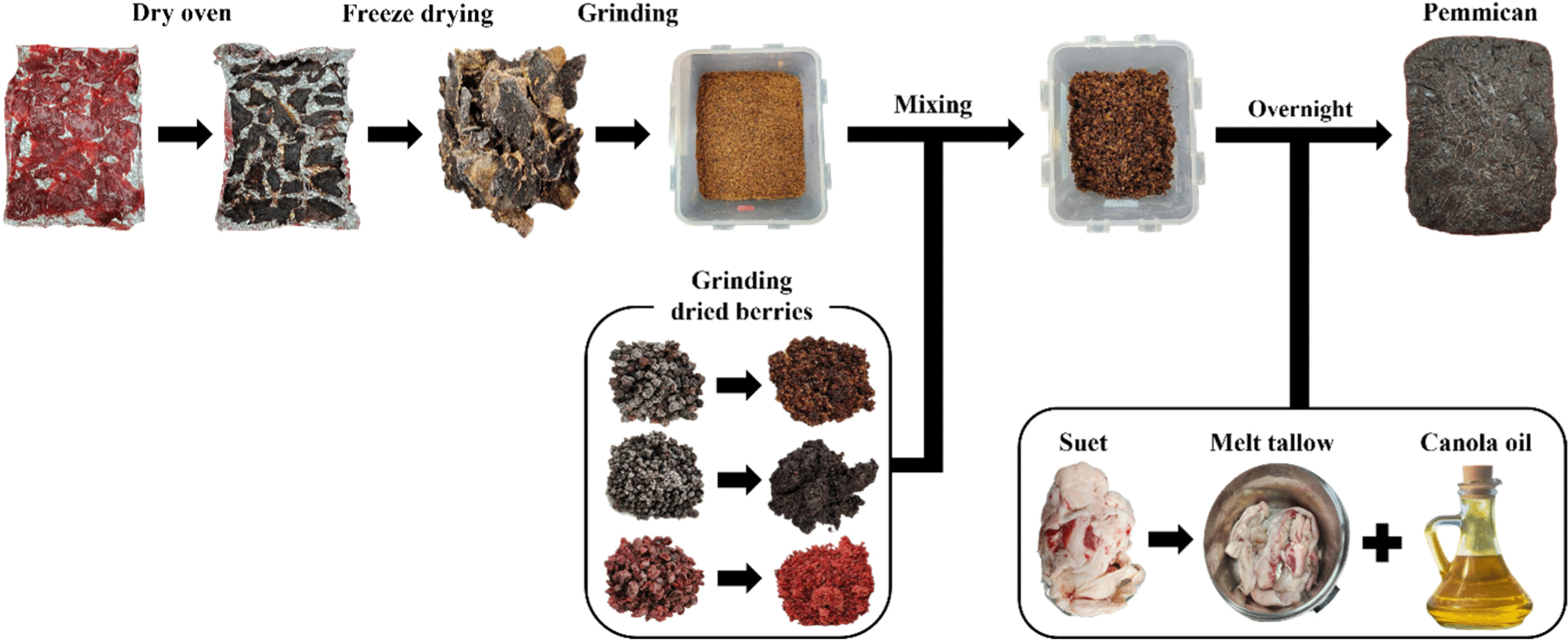
The process of manufacturing pemmican is shown in Fig. 1. First, the Hanwoo top round meat was thinly sliced 20×0.5×20 cm (L×W×H) and then cut into wide slices before being dried in a 77°C dry oven (SH-DO-360 FH, Samheung, Seoul, Korea) for 17 h. Next, the dried meat was freeze-dried using a freeze dryer (FDU-2100, EYELA, Tokyo, Japan). The meat was dried twice to prevent ice crystal formation and to improve drying efficiency during freeze-drying. The dried meat, raisins, dried blueberries, and dried cranberries were ground using a blender (HMF-4010SS, Hanil Electric, Seoul, Korea) and mixed together. Beef fat was rendered at 120°C for about 30 min to produce beef tallow, which was then double-strained through two stain-resistant sieves to remove impurities. Afterwards, the mixture and the beef tallow in liquid form, along with CA added according to the blending ratios shown in Table 1, were mixed and shaped before being frozen overnight at –20°C. Finally, the frozen mixture was cut into samples measuring 2×2×4 cm (L×W×H) for the experiment. A total of six treatment groups (CON, beef tallow 25%; CA1, beef tallow 22.5%+CA 2.5%; CA2, beef tallow 20%+CA 5%, CA3, beef tallow 17.5%+CA 7.5%; CA4, beef tallow 15%+CA 10%; CA5, beef tallow 12.5%+CA 12.5%) were manufactured.
The method described by Lepage and Roy (1986) was used to methylate the samples at 100°C for 1 h. Hexane was added after cooling to separate the fatty acid methyl esters. The upper layer of the sample was collected. A gas chromatograph with a capillary column (100 m×0.25 mm i.d.×0.20 μm film thickness) was utilized to quantify the fatty acid methyl esters. The carrier gas used was nitrogen. The initial oven temperature was maintained at 180°C, and the final temperature was maintained at 240°C (2°C per min). The temperatures of both the injector and detector were kept at 250°C.
The contents of moisture, crude fat, crude protein, crude ash and carbohydrates were measured using AOAC (2012). The 105°C air oven drying method was used to determine the moisture content, crude protein content was analyzed using the Kjeldahl method, crude ash content was determined using the dry ashing method at 550°C and crude fat content was determined using the Folch method, and the carbohydrate content was determined by subtracting the moisture, crude ash, crude fat, and crude protein from the sample, as outlined in the method by Hussain et al. (2009).
The color of the inner surface of the pemmican was measured using a standardized Spectro colorimeter (CM-26d, Konica, Tokyo, Japan) against a white plate (CIE L*, 89.39; CIE a*, 0.13; CIE b*, –0.51). The CIE L*, CIE a*, and CIE b* values were obtained, and used a D65 illuminant.
Samples were placed in moisture activity sample cups, sealed, and equilibrated at room temperature for 12 h to ensure consistent experimental conditions. Water activity was then measured using an AquaLab 4TE (METER Group, Pullman, WA, USA).
Pemmican cubes, measuring 1.00×1.00×1.00 cm (L×W×H), were analyzed using a rheometer (Model Compac-100, Sun Scientific, Tokyo, Japan). The probe utilized had an area of 3.14 cm2, with a load cell weight of 10 kg and a cross-head speed of 200 mm/min. To determine the hardness, springiness, cohesiveness, chewiness, and gumminess, the calculations followed the methodology outlined by Bourne (1978).
To measure the pH value of pemmican, 6 g of sample was mixed with 54 mL of distilled water, homogenized at 17,740×g for 60 s using a Bihon Seiki Ace homogenizer (Osaka, Japan), and subsequently measured using a pH meter (Orion StarTM A211, Thermo Scientific, Waltham, MA, USA).
2-Thiobarbituric acid reactive substance (TBARS) were measured using the method described by Witte et al. (1970). A 10 g sample was homogenized with 70% perchloric acid (Samchun Chemicals, Pyeongtaek, Korea) diluted to make 10% perchloric acid 15 mL, and then 20 mL of distilled water, at 17,740×g for 30 s. The homogenate was then filtered through Whatman No. 2 filter paper to obtain the filtrate. Next, 5 mL of the filtrate was mixed with 5 mL of 2-thiobarbituric acid (Sigma Aldrich, Darmstadt, Germany) and left to stand in the dark for 16 h. After 16 h, absorbance was measured at 529 nm using a Spectrophotometer (mobi, MicroDigital, Seongnam, Korea). The standard curve for malondialdehyde used in the experiment was calculated with x-0.0011 (r=0.999), y=0.1975, where x=TBARS value and y=absorbance.
Pearson (1968) was used for measuring volatile basic nitrogen (VBN) levels. Initially, a 3 g sample was homogenized with 45 mL of distilled water at 17,740×g for 60 s. The resulting mixture was then filtered through Whatman No. 2 filter paper. Subsequently, 3 mL of the filtrate was transferred to the outer chamber of a conway unit. In the inner chamber, 1 mL of 0.01 M appropriate reagent (Sigma Aldrich) and 4 drops of indicator solution (0.066% methyl red+0.066% bromocresol green) were added. Additionally, 1 mL of 50% K2CO3 (Samchun Chemicals, Pyeongtaek, Korea) was added the outer chamber. The mixture was allowed to culture at 37°C for 120 min. Following culturing, the solution in the inner chamber was titrated with 0.01 M sulfuric acid. Ultimately, VBN was quantified as mg per 100 g of sample (mg%).
A: the amount of sulfuric acid injected (mL)
B: the amount of H2SO4 injected into the blank (mL)
F: 0.02 N H2SO4 standardized index
28.014: amount of N required to titrate 1 mL of 0.02 N H2SO4
The experiment results were analyzed with three or more repetitions, and all statistical analyses were conducted using SPSS (26.0, IBM, Armonk, NY, USA). To compare the significance of treatment groups and storage periods, one-way analysis of variance (ANOVA) analysis was performed, followed by one-way ANOVA and Duncan’s multiple range test (p<0.05) for mean and SD.
Results and Discussion
The fatty acids composition of pemmican, in which beef tallow was replaced with CA is shown in Table 2. As the level of CA increased, there was a significant decrease in the content of SFA, while the content of MUFA and PUFA significantly increased (p<0.05). The main SFA in pemmican were stearic acid (C18:0) and palmitic acid (C16:0), while the major unsaturated fatty acids (UFA) were oleic acid (C18:1n9) and linoleic acid (C18:2n6). These findings align with the results reported by Lee et al. (2010), which indicated a similar fatty acid composition in Hanwoo beef fat and pemmican. CA is known to primarily contain UFA, with oleic acid at 62.41% and linoleic acid at 20.12% (Zambiazi et al., 2007). The increase in the content of these fatty acids in the CA treatment groups can be attributed to the high ratio of oleic acid and linoleic acid in CA. This corresponds to the results reported by Koo et al. (2009), which demonstrated an increase in UFA, such as oleic acid, in hamburger patties produced with CA, and the results reported by Moon et al. (2021), which showed an increase in MUFA with increasing CA content in emulsified sausages produced with varying ratios of horse fat and CA. However, it should be noted that UFA are relatively susceptible to oxidation compared to SFA, which poses a risk of reducing fat hardness and deteriorating fat color during storage (DeLany et al., 2000). Therefore, it is necessary to determine an appropriate ratio of mixed oils to ensure oxidative stability.
The quality characteristics of pemmican, in which beef tallow was replaced with CA is shown in Table 3. Among all the treatment groups, there were no significant differences observed in moisture, crude fat, crude protein, crude ash and carbohydrate content (p>0.05). These findings are in line with previous studies that found no significant differences in the proximate composition of pork patties when 50% of animal fat was replaced with plant-based oil, compared to the control group (Lu et al., 2017). Furthermore, when animal fat was replaced with CA in hamburger patties, there were no significant differences observed in the proximate composition compared to the control group (Koo et al., 2009). Additionally, the fat and protein content in beef burgers with added CA did not differ significantly from the control group (Onopiuk et al., 2022). Therefore, it can be concluded that replacing up to 12.5% of animal fat with CA does not affect the proximate composition of pemmican.
CON, beef tallow 25%; CA1, beef tallow 22.5%+CA 2.5%; CA2, beef tallow 20%+CA 5%; CA3, beef tallow 17.5%+CA 7.5%; CA4, beef tallow 15%+CA 10%; CA5, beef tallow 12.5%+CA 12.5%.
In terms of color, no significant differences were observed in CIE L* and CIE a* among the treatment groups (p>0.05). Pemmican inherently exhibits a very dark color. Therefore, the addition of CA does not seem to significantly affect the CIE L* and CIE a*. The lowest CIE b* value was observed in the CON group, and there were no significant differences in the CA 1–5 groups (p>0.05). The CIE b* increased as the level of CA increased, suggesting that the yellow hue of CA itself may have influenced the color. CA's CIE b* hue is known to be caused by natural pigments like carotenoids and chlorophylls found in oil (Przybylski et al., 2005), and these compounds have been reported to impact the CIE b* of meat products (Bolognesi and Garcia, 2018). However, the color of meat products is primarily affected by variations in raw materials rather than changes in color due to the type of animal fat used. Therefore, the color changes in pemmican are considered minimal.
Water activity is a critical parameter in food that affects stability, microbial reactions, and the types of microorganisms present (Tapia et al., 2020). Dried meat products need to maintain a stable aw to prevent quality changes during storage (Sun et al., 2002). In all treatment groups, the aw values of pemmican were consistently low, at 0.40 or below. Furthermore, there was a decreasing trend in aw with increasing levels of CA addition, with CA5 showing significantly the lowest value (p<0.05). Animal fat is retained more efficiently within the protein matrix, and its particles act as a barrier against water, allowing the meat to retain moisture better (Kumar, 2021). Therefore, it is determined that as the level of beef tallow decreases, aw decreases. Low moisture activity foods are often lightweight and stable at room temperature, making them convenient for consumers as they can be easily carried and stored at ambient temperature, such as snacks, dried fruits, and jerky. The results of this study suggest that as the level of CA addition increases, there is a decrease in aw, indicating better inhibition of microbial growth and quality changes. This implies that the addition of CA contributes to enhancing storage safety, extending shelf life, and preserving product quality.
Hardness, springiness, chewiness, and gumminess showed a decreasing trend as the level of CA increased. However, cohesiveness did not exhibit any significant differences (p>0.05). These findings are consistent with Park et al. (2005), who observed that replacing animal fat with vegetable oil reduced the hardness of pork patties. Wood et al. (2004) also reported that the hardness of adipose tissue is greatly influenced by the physical properties of fat, which are determined by fatty acids, and this can impact the meat quality. The major fatty acids composing pemmican have specific melting points: palmitic acid (16:0) at 62°C, stearic acid (18:0) at 70°C, oleic acid (18:1) at 13°C, and linolenic acid (18:3) at –11°C (Knothe and Dunn, 2009). It is inferred that the decrease in hardness is due to the inability of fat to retain moisture because of the low melting point of unsaturated fatty acids. Furthermore, it has been reported that hardness decreases as the ratio of unsaturated fatty acids, which have weak intermolecular forces due to their molecular structure, increases compared to SFAs (Hur et al., 2005) and it has been reported that adding vegetable oil to meat products can soften the protein matrix structure, resulting in a smoother texture (Cho et al., 2023). Therefore, the results of this study suggest that pemmican, in which animal fat was replaced with CA containing high levels of unsaturated fatty acids, will exhibit a softer texture compared to the CON.
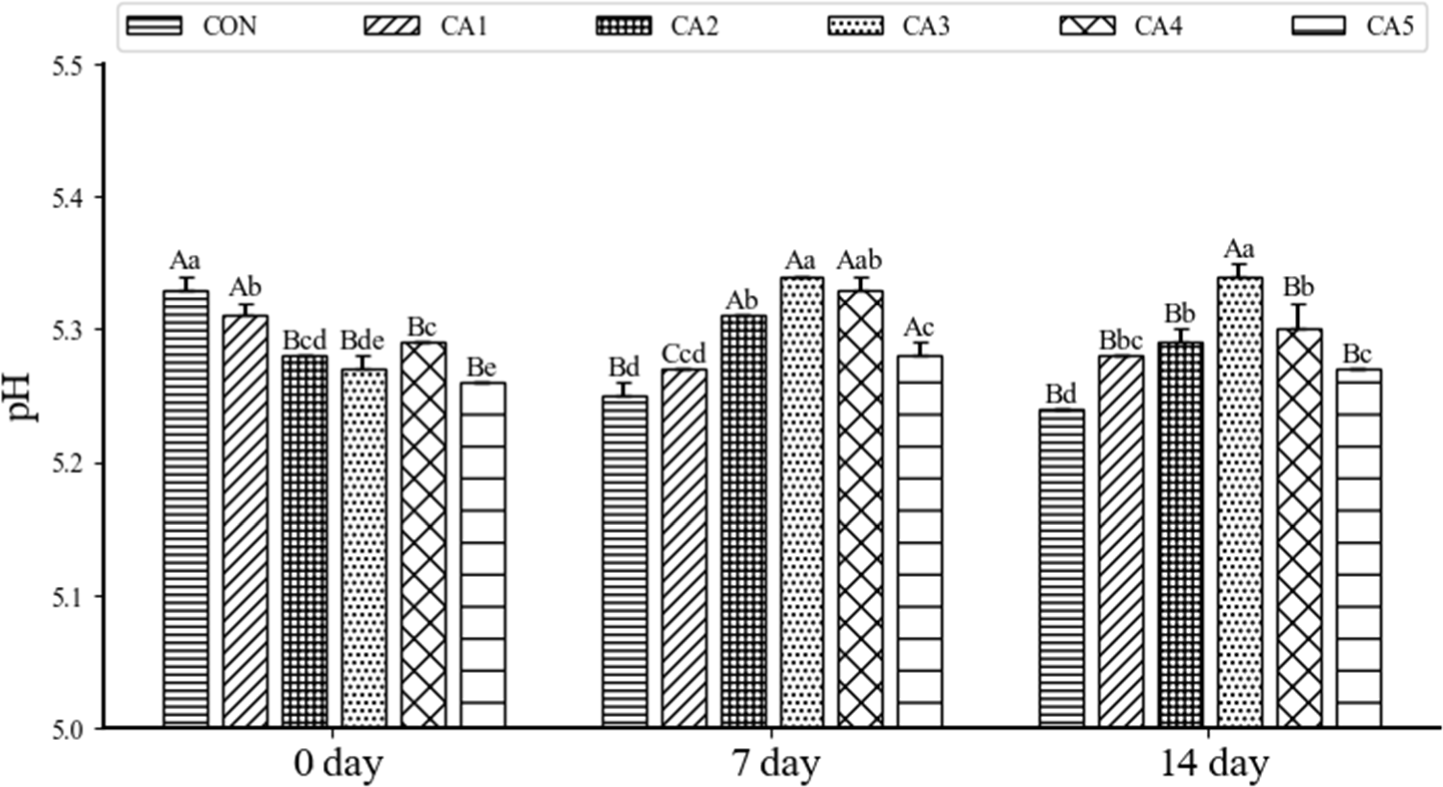
The pH of pemmican, in which beef tallow was replaced with CA, during the 14 days of storage is shown in Fig. 2. The pH of meat products is a significant factor in evaluating freshness, color, and shelf life, serving as an important indicator for assessing the freshness of meat products (Tamkutė et al., 2021). The treatment groups with added CA exhibited significantly lower pH values compared to the CON group at day 0 (p<0.05). This finding aligns with the results of a study by Lee et al. (2015), which observed a decrease in pH of sausage emulsion with 16% CA addition, replacing animal fat with vegetable oil. This decrease in pH enhances the safety of meat products by deactivating pathogens and inhibiting quality changes caused by spoilage microorganisms, thereby improving product stability and extending shelf life (Ammor and Mayo, 2007). Significant increases in pH were observed in the CA2, CA3, CA4, and CA5 treatment groups until day 7 (p<0.05), followed by a decreasing trend at day 14. Hydrolysis, a chemical reaction that breaks the ester bonds of triglycerides in vegetable oils, results in the formation of free fatty acids and glycerol, which can subsequently lower the pH of the product (Fakhri and Qadir, 2011). Therefore, it can be predicted that triglyceride hydrolysis of CA occurred from day 7 onwards. This is consistent with reported results showing that the pH increased until the 7th day and then decreased in press ham with added vegetable oil (Dzudie et al., 2004), as well as in beef patties with animal fat replaced by brown rice oil and olive oil, where the pH increased until the 7th day of storage (Seo et al., 2011).
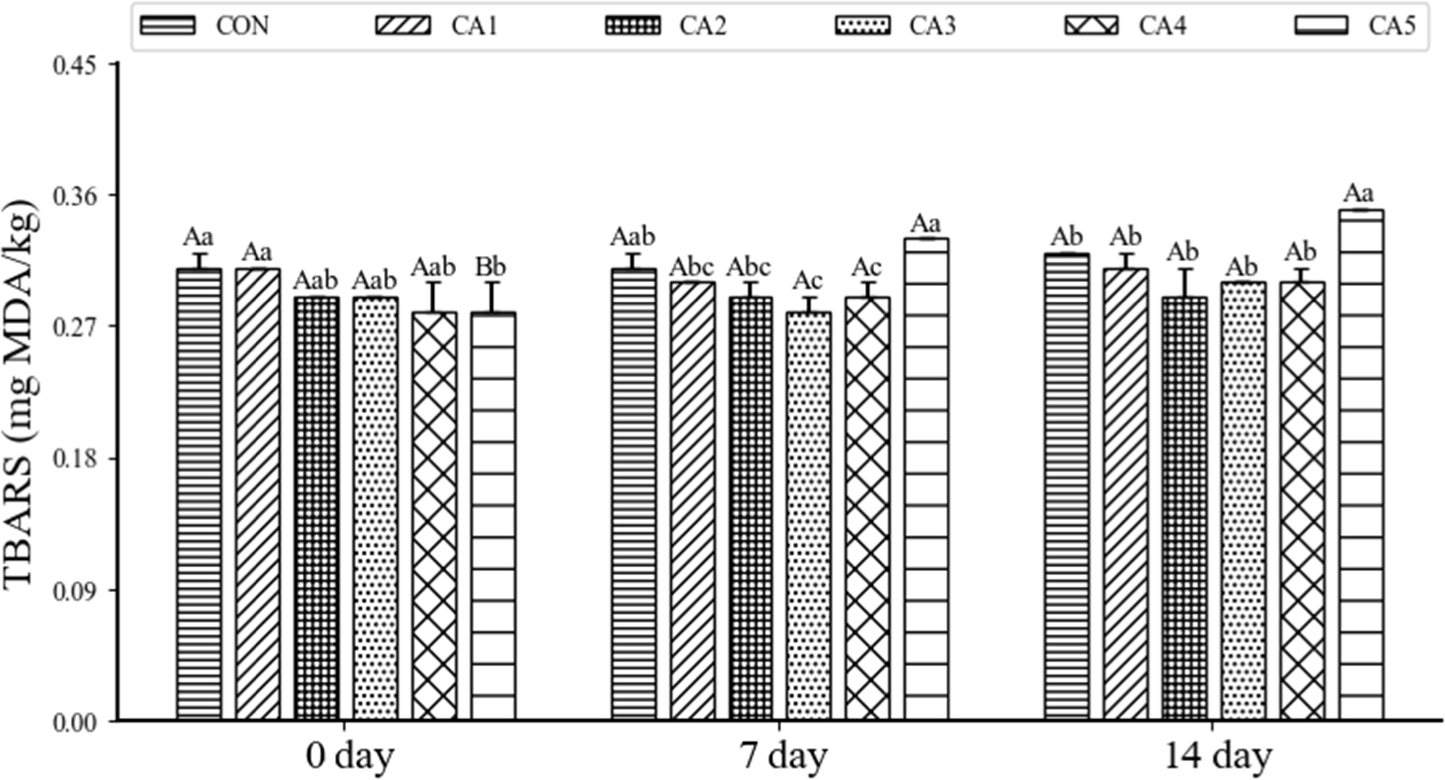
The TBARS values of pemmican, in which beef tallow was replaced with CA, during the 14 days of storage is shown in Fig. 3. A major cause of quality deterioration in meat products is lipid oxidation, which leads to undesirable changes in nutritional value, taste, appearance, and texture, and can potentially generate toxic substances (Sun et al., 2011). In the case of TBARS in pemmican, there was a decreasing trend in TBARS values as the level of CA increased at day 0, and no significant increase in TBARS values was observed as the storage period elapsed in the other treatment groups, except for CA5 (p>0.05). CA contains a significant amount of tocopherol, also known as vitamin E (Matthaus et al., 2016). α-tocopherol primarily protects unsaturated fatty acids from lipid radicals (Monahan et al., 1992). Therefore, higher levels of α-Tocopherol in meat products indicate better antioxidant activity, enhancing oxidative stability. Carotenoids present in CA also scavenge peroxyl radicals, protecting PUFA from oxidation and stabilizing carbon-centered radicals by resonance (Domínguez et al., 2019). However, CA5 showed significantly the highest values at both day 7 and day 14 (p<0.05). Unsaturated fatty acids are more susceptible to lipid oxidation compared to SFAs (Rael et al., 2004). The oxidation of PUFA deteriorates the color, flavor, and quality of meat (Adeyemi and Olorunsanya, 2012). This aligns with the reported decrease in oxidative stability when using vegetable fats in meat products (Kılıç and Özer, 2019). The lipid peroxidation inhibition provided by phenolic compounds can help reduce oxidative stress at low replacement ratios. However, as the amount of oil and unsaturated fatty acids increases, so does oxidative sensitivity, which can diminish this benefit, and finding the optimal ratio is crucial (Xu et al., 2015). Therefore, it can be inferred that lipid oxidation occurred due to the susceptibility of unsaturated fatty acids to spoilage when CA was replaced at levels above 10%.
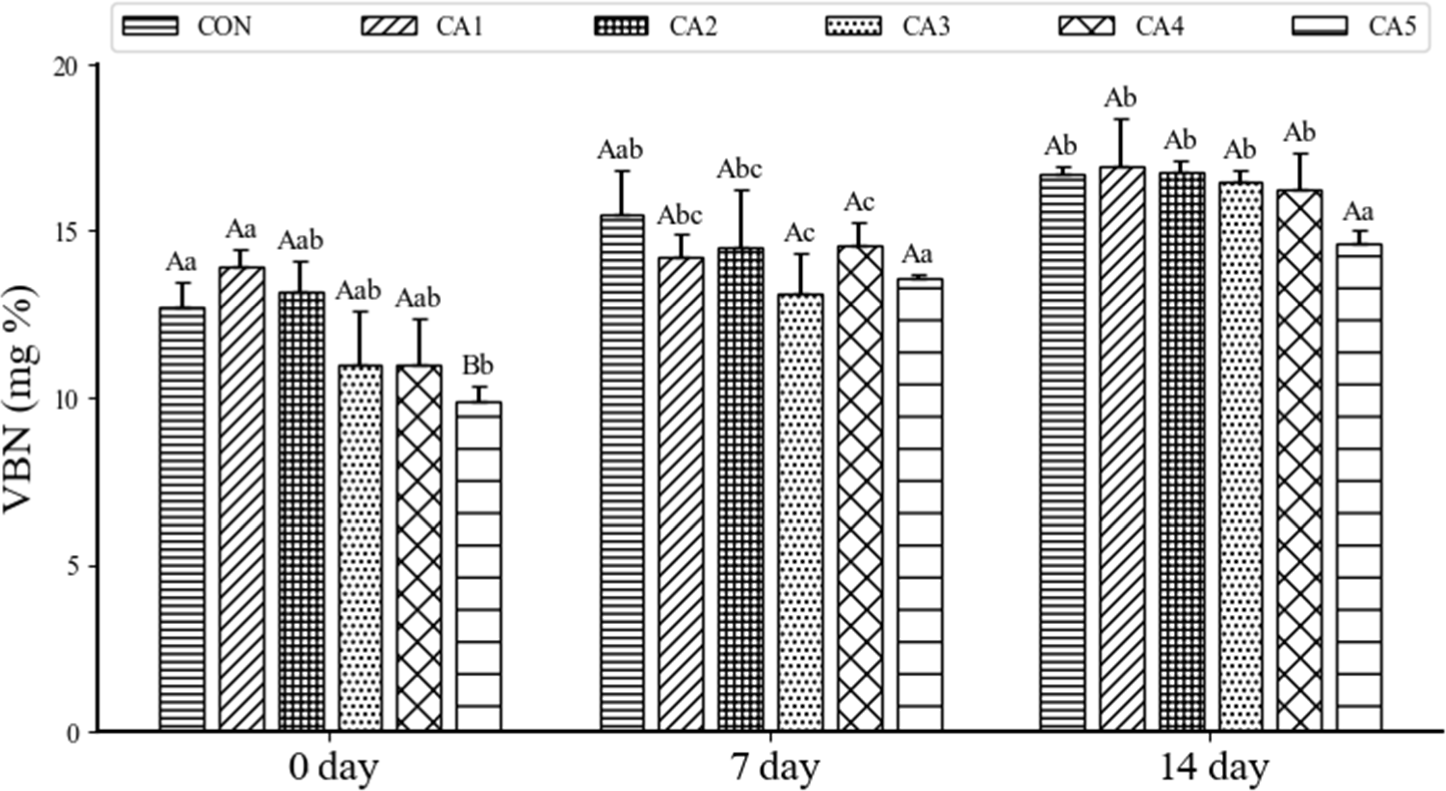
The VBN values of pemmican, in which beef tallow was replaced with CA, during the 2 weeks of storage is shown in Fig. 4. VBN is a numerical indicator that measures the presence of volatile amines like ammonia nitrogen and trimethylamine. It reflects the freshness of meat during refrigerated storage, and in South Korea, the permissible limit for VBN in meat products is regulated to be 20 mg% (Jeon and Choi, 2012). For pemmican, there was a decrease in VBN as the proportion of CA increased, both at 0 and 14 days. This can be attributed to the antimicrobial and antioxidant effects of phenolic compounds and tocopherol present in CA, which inhibit protein degradation (Li et al., 2021). Also, the aldehydes and ketones generated from the oxidation of fatty acids affect the quality and shelf life of meat, and these compounds can influence VBN levels (Geng et al., 2024). These results suggest that the decrease in TBARS also likely contributed to the reduction in VBN levels. These findings are consistent with studies that found low VBN values in ground pork with added vegetable fats such as pork fat, olive oil, and soybean oil (Youn et al., 2007). In conclusion, replacing animal fat with CA in pemmican appears to reduce the VBN content, thereby improving the product's shelf life.
Conclusion
This study aimed to improve the fatty acid composition of a dried meat product called pemmican by replacing beef tallow with CA. The study also examined the quality characteristics and storage stability of pemmican based on the level of CA substitution.
The proximate composition of pemmican with CA replacing animal fat did not vary significantly across all treatment groups. However, as the proportion of CA increased, the pH and water activity (aw) decreased. The addition of CA did not impact the CIE L* of the product but did slightly increase its CIE b*. Furthermore, as the level of CA increased, the hardness, springiness, chewiness, and gumminess of the pemmican decreased, resulting in a softer texture. The substitution of animal fat with CA led to an increase in MUFAs and PUFAs content and a decrease in SFAs content and notably, there was a significant increase in oleic acid and linoleic acid content. Storage evaluation conducted at 4°C on days 0, 7, and 14 showed no significant differences in TBARS, except for the CA5 treatment. In terms of VBN, a decreasing trend was observed with increasing levels of CA addition.
In conclusion, replacing animal fat with CA in the production of pemmican improves the fatty acid composition and enhances stability against microbial growth, thanks to the decreased pH and aw. Additionally, it inhibits protein degradation and lipid oxidation, although an increase in TBARS was observed in the CA5 treatment, indicating lipid deterioration. Overall, substituting animal fat with CA in pemmican increases the content of unsaturated fatty acids, suggesting superior nutritional quality. The CA4 treatment at a concentration of 10% is considered the most optimal.













