Introduction
The use of probiotic lactic acid bacteria (LAB) is a current topic of interest and a growing trend in the dairy industry. Probiotic bacteria are primarily used to manufacture dairy products. As reported by several authors, cheese is an excellent medium for addition of these probiotics. However, individual probiotic strains should be evaluated to determine whether they alter the sensory characteristics of a cheese, and to determine the effects of cheese production and storage on survival of the probiotic cells (Grattepanche et al., 2008; Vinderola et al., 2009; Yerlikaya and Özer, 2014). Cheese has been shown to be a good medium for transfer of probiotics into the intestine, as the cheese creates a buffer against the highly acidic conditions in the gastrointestinal tract (GIT) and thus creates a favorable environment for bacterial survival during gastric transit (Karimi et al., 2012a; Karimi et al., 2012b; Ortakci et al., 2012). Supplementation of cheeses with probiotic LAB adds value and provides potential health benefits (Gomes et al., 2011; Minervini et al., 2012). Intake of cheese supplemented with probiotic bacteria has been associated with a variety of health-promoting benefits, such as immune system improvement, oral and gut health effects in the elderly, prevention of food allergies, and strengthening of intestinal immunity (Albenzio et al., 2013a; Albenzio et al., 2013b; Hatakka et al., 2007; Ibrahim et al., 2010; Lollo et al., 2012; McFarland, 2000; Medici et al., 2004; Modzelewska-Kapituła et al., 2010). In a previous study, we isolated probiotics from fecal samples of healthy Korean neonates. We have used one of the bacteria isolated in the previous study in this study, Bifidobacterium longum KACC 91563, a subspecies of B. longum, as it is a well-known probiotic strain that exhibits positive host effects (Shanahan, 2010). In addition, B. longum KACC 91563 produces family 5 extracellular solute-binding protein (ESBP), which not only reduces food allergies (Kim et al., 2016), and also exhibits antioxidant activity (Chang et al., 2013), and capacity for production of antihypertensive peptides (Ha et al., 2015) by degrading milk proteins. Kwark, also known as quark or quarg, is a natural, soft, white, and un-ripened variety of fresh cheese (≥50% moisture) originating from Central Europe, where it is generally manufactured from cow milk only. These fresh cheeses appear to be ideally suited for use in delivery of probiotic organisms. Because they are stored at refrigeration temperatures, prolonged periods of ripening are not necessary (Heller et al., 2003). Kwark is generally made from acid milk gels that are concentrated after fermentation with lactic cultures to ~pH 4.6. Kwark is snowy white in color, with a subtle taste similar to sour cream, but a soft texture similar to cottage cheese. The health-enhancing properties of Kwark cheese can be improved by incorporation of functional probiotic bacteria. Several probiotics, which are well established in terms of their positive health effects, have been used in various dairy foods including Kwark cheese (Kadiya et al., 2014; Kelly and O’Kennedy, 2001; Kosikowski, 1982; Lake et al., 2005). However, the combined use of functional probiotic bacteria with the Kwark cheese starter has seldom been reported. Therefore, in this study, Kwark cheese was manufactured with commercial starter and B. longum KACC 91563, and its chemical and sensory properties, as well as the survival of the probiotic bacteria, were evaluated.
Materials and Methods
A cheese starter culture consisting of freeze dried CHN-11 (Chr. Hansen, Denmark) and B. longum KACC 91563 was used. Raw fresh cow’s milk was obtained from the National Institute of Animal Science.
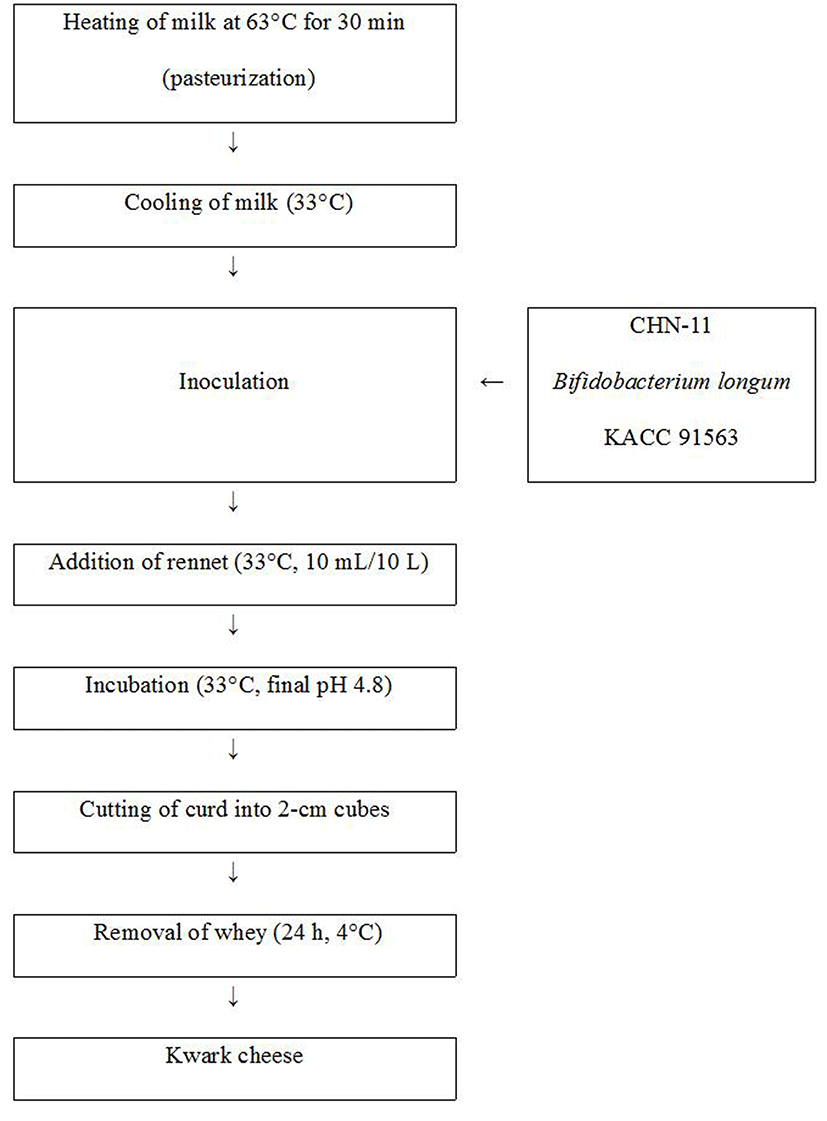
Kwark cheese was manufactured using the method described by Davis (1976), with some modifications, as shown in Fig. 1. Kwark cheese was made using 10 L of pasteurized milk (63°C, 30 min) that was then cooled to 33°C using a cheese vat. The milk was inoculated with starter culture (0.002% CHN-11, v/v), and then the same amount of B. longum KACC 91563 (approximately 105-106 CFU/g) was separately added to the milk. The control was produced with starter culture alone. Rennet (100 µL/10 mL) was added and mixed thoroughly. The cheese was incubated at 33°C until it reached pH 4.8. The resulting curd was cut into 2-cm cubes with cheese knives. The whey was removed, and the curds were cooled at low pressure, and then stored at 4°C.
Moisture, protein, fat, and salt contents were analyzed using a Food Scan (Food ScanTM Lab 78810, Foss Tecator Co. Ltd., Denmark).
The pH of the samples was measured using a pH-meter (CH/SevenEasy S20K, Mettler-Toledo, Switzerland). Acetic acid, d-lactic acid, l-lactic acid and lactose were analyzed using a Automated Chemistry Analyzer (Thermo Scientific, Finland).
Samples were serially diluted and then used for viable plate counts. Viable counts of lactic acid bacteria in the cheese samples were determined using de Man, Rogosa, and Sharpe agar (MRS; Difco, USA). Viable counts of bifidobacteria in cheese samples were determined using Bifidobacterium Selective Medium (BSM; TOS supplemented with mupirocin, Thitaram et al., 2005). Kwark cheese (10 g) was homogenized for 1 min in a sterile stomacher bag with 90 mL of sterile distilled water using a stomacher (Bagmixer® 400W, Interscience, France) for 1 min at high speed to obtain a slurry for the first dilution, and subsequent serial dilutions were made in diluent before spread plating on BSM and MRS plates. The BSM plates were incubated for 48 h at 37°C under anaerobic conditions (GasPak™ EZ Anaerobe Container System, Dickinson and Company, USA). The MRS agar plates were incubated under aerobic conditions for 24-48 h at 37°C. Colony forming units (CFU) per gram were counted per plate.
Semi-trained panelists (n=10) evaluated and analyzed samples of Kwark cheese with and without B. longum KACC 91563. The panelists used were chosen among the members of Animal Products Development Division, and based on their previous experiences in sensory evaluation of dairy products. The panelists were asked to score the samples for color, flavor, texture, taste, and overall acceptance using the following hedonic 9-point scale: like extremely (9), like very much (8), like moderately (7), like slightly (6), like moderately (5), neither like nor dislike (4), dislike moderately (3), dislike very much (2), dislike extremely (1) (Jaclyn et al., 2014).
Data were analyzed using the Statistical Analysis System program (version 9.2) (SAS, 2010). Means were compared by analysis of variance (ANOVA) followed by Duncan’s multiple range test and the difference after 10 d storage were compared by a Student’s t-test. Significance of differences was defined at the 5% level (p<0.05). All of the experiments were performed twice in duplicate (n=4).
Results and Discussion
The proximate composition of Kwark cheese with and without addition of probiotic bacteria is presented in Table 1. Control was made with using commercial starter but, treatment was made with commercial starter and B. longum KACC 91563. The average moisture content of Kwark cheese ranged between 68.27 and 67.10% and no differences were observed between the treatment and control. The protein and fat content of treatment was lower than in control. The salt content of treatment was 0.70±0.08%, and showed no difference from that of control. This finding was in agreement with the results reported by Gursoy et al. (2014). The addition of B. longum KACC 91563 did not change the cheese composition compared with control. Thus, the treatment and control showed no significant differences in moisture, protein, fat, or salt contents.
| Kwark cheese | Moisture (%) | Protein (%) | Fat (%) | Salt (%) |
|---|---|---|---|---|
| C1 | 66.27±4.73 | 12.46±1.89 | 17.50±2.43 | 0.65±0.02 |
| T2 | 67.10±3.08 | 12.10±1.77 | 16.79±1.29 | 0.70±0.08 |
1Control, Kwark cheese added with commercial starter; 2Treatment, Kwark cheese supplemented with commercial starter and B. longum KACC 91563
Data are expressed as mean±standard deviation (n=4).
Values in the same column are not significantly different (p>0.05).
As shown in Table 2, pH and chemical composition of Kwark cheese with and without addition of probiotic bacteria are examined during the storage. pH of treatment was lower than that of control. Magdoub et al. (2005) investigated that the decrease of pH may be due to the convert to residual lactose in cheese to lactic acid and free fatty acid which had developed in the cheese. After 10 d, pH of treatment and control cheese was increased from 4.36 to 4.54, and 4.52 to 4.63, respectively. The level of acetic acid and d-lactic acid of treatment was higher than that of control. Treatment and control were increased during storage days in acetic acid and d-lactic acid. The control and treatment revealed the level of l-lactic acid increasing over 10 d. Lactose of treatment was higher than that of control. However, it was seen that the lactose of all the samples has decreased during storage days. Lactose of fresh cheese like quarg was 2 to 4% (Park, 2003). Thus, the results obtained that pH of Kwark cheese with commercial starter and B. longum KACC 91563 seemed to be reduced due to more the production of lactic acid and other organic acids than that of control.
1Control, Kwark cheese added with commercial starter; 2Treatment, Kwark cheese supplemented with commercial starter and B. longum KACC 91563
Data are expressed as mean±standard deviation (n=4).
*Values in the same group are significantly different by t-test (p<0.05).
The growth of CHN-11 and B. longum KACC 91563, based on viable cell counts in Kwark cheese, are shown in Table 3. According to Table 3, the number of lactic acid bacteria and B. longum KACC 91563 of cells increased about 2 and 1 Log CFU/g immediately after inoculation, respectively. In treatment, the number of lactic acid bacteria was lower than in the control, but the difference was not significant (p>0.05). Bifidobacterial counts on selective agar plates incubated under anaerobic conditions showed successful incorporation into Kwark cheese at a level of 7.58 Log CFU/g (Table 3). Thus, Kwark cheese with and without addition of probiotics showed no significant difference (p>0.05) viable cell counts. It should be noted that different Bifidobacterium species will exhibit different survivability or have different impacts on the sensory attributes of dairy products because bifidobacteria species differ in their nutrient requirements, growth characteristics, and metabolic activity.
1Control, Kwark cheese added with commercial starter; 2Treatment, Kwark cheese supplemented with commercial starter and B. longum KACC 91563
Data are expressed as mean±standard deviation (n=4).
Values are not significantly different (p>0.05).
Several factors must be considered when adding probiotics to fermented foods such as cheese. Mainly, the probiotics must be present at high viable cell counts at the time of consumption to achieve the desired benefits (Gomes et al., 1995). For maximal benefit, a probiotic dairy product should contain at least 106-107 CFU/g probiotic bacteria at the time of consumption, and should be consumed regularly at a quantity of higher than 100 g per day (Boylston et al., 2004; Gomes and Malcata, 1999; Matijević et al., 2009; Medici et al., 2004). According to these criteria, daily consumption of 10 g of Kwark cheese supplemented with B. longum KACC 91563 (containing 107 CFU/g) would meet the minimum probiotic bacteria requirements.
The results of the sensory evaluation of the Kwark cheese samples are shown in Table 4. In sensory properties, flavor, texture, taste and overall acceptance were decreased generally after 10 d. The results seemed to be due to loss of freshness of the cheese. The individual effect of supplementation of Kwark cheese with or without B. longum KACC 91563 on taste and overall acceptance was statistically significant during storage days (p<0.05). Mahmoudi et al. (2012) found that supplementation of Iranian white cheese with B. animalis and Lactobacillus rhamnosus had no significant effect on the texture and flavor of the cheese. These results are consistent with the findings of previous studies by Gursoy and Kinik (2010) and Zomorodi et al. (2010). In addition, cheese supplemented with bifidobacteria shows higher levels of acetic acids compared with controls (Ong et al., 2007); however, the organoleptic properties were unchanged (Gobbetti et al., 1998). The inclusion of some probiotic bacteria in dairy foods, such as cheese, does not markedly change the sensory profile of the food (Champagne et al., 2005; Cruz et al., 2009). Escobar et al. (2012) suggested that the probiotic supplementation of Panela cheese had no perceived effect. In addition, Buriti et al. (2005) reported that the addition of L. acidophilus to Minas fresh cheese had no effect on flavor compared with a control. In agreement with these results, our study showed that cheese supplemented with probiotic bifidobacteria attained equal or greater acceptance in sensory evaluation compared with control, and the addition of B. longum KACC 91563 to Kwark cheese did not create any sensorial defects.
1Control, Kwark cheese added with commercial starter; 2Treatment, Kwark cheese supplemented with commercial starter and B. longum KACC 91563; panel=10
Data are expressed as mean±standard deviation (n=4).
*Values in the same group are significantly different by t-test (p<0.05).
Conclusions
In this study, we produced Kwark cheese supplemented with B. longum KACC 91563 to investigate the effects on the chemical and sensory characteristics of the cheese. The compositional analysis showed that any differences were not significant (p>0.05). The chemical analysis showed that pH of Kwark cheese with commercial starter and B. longum KACC 91563 was lower than that of control. In addition, no significant differences (p>0.05) in lactic acid bacterial counts were detected between Kwark cheese with and without addition of probiotics. Kwark cheese supplemented with B. longum KACC 91563, which has the ability to alleviate food allergies, retained a viable cell count >107 CFU/g of bifidobacteria. Thus, daily consumption of 10 g of Kwark cheese would meet the minimum probiotic requirement. Kwark cheese supplemented with B. longum KACC 91563 was preferred over the control, but addition of probiotics did not significantly alter the color, flavor, texture, taste, or overall acceptance of the Kwark cheese. Based on these findings, addition of B. longum KACC 91563 improved product quality without significant negative effects on the characteristics of the Kwark cheese. Therefore, Kwark cheese supplemented with B. longum KACC 91563 shows promise for use as a probiotic or functional cheese against food allergies.