Introduction
Eggshell matrix proteins, an essential part of the avian eggshell (Tian et al., 2010) and accounting for about 2% of the eggshell weight (Hincke et al., 2012; Rose and Hincke, 2009), are secreted by cells in the red isthmus of the oviduct where eggshell mineralization is initiated. Previous in vitro and in vivo studies showed that the organic matrix plays a key role in shell formation (Gautron and Nys, 2007; Hincke et al., 2012; Lavelin et al., 2000; Nys et al., 2004). These matrix proteins are composed of soluble and insoluble proteins, glyco- and phospho- proteins, and proteoglycans (Gautron et al., 1997). The functions of the majority of these eggshell proteins have not yet been analyzed (Marie et al., 2015), but bioinformatics indicates that the eggshell-specific matrix proteins may have many biological functions such as biological combination, catalysis, enzyme modulation, biomineralization, antibacterial, molecular transduction, and channel regulation. Besides, they also play an important role in biological control, cell growth and location, stress reaction, and metabolic process (Brionne et al., 2014; Sun et al., 2013). More than 500 proteins termed Ovocleidins or Ovocalyxins have been identified in the eggshell by recent transcriptomics and proteomics approaches (Gautron et al., 2011a; Mann et al., 2006; Mann et al., 2007; Mikšík et al., 2007; Mikšík et al., 2010). Ovocalyxin-36 (OCX-36), Ovocleidin-17 (OC-17) and Ovocleidin-116 (OC-116) are the most abundant eggshell matrix components.
OC-17, the first isolated and characterized eggshellspecific matrix protein (Hincke et al., 1995), is a C-type, lectin-like protein of 17 kDa that also occurs as a glycosylated form ovocleidin-23 (Mann, 1999; Mann and Siedler, 1999). A large number of arginine and lysine residues are present on the surface of OC-17 protein, forming a classic structure of cationic antimicrobial peptides, conferring the protein high antibacterial activity (Freeman et al., 2010; Freeman et al., 2012; Wellman-Labadie et al., 2008). OC-116, the first cloned eggshell-specific matrix protein, corresponds to the core protein of an eggshell dermatan sulfate proteoglycan (Hincke et al., 1999). OCX-36 is the most abundant in the basal shell and associated membranes. Sequence comparison analysis reveals that OCX-36 is related to the antibacterial protein superfamily BPI/LBP/PLUNC and shares 20-27% identity with BPI/LBP/PLUNC members (Gautron et al., 2007; Gautron et al., 2011b). Additionally, OCX-36 is a pattern recognition molecule, which recognizes bacterial endotoxins as the first step to eliminate pathogens, and it also plays an important role during calcification (Cordeiro et al., 2013; Gautron et al., 1997; Hincke et al., 2010; Kovacs-Nolan et al., 2014).
Currently, for the purification of those proteins, eggshells were usually treated first with EDTA or acetic acid/HCl to remove the associated shell membranes, then shells were decalcified and extracted against calcium chelation (EDTA or EGTA) or acid demineralization (acetic acid or HCl), and finally, the supernatant of extraction was purified through diethylaminoethyl (DEAE) Sepharose or carboxymethyl (CM) Sepharose (Cordeiro et al., 2013; Gautron et al., 2001; Hincke et al., 1995; Hincke et al., 2003; Mann et al., 2002; Mikšík et al., 2007; Mikšík et al., 2010; Rose and Hincke, 2009). Nevertheless, the acid extraction is complicated and time-consuming, and some portion of the activity is damaged during extraction. Relevant studies indicated that proteins extracted with aqueous solutions are highly different from those extracted by EDTA or acid solutions (Mikšík et al., 2007; Mikšík et al., 2010), with higher activity observed in the aqueous protein (Harnedy and FitzGerald, 2013). Therefore, it is necessary to develop a facile, time-saving and efficient extraction method.
In this study, we attempt to develop a rapid eggshell-membrane separation process using water as a medium to extract matrix proteins with high bioactivity, and co-purify them through two columns in series (Hitrap DEAE-Sepharose Fast Flow and CM-Sepharose Fast Flow). The separation efficiency was improved greatly by using this simple and convenient method.
Materials and Methods
Fresh chicken eggshells (Hy-Line Brown and White Leghorns) were collected from a local hennery (Jiufengshan Farm, China). Acetic acid, hydrochloric acid, sodium hydroxide, sodium chloride, ethylene diamine tetraacetic acid (EDTA), guanidine hydrochloride, tris, potassium dihydrogenphosphate and disodium hydrogen phosphate were obtained from Sinopharm Chemical Reagent Co., Ltd. (China), and all the chemicals were of analytical reagent grade. Dithiothreitol (DTT), phenylmethylsulfonyl fluoride (PMSF) and 4-(2-hydroxyethyl)-1-piperazine ethanesulphonic acid (HEPES) were supplied by Biosharp Co. Ltd., and all of them were of biochemical reagent grade. Deionized water was used for aqueous solution preparation. Prior to use, all solutions prepared were filtered through a 0.22 μm membrane. CM Sepharose Fast Flow and DEAE Sepharose Fast Flow were supplied by General Electric Company (USA).
A method modified from a previous work (Li, 2009) was used to separate the eggshell from the membrane and minimize the impact on proteins during the process. Briefly, fresh eggshells were rinsed with running tap water to remove the dirt and residual egg white on the surface, followed by washing in 1 M NaCl, then rinsing in 100 mM EDTA, pH 8.5 for 2 h, and drying at 25℃. Next, the dried eggshells were smashed quickly (10 s), followed by passing through a 200 screen mesh and stirring at a solid/water ratio of 1:10 for 15 min at 600 rpm. After these treatments, eggshell membranes were in the upper layer while eggshell powders were at the lower layer, which were filtrated and dried to obtain membranes and eggshell powders, respectively.
Eggshell matrix proteins were extracted in three different ways:
A. Acetic acid (HAc) decalcification was performed as previously described (Gautron et al., 2007; Mann et al., 2003; Mine et al., 2003). Briefly, 50 g eggshell powders were extracted overnight at 4℃ with 4 M guanidine hydrochloride in 50 mM sodium acetate, pH 4.8, containing protease inhibitors (5 mM benzamidine-HCl, 100 mM amino-n-caproic acid, 10 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride). After centrifugation, the supernatants were dialyzed against water (cut-off of 3500 Da) and then concentrated, which was referred to as HAc extract.
B. EDTA decalcification was performed as previously described with minor modifications (Mikšík et al., 2007; Mikšík et al., 2010). Briefly, 50 g of eggshell powders was rinsed in 250 mL Tris-HCl at 50 mM, and soaked for 72 h in 4 M guanidine hydrochloride. After centrifugation, the supernatant was collected as A, and the precipitate was soaked for 48 h with 200 mL of Tris-HCl at 50 mM, 4 M guanidine hydrochloride, and 0.5 M EDTA (pH 7.4). After centrifugation, the supernatant was collected as B. The mixture of A and B was supplemented with 1 mM PMSF, followed by dialysis and lyophilization. The final preparation was designated as EDTA extract.
C. Na2HPO4-KH2PO4 (Phosphate Buffer, PB) extraction was performed as follows. Briefly, 50 g of eggshell powders was stirred for 72 h at room temperature with 500 mL of PB at 2 mM, pH 7.0, and then dialyzed (cutoff of 3500 Da) and freeze-dried. The eggshell matrix protein extract was named as PB extract.
All the extracts were stored at 4℃ for further use.
Eggshell matrix protein showed antibacterial activity against Gram-positive and Gram-negative bacteria (D’Alba and Shawkey, 2015; Mine et al., 2003; Wellman-Labadie et al., 2010). For antimicrobial activity analysis, all the extracts were dialyzed and lyophilized, followed by dilution to a final concentration of 1.0 mg/mL protein in PBS (pH 7.2, 10 mM phosphate buffer, containing 0.15 M NaCl), prepared as a stock solution and sterilized by sterile membrane syringe filter (0.22 μm).
The activity of the three eggshell matrix protein extracts was evaluated by the standard agar well diffusion assay (Perez et al., 1990) using Escherichia coli, Salmonella Enteritidis, and Staphylococcus aureus. Briefly, 100 μL of each test bacterium was grown in 10 mL LB media until the concentration of bacterial colony-forming units (CFU) reached 108 U/mL, then wells of 6 mm diameter were punched into the LB agar plate with a sterile Pasteur pipette. Next, the three kinds of extracts were individually added to each well. Finally, the plates were incubated at 37℃ for 24 h and the antibacterial activity of each extract was examined in terms of the mean diameter of inhibition zone produced by each extract with three replicates.
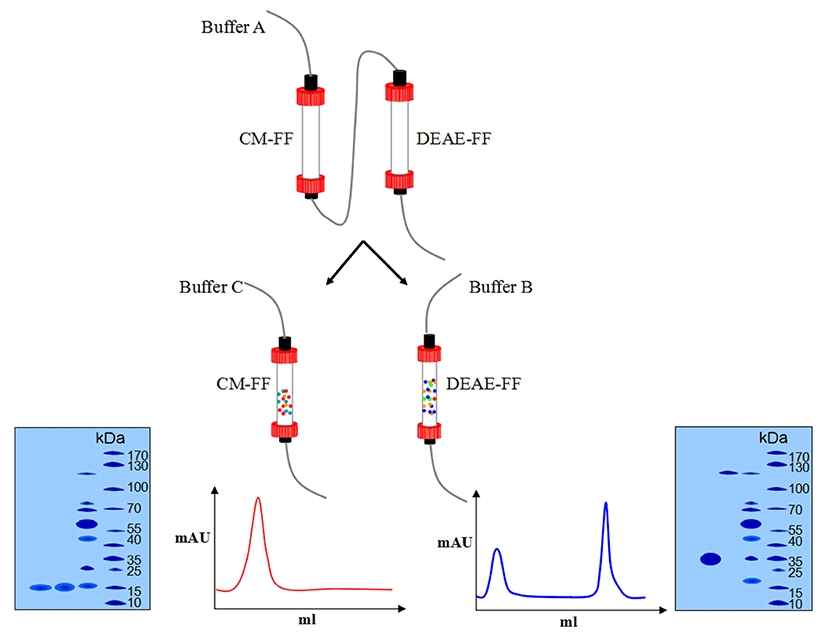
The supernatant of extraction was passed through two columns (CM Sepharose Fast Flow and DEAE Sepharose Fast Flow) connected in series. Both columns were preequilibrated with 50 mM Tris-HCl, pH 8.5 at a flow rate of 1 mL/min. The supernatant was loaded onto the columns and eluted with 50 mL of Tris-HCl, pH 8.5, 2 mM of DTT at a flow rate of 1 mL/min. Next, the CM Sepharose column was disconnected, and the bound proteins were eluted from the DEAE column with 50 mM NaHepes, pH 7.5, 2 mM DTT, and 200 mM to 400 mM NaCl at a flow rate of 1 mL/min. The CM column was eluted with 100 mM NaCl, 50 mM Tris, pH 8.0, at a flow rate of 0.5 mL/min. The eluted fraction from the DEAE column was OCX-36 and those from the CM column were OC-116 and OC-17. Finally, they were dialyzed and lyophilized for further analysis. The purification flow diagram is shown in Fig. 1.
A 12% polyacrylamide gel was used for sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) (DYY-12, Beijing Liuyi Instrument Factory, China). The amount of protein loaded was approximately 4-8 μg of the sample. Page RulerTM Plus Prestained Protein Ladder (#SM0671, Fermentas Instrument Inc., Canada) with a wide range of molecular weight markers was loaded for comparison of the molecular weights. The protein bands were stained with Coomassie Brilliant Blue R-250 (0.1% in the staining solution, containing 25% ethanol and 8% acetic acid) for 30 min at 45℃ and destained by diffusion in destaining solution (25% ethanol and 8% acetic acid) (Fang et al., 2012).
Protein concentration was determined using a commercial Bradford Protein Assay Kit (Bio-Rad, Hercules, USA), with bovine serum albumin as a standard (Bradford, 1976). The absorbance of the sample was measured at 595 nm in triplicate.
Results from at least three independent experiments were expressed as the mean±standard deviation (SD). Differences between groups were determined by one-way analysis of variance (ANOVA). Statistical analyses were performed using Social Sciences software (SPSS version 19 for Windows; SPSS Inc., USA). A value of p<0.05 was considered statistically significant.
Results and Discussion
To reduce the impact of the separation method on the eggshell matrix proteins and shorten the separation time, we improved our original process (Li, 2009) by adding EDTA.
As the eggshell moisture increased, the recovery and residual rates of eggshell showed a decreasing trend (Figs. 2A, B). When the shell/water proportion was less than 6%, the membrane recovery increased slightly, but when it was higher than 6%, the recovery rates of both eggshell and membrane declined sharply, and the residual rate of eggshell membrane increased significantly. Thus, the proper shell/water proportion was 6%. Eggshell is brittle compared with the membrane, suggesting that eggshell membrane has a larger particle size distribution. At a higher water content, the eggshells cannot be completely crushed or fully recovered because part of them cannot be separated by flotation, resulting in a lower recovery rate but a higher residual rate. With increasing drying time, eggshell and membrane release the adsorbed water steadily, become increasingly brittle, and are more likely crushed into smaller particles, but the eggshell powders, when too small, are hard to separate, leading to the increase of the residual rate. As shown in Figs. 2C, D, after stirring for 5 min, the recovery rate was highest for the eggshell but lowest for the eggshell membrane because the force between water and eggshell was not strong enough to separate them. When stirred for 15 or 20 min, the membrane showed the minimum level of eggshell powders, indicating that shell and membrane were well separated with a similar recovery rate, and for energy saving, 15 min was considered as a proper stirring time. Figs. 2E and F show the effects of different concentrations on the shell-membrane separation, demonstrating a positive correlation between recovery of membrane and concentration. However, the inflection point occurred at 150 mM concentration, at which the eggshell was dissolved and the free calcium ions in solution increased, leading to the decrease of recovery. Therefore, 100 mM was defined as a suitable concentration for the EDTA solution.
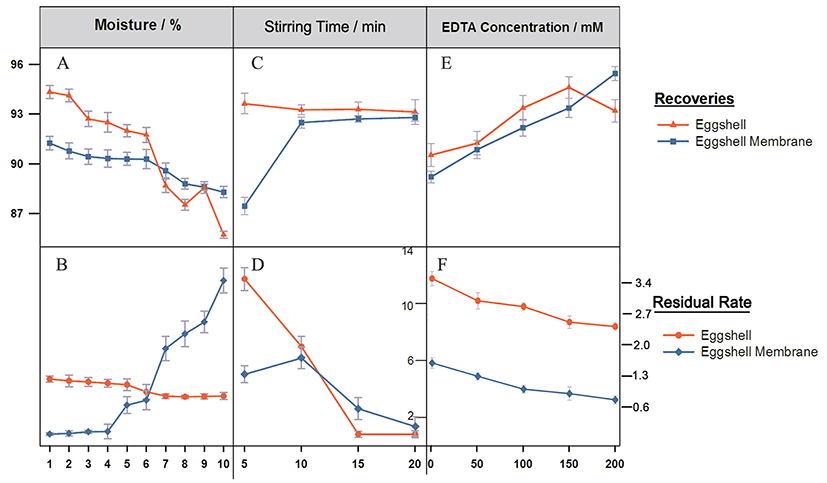
Collectively, eggshells were first treated with 100 mM EDTA, pH 8.5 for 2 h, and dried at 25℃. Then, the dried eggshells were smashed for 10s, followed by passing through a 200 screen mesh and stirring at a solid/water ratio of 1:10 for 15 min at 600 rpm. The upper layer was filtrated and dried to obtain the membrane, while the lower layer was the eggshell powder. The recovery rates of eggshell and membrane were 93.88% and 91.15%, and the residual rates were 1.01% and 2.87%, respectively.
Three different solutions (HAc, EDTA and PB) were used to extract eggshell proteins. As shown by SDS-PAGE in Fig. 3, proteins with a wide range of molecular weights were extracted by HAc, because the matrix proteins are usually acidic. Different from HAc, PB extracted the proteins within the molecular weight range of 25 to 70 kDa. In contrast, fewer proteins were extracted by EDTA as indicated by the bands in Lane 1, and only a few water-soluble proteins were extracted by deionized water.
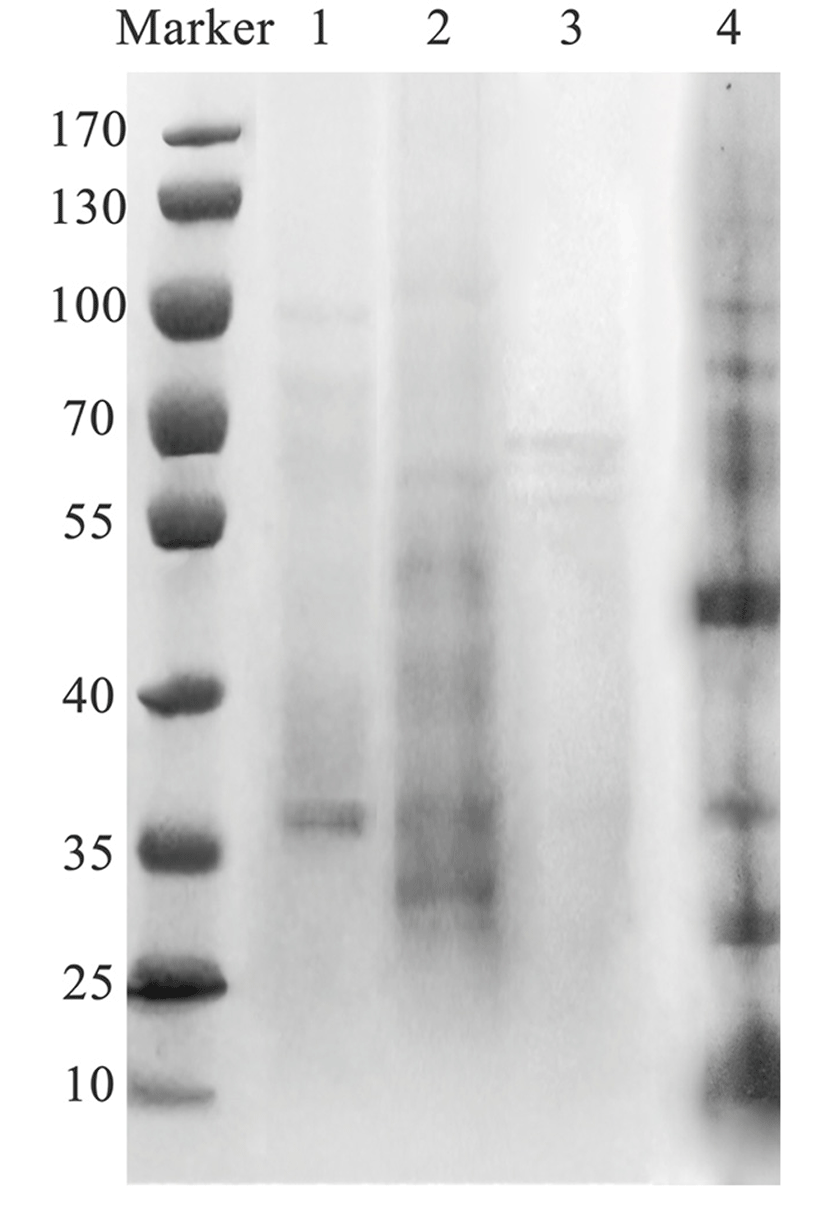
The eggshell matrix protein content reached 2.03% when extracted by PB, which was approximately 1.1-fold that of HAc and 1.2-fold that of EDTA, probably due to the similar neutral pH value of PB to that of the physiological conditions of hens, thus dissolving more matrix proteins. Extraction is an important step before isolation and purification, which should not only dissolve as much of the target protein as possible, but also ensure the integrity and physiological activity of the protein. Currently, acid or/and EDTA solutions are used to extract eggshell matrix by dissolving calcium carbonate crystals to dissociate the matrix proteins which are tightly bound (Gautron et al., 2007; Mine et al., 2003; Nys et al., 2004; Rose and Hincke, 2009). During this process, some proteins may denature irreversibly and lose activity. In this experiment, we used phosphate to maintain the pH similar to that of the in vivo physiological environment during extraction. Additionally, recent studies indicate that aqueous extracts can promote osteoblast differentiation in vitro, because of the solubility differences between the matrix proteins extracted by aqueous solutions and those extracted by acid or EDTA solutions (Almeida et al., 2000; Milet et al., 2004; Pereira-Mouriès et al., 2002), which is meaningful to proteomics.
| Extract solution | HAc3) (%) | EDTA (%) | PB (%) |
|---|---|---|---|
| Yield2) | 1.88±0.15% | 1.67±0.41% | 2.03±0.12% |
1)Values are given as means ± SD (n=3).
2)Yield = (mass of matrix proteins / mass of eggshell powder) × 100%
3)HAc, Acetic Acid; EDTA, Ethylenediaminetetraacetic acid; PB, Phosphate Buffer.
The DEAE column was eluted at a flow rate of 1ml/min with the buffer containing NaCl solution from 250 mM to 400 mM. The elution peak was not obvious at 250 mM NaCl, and two peaks were not separated at 300 mM (data not shown). However, at 350 mm, the two peaks were well separated from each other (Fig. 4), indicating that they were OCX-36 and OC-116, respectively.
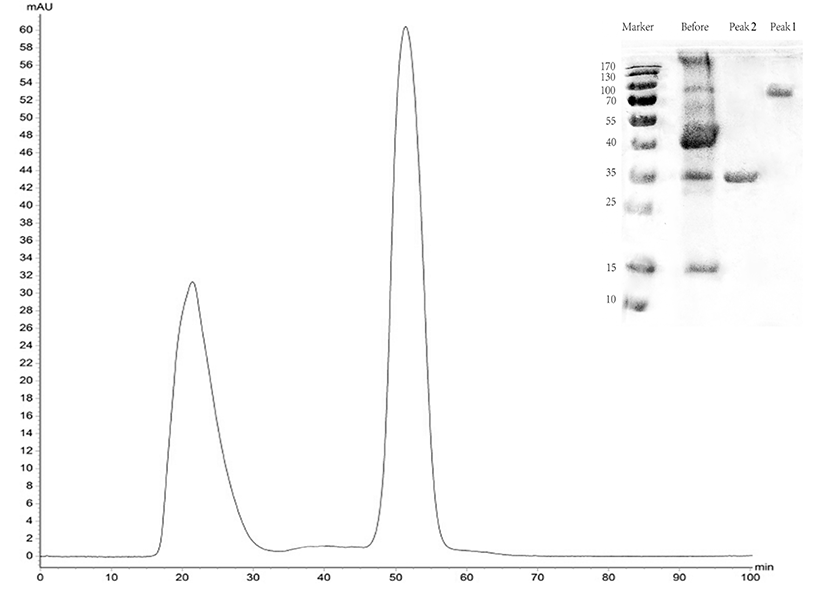
The protein was isolated and purified to homogeneity by CM-FF column with 100 mM NaCl, 50 mM Tris, and pH 8.0 at a flow rate of 0.5 mL/min. The apparent molecular weight of the protein was 17 kDa as determined by SDS-PAGE (Fig. 5).
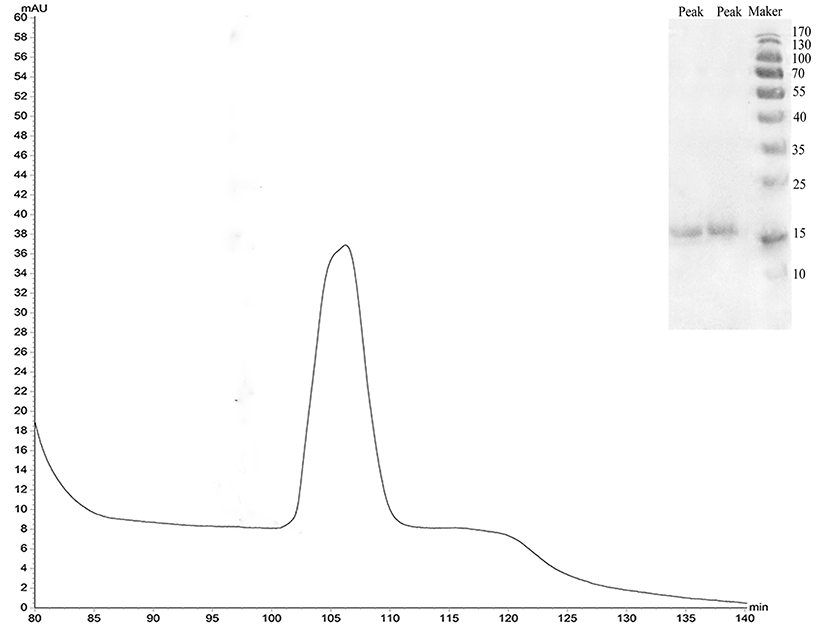
The protein recovery rate was calculated according to the protein assay standard curve as shown in Fig. 6, and the peaks were integrated to calculate the purity using Unicorn Version 7.0. Results are shown in Table 2.
| Protein | Recovery (%) | Purity (%) |
|---|---|---|
| OCX-36 | 55.27 | 96.82 |
| OC-17 | 53.38 | 80.15 |
| OC-116 | 36.34 | 73.22 |
Eggshell matrix proteins were mostly acidic, with an isoelectric point (pI) of 5.74 for OCX-36 and a pI of 6.6 for OC-116 (data were obtained from NCBI and ExPASy), indicating that they have a weak negative charge, and in a certain pH range, they can bind to a weak cation exchange column like DEAE Sepharose column. However, OC-17 had a pI of 9.82, indicating that it has a weak positive charge and can bind to a weak anion exchange column like CM Sepharose column.
Compared with the isolation method for each protein, the co-purification method proposed in this study is time-saving and efficient, because three different proteins were isolated and purified simultaneously. The purity of OCX-36 (96.82%) was lower than that previously reported (98%), which had been extracted and purified from the eggshell membrane (Cordeiro et al., 2013), but thus far, no data are available about the purified OCX-36 protein from the eggshell. And also no data is available about OC-116 (Mann et al., 2002). It is reported that the purity of OC-17 was >90% (Hincke et al., 1995), which is higher than that of the present study (80.15%), but the present result is reasonable for time-saving and cost-effectiveness without further purification steps. If higher purity is required, further purification scheme can be adopted.
Gram-negative (E. coli, S. Enteritidis) and Gram-positive bacteria (S. aureus) were selected to assess the antibacterial activity of the extracts, which was expressed in terms of the mean diameter of inhibition zone, and the results are shown in Table 3.
1)Different superscripts (a, b, c) indicate statistical significant difference between groups (p<0.05).
2)Values are mean ± SD of three independent experiments performed in duplicate. In each well, the sample was 100 μL.
3)HAc, Acetic Acid; EDTA, Ethylenediaminetetraacetic acid; PB, Phosphate Buffer.
The pattern of inhibition varied with the type of extraction solvent used and the bacterium tested. All the three extracts showed inhibitory activity against all the test bacteria. PB extract exhibited significantly higher antibacterial activity to the test bacteria than HAc extract or EDTA extract. HAc extract had lower activity than the other two extracts, and S. aureus was comparatively the most susceptible bacterium to the three extracts. According to the SDS-PAGE results, more bands were observed in PB extract than the other two extracts, suggesting that PB solution may extract much more eggshell matrix proteins, which we assume may be related to high antibacterial activity. This hypothesis should be tested by further experiments.
Proteins will be damaged by acid extraction, or form a coacervate easily by EDTA solution, and when purified, both EDTA and acidic proteins are adsorbed to the DEAE column because of their negative charges, thus EDTA may block the binding of acidic protein to the beads. However, dialysis to remove EDTA is time-consuming.
OC-17, OC-116 and OCX-36 purified from the three extracts were separately mixed with each other to investigate their antimicrobial activity using the method described before, and results are shown in Table 4.
1)Different superscripts (a, b, c) indicate statistical significant difference between groups (p<0.05).
2))Values are mean ± SD of three independent experiments performed in duplicate. In each well, the sample was 100 μL.
3)HAc, Acetic Acid; EDTA, Ethylenediaminetetraacetic acid; PB, Phosphate Buffer.
As shown in Table 4, all the three mixtures exhibited antimicrobial activity as expected. Statistical analysis of the data revealed no significant difference in their activity against Salmonella Enteritidis and Staphylococcus aureus. The mixed proteins from HAc extract showed the lowest activity, while those from PB extract had slightly higher activity than those from EDTA extract. The results demonstrated that there were negligible differences in the mixtures of major eggshell matrix proteins purified from differently treated solutions. However, our previous study showed a statistically significant difference among the unpurified extracts. Further investigation revealed that, besides OC-17 and OCX-36, some other antimicrobial proteins or peptides were present in the unpurified extracts, mostly in the PB extract, leading to higher activity. This result suggested that the eggshells are enriched in proteins with antimicrobial or similar properties, which facilitate the development of the chick embryo and protection of the embryo and unfertilized egg against pathogen invasion. Besides the major matrix proteins of OCX-36 and OC-116 or OC-17, some unknown low-abundance proteins also displayed antimicrobial activities, and we need to develop a new method to analyze the components.
Conclusions
In this study, we first modified the eggshell-membrane separation process by adding EDTA, then extracted the matrix proteins by PB solution, and finally obtained the damage-free proteins with a higher yield relative to HAc or EDTA solution. Three eggshell matrix proteins (OC-116, OC-17, and OCX-116) were isolated and purified through the CM-DEAE columns connected in series. Bioactivity test of the three extracts showed that the PB solution extract exhibited significantly higher antibacterial activity against the test bacteria. The results suggest that this modified method is feasible and efficient in separating eggshell and membrane as well as purifying matrix proteins from eggshells.