Introduction
Biologically active peptides are oligopeptides that can exert biological activities beyond their expected nutritional values that are proven beneficial to humans due to its health-promoting properties (Erdmann et al., 2008). These biologically active peptides–made up of sequences of amino acids encoded in the parent protein molecules – remain inactive until released via enzymatic hydrolysis by peptidases during food processing and/or during gastrointestinal digestion. An example of a parent protein molecule with high biological value is elastin (Hattori et al., 1998). Besides the apparent nutritional value of its amino acids, the protein elastin itself has been a candidate for an active source of value-added product due to its functional properties such as antihypertensive, antimicrobial, antioxidative and antiaging (Agrawal et al., 2016). Elastin is the insoluble elastic fibrous protein which forms the framework that holds the connective tissues in animals (Antonicelli et al., 2007). An elastin core is surrounded by layers of microfibrils forming elastic fibers which provides elasticity and resilience of connective tissues such as blood vessels, lungs, aorta, and skin, which are essential for human health and development (Levillain et al., 2016). Apart from the manifold components, the elastic fibers are also maintained by highly complex molecular activities firmly controlled development, multiple stages of assembly, distinct biochemistry. However, the complexity is being unraveled and understood through studies of mouse models (Kielty et al., 2002). Elastin has been investigated in fields of biomedical and tissue engineering, molecular therapy, and even cosmetics (Ganceviciene et al., 2012; Lescan et al., 2018; Yeo et al., 2015). As such, elastin is involved in multiple levels of studies and analysis, ranging from topical applications to mRNA-synthesis, thus creating a demand for elastin in various forms. Commercially, elastin is distributed in powder form and of animal origin such as bovine and porcine. The protein accumulates in the animal parts postnatally, making the extraction more intricate, hence the final product expensive (Mecham, 2008). So, finding cheaper and easier alternative with high throughput is a major concern for elastin extraction.
Generally, highly meat productive birds or poultry breeds are called broiler poultry. Broilers are young chicken (Gallus gallus domesticus) of either sex of six to eight weeks of age. There are studies suggesting the potential of functional protein extraction from by-products of poultry industry (Fauzi et al., 2016; Mohammad et al., 2014; Munasinghe et al., 2014). The study suggests that collagen – another example of parent protein molecule – could be extracted from chicken skins and bones, which then can be utilized in various fields such as pharmaceuticals, biomedicine and food. As collagen shares similar characteristics with elastin, it is worth investigating the potential of chicken skin for elastin extraction (Mecham, 2008; Munasinghe et al., 2014).
Still, there is minimal information on the extraction of elastin from chicken skin. Exploring this prospect would improve by-products management and make elastin more accessible. Also, elastin from chicken skin could be an alternative to conventional elastin as essential properties such as antihypertensive was present and have been investigated earlier (Yusop et al., 2016).
Antioxidants protect human health against ROS (reactive oxygen species) and can increase the stability of food lipids (Martinez-Maqueda et al., 2012; Nazeer and Deeptha, 2013). It is found that certain bioactive peptides do indeed have antioxidative properties and can be used as a natural substitute to synthetic antioxidants to improve health (Ryan et al., 2011). Endogenous antioxidants that are mainly enzymes – catalase, superoxide dismutase and glutathione peroxidase – must be able to control the free radicals to protect the cellular environment against oxidative stress. The biological actions of proteins can be increased by using enzymatic hydrolysis; resulting in the targeted peptides or fractions being more bioactive than the others (Babji et al., 2018). Elastase is the enzyme found to be able to break down elastin, potentially activating the peptides that possess antioxidant properties (Antonicelli et al., 2007).
The objective of this study was to isolate elastin from broiler hen skin. Furthermore, to get access to its bioavailability, its hydrolysate was produced by incorporating enzymatic hydrolysis using Elastase. Ultrafiltration and gel filtration chromatography were used preceding reversed phase high performance liquid chromatography (RP-HPLC) to further purify the antioxidative peptides. The sequential analysis of the peptides was also carried out through MALDI-TOF-TOF-MS/MS. For ease of reference, the names of the samples analyzed were listed in Table 1.
Materials and Methods
Broiler’s skin was purchased consistently from a supplier in a local market in Bangi, Malaysia. The skins were kept in the freezer at –18°C and thawed approximately 1 h before further use.
Elastin extraction from broiler was done based on the modification of Lansing method from Lansing et al. (1952) and Nadalian et al. (2015). Broiler’s skins were suspended in 1 M NaCl. The solution was put in a cold room with constant stirring for 24 h. Then, the homogenate was centrifuged (5804R, Eppendorf, Hamburg, Germany) at 13,000×g for 20 min. Afterwards, the pellet was washed with distilled water and defatted with acetone for 1 h. The treated sample then was suspended in 0.1 M NaOH and heated for 15 min in a boiling water-bath with constant shaking. After cooling and centrifugation, the residue was extracted again for 45 min in 0.1 M NaOH at 100°C. The residues of NaOH-insoluble material were then washed several times in water and lyophilized. The sample was freeze dried by using bench top freeze dryer (Labconco, Kansas City, MO, USA) at temperature −80°C and vacuum pressure of 4.5 Pa. Next, the powder obtained was immersed in oxalic acid, relative to the insoluble elastin weight, at 100°C for 40 min. The residue of insoluble elastin re-submersed for solubilizing step as water-soluble elastin.
Elastin powder was hydrolyzed by Elastase (Sigma-Aldrich, St Loius, MO, USA) at optimal conditions of combined pH (pH 8.5) and temperature (37°C). The enzyme was added to the elastin powder with the ratio of 100:1 (w/w). The freeze-dried elastin was grounded into powder, then suspended in deionized water with a ratio of 1:100 (w/v), adjusted to pH 8.5 with 0.1 M HCI and temperature of 37°C. Lastly, all the hydrolysates were heated at 95°C for 5 min for enzyme inactivation, before being centrifuged (3,000 g, 4°C, 15 min; 5804R, Eppendorf) to separate soluble hydrolysates from any non-soluble materials. The supernatants were lyophilized into powders, which then stored at −18°C.
Determination of amino acid composition was done based on the method from Alaiz et al. (1992) with some modification. Broiler skin was subjected to acid hydrolysis, performic acid and alkaline hydrolysis using water AccQ.Tag Amino Acid Analyzer (Waters, Dublin, Ireland). Hydrolysis of samples began by adding 5 mL of 6 M HCl at 110°C for 24 h. Then, α-aminobutyric acid was added and filtered through 0.2 m cellulose acetate membrane (Whatman No. 1). Derivatization of amino acids was done for 10 min at 55°C. The amino acids were then run at 37°C with a flow rate of 1 mL/min using C18 AccQ-Tag Amino Acid Analysis Column (150×3.9 mm; Waters, Milford, MA, USA). For amino acid quantification, measurement of the absorbance was done at 248 nm, while the associated fluorescence detector was measured at excitation and emission of 250 nm and 395 nm respectively. Tryptophan level was quantified by alkaline hydrolysis.
Determination of free radicals scavenging activity of elastin from broiler hydrolysate (EBH) was done based on the method from Brand-Williams et al. (1995) with some modification. Elastin powder was dissolved in distilled water. 2 mL of 0.2 mM 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) was added to 100 μL of sample solutions and mixed rigorously. After incubation at 0.5, 5, and 24 h, the absorbance was measured at 517 nm using a microplate reader (Biotek 259037), with distilled water as a control. DPPH scavenging activity was measured as the equation:
IC50 values (the concentrations of the test compounds required to reduce the produced hydroxyl radical to one-half) were calculated.
Determination of the 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radicals scavenging activity was done based on the method from Re et al. (1999). ABTS solution (7.4 mM) was prepared in 100 mM Phosphate Buffered Saline (PBS) at pH 7.4 with 0.15 M NaCl and oxidized using 2.6 mM of potassium persulfate (K2S2O8) for 12 h in the dark. The ABTS was then diluted to 734 nm absorbance with ethanol. 50 μL of the sample was mixed with 950 μL of the diluted ABTS, before being shaken rigorously for 30 s and held in a dark environment for 10 min. 50 μL of distilled water was used instead of the sample for control. Absorbance was measured at 734 nm. The percentage inhibition of ABTS.+ to ABTS was calculated using the following equation:
IC50 values (the concentrations of the test compounds required to reduce the produced hydroxyl radical to one-half) were calculated.
Determination of Fe2+ chelating activity was done based on the method from Decker and Welch (1990) with some modification. 2 mL reaction composition made up of 200 μL of the sample, 50 μL FeCl2 (2 mM), and 1.75 mL deionized water was shaken and left at room temperature for 5 min to stand. 50 μL ferrozine (5 mM in methanol) was then added, mixed, left standing for another 5 min. The Fe2+ ferrozine complex was read through absorbance of 562 nm, with the positive control being EDTA. The Fe2+ chelating activity of the extract was measured as the equation:
Where A0 was the absorbance of the control (without sample) and A1 was the absorbance of the sample. IC50 values were calculated.
The hydrolysate solutions of elastin were separated into large and low molecular weight fractions by ultrafiltration at 4°C using 10 kDa Molecular Weight Cut-Off (MWCO) membrane (Vivaflow 200) (Sartorius, Göttingen, Germany), followed by a 3 kDa MWCO (Vivaflow 50) membrane. The hydrolysates were divided into 3 fractions. Fractions were referred to as the less than 10 kDa ultra-filtrated (10 kDa UF), between 3 to 10 kDa ultra-filtrated (3–10 kDa UF) and less than 3 kDa ultra-filtrated (3 kDa UF) hydrolysates, respectively. All fractions were freeze-dried and stored at –18°C until further use.
After ultrafiltration, a gel filtration chromatography column, Hiprep 26/60 sephacryl S-100HR (26×600 mm) (GE Healthcare, Buckinghamshire, UK) was used to further purify fractions with the highest ABTS radicals scavenging activity. Fractions of 250 mg sample were dissolved in 2 mL sodium phosphate buffer (10 mM, pH 7.2) prior to column loading and elution with sodium phosphate buffer (10 mM, pH 7.2) at flow rate 1.5 mL/min (You et al., 2010). 5 mL of the eluted fractions were collected into several fractions based on the peaks observed by absorbance at 280 nm and freeze-dried. Each fraction was tested for ABTS radical scavenging activity assay to determine antioxidants activity.
Fractions from gel-filtration chromatography with the highest antioxidant activity were further separated using RP-HPLC (Waters, Milford, MA, USA). 5 mg of the peptide fractions were dissolved in 2 mL of sodium phosphate buffer (10 mM, pH 7.2) before being filtered with a 0.22 μm filter. 200 μL of the sample was then loaded onto XBridge BEH130 Prep C18 (10×250 mm, 5 μm) (Waters, Milford, MA, USA). The solvents involved are; solvent A, 0.1% (v/v) trifluoroacetic acid (TFA) in deionized water; solvent B, 0.1% (v/v) TFA in 100% (v/v) acetonitrile solution. The flow rate was 4.73 mg/min and the UV absorbance of the eluents was measured at 214 nm. The samples are then put into a centrifugal concentrator at 380×g for 3 h, which are then used for ABTS radical scavenging activity.
The trypsin-digestion and peptides extraction of the targeted fractions was done based on several studies (Bringans et al., 2008; Garg et al., 2013; Sharma et al., 2007). Analysis of peptides was done by MALDI TOF/TOF (matrix-assisted laser desorption/ionization time-of-flight) mass spectrometer. This method is directly derived from Garg et al. (2013), as the method is deemed the most suitable for this experiment. The full, detailed approach could be obtained from the study. This method resulted in the recognition of the peptide sequences based on the matching identity ’protein spots’ with the highest ion score.
All analyses were directed in triplicate. A one-way analysis of variance (ANOVA) was implemented, and the mean comparisons were analyzed conferring to Tukey range test at significant level 95% (p<0.05). The statistical analyses were completed by using SPSS package (SPSS 23.0 for windows, SPSS IBM, Chicago, IL, USA).
Results and Discussion
The hydrolysates were measured for antioxidant activities through DPPH, ABTS and metal-chelating activity assays. It was evident that the elastin hydrolysates possess antioxidative potentials, with ABTS value reflects the radicals scavenging activity (IC50 of 1.10±0.08 mg/mL), DPPH (2.80±0.37 mg/mL) and metal chelating activity (1.21±0.09 mg/mL) as shown in Table 2. Antioxidant properties of food protein hydrolysates are found to be influenced by their amino acid composition. A high value of DPPH could be correlated to a high amount of hydrophobic peptide fraction of the peptides involved, especially the ones derived from natural protein sources (Li et al., 2008; Pownall et al., 2010). A similar point was made in a study of rapeseed peptides, where the DPPH radical scavenging activities of the peptides are found to have a direct correlation with hydrophobicity (Zhang et al., 2008). In addition, the peptides could serve as food preservatives by protecting food lipids from metal ion-dependent oxidative damage. Side chains of amino acids with carboxyl and amino groups could potentially be essential in chelating metal ions (Saiga et al., 2003).
Bioactivity of protein hydrolysates could be affected by the molecular weights of the peptides (Mohan et al., 2016). Table 3 outlines the ABTS radical scavenging activity and the IC50 value of EBH of the three fractions with different molecular weights. Significant differences in IC50 values were observed in all membrane cut-off MW sizes (p<0.05) with the lowest IC50 showed by EBH III fraction (<3 kDa) (0.66 mg/mL). It has been described that temperature treatment through hydrolysis surges liability of peptide bonds to the elastase and consequently releasing significant antioxidant peptides (Cudennec et al., 2016). The results are in line with a study confirming that the sanitized peptide fraction of fish proteins containing molecular weight less than 1,000 Da has the strongest antioxidant activity compared to other hydrolysates fractions (Centenaro et al., 2014).
| Membrane MWCO (kDa) | ABTS scavenging activity (%) | IC50 value (mg/mL) |
|---|---|---|
| <10 UF | 50.36±0.38c | 0.96±0.03a |
| 3–10 UF | 52.84±0.38b | 0.82±0.02b |
| <3 UF | 55.58±0.38a | 0.66±0.01c |
Gel filtration is a method that separates substances based on differences in molecular dimensions. EBH III fraction (<3 kDa) from ultrafiltration which showed the highest ABTS radical scavenging activity was separated through gel filtration chromatography.Fig. 1A shows the ABTS radical scavenging activity (IC50) and gel filtration chromatography elution profile of the EBH III fraction. It is found that EBH III fraction was separated into four fractions (EBI-EBIV), where EB-II fraction exhibits the strongest ABTS radical trapping activity with the lowest IC50 values (0.42 mg/mL) compared to other fractions (p<0.05).
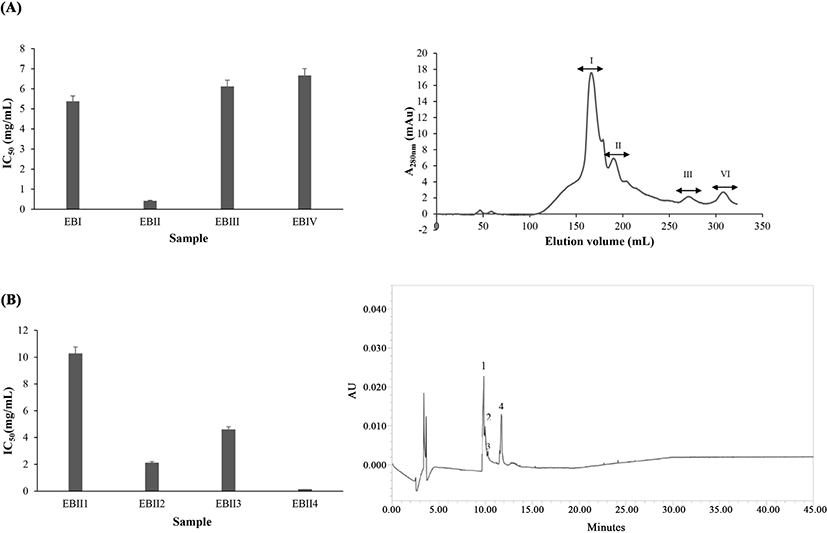
The amino acid composition of non-hydrolysed elastin (UEB) and fractions obtained from ultrafiltration (EBH III) and gel filtration chromatography (EB-II) are summarized in Table 4. The main amino acids of elastin (EBH III) fraction and potent fraction EB-II are Gly, Glu, Ala, and Pro where the highest was showed by Glycine content. The Glycine in EBH III, EB-II and UEB fractions are 20.64%, 19.63%, and 18.36%, respectively (p<0.05). While it is unknown what are the contents found in this stage, there are assumptions that could be done based on studies that found out that elastin extracts from broiler consist of amino acids of two main types; the basics, and the radical-scavenging. The free radical-scavenging amino acids (Glu, Cys, Met, Tyr, and Lys) are found in elastin (Cao et al., 2009; Chen et al., 1996). From the results, it is found that these amino acids could increase the antioxidant activity of the corresponding peptides; UEB=38.66%, EBH III=37.33% and EB-II=41.98%, suggesting that the hydrophobic amino acids found are indeed the radical-scavenging types.
Observation of antioxidant properties in amino acids is not necessarily new (Chen et al., 1996). Amino acids such as Met, Lys, Tyr, His, and Trp has been found to show antioxidant properties in safflower oil (Riisom et al., 1980). There are reports suggesting aromatic amino acids (Phe, His, Tyr, and Trp) also portray antioxidative property – converting radicals into stable molecules – while simultaneously maintaining own stability due to their resonance structure. In other words, aromatic amino acids have better radical-scavenging properties compared to other amino acids (Rajapakse et al., 2005; Sarmadi and Ismail, 2010).
The fractions of EB-II from gel filtration with the highest radical scavenging activity were separated by RP-HPLC. The sample solution containing differences in molecular polarity form the basis of RP-HPLC, where the antioxidant peptide of EB-II fraction of EBH was separated and thus, identified. Fig. 1B portrays ABTS radical scavenging activity (IC50) and the elution profile of RP-HPLC for the EB-II fraction that generates four fractions (EB-II1-EB-II4). The EB-II4 has the elution time of 11:94 min and has been verified with the highest ABTS radical scavenging activity, with IC50 of 0.12 mg/mL (p<0.05).
The timing in the hydrophobic chromatography column is essential in determining antioxidative peptides based on hydrophobic properties. Longer elution time means the sample is more hydrophobic, which correlates with antioxidative activity. The time taken for peptides in EBH reflects the antioxidative activity in the peptide sequences, which is further supported by the ABTS assay. Another study shows a similar idea of hydrophobic chromatography column where loach peptides with a higher amount of hydrophobic amino acids have longer retention time (Rajapakse et al., 2005).
The EB-II4 fraction which has the highest antioxidant activity was analyzed by matrix-assisted laser desorption/ionization time-of-flight/time-of-flight mass spectrometry (MALDI-TOF/TOF-MS) for identification of amino acid sequence. Fig. 2 shows the mass spectra of the antioxidant peptide from elastin hydrolysate (EBH). The amino acid sequence of the antioxidant peptides from EBH, as shown in Table 5 is characterized by the Ludwig NR databases. The following peptides were found from the results of mass spectral analysis: Gly-Ala-His-Thr-Gly-Pro-Arg-Lys-Pro-Lys-Pro-Arg (GAHTGPRKPKPR), of the parent protein trA0A021W2G6AZ78_21380 RNA helicase; peptide Gly- Pro-Gly-Phe-Asp-Val-Arg (GPGFDVR), of the parent protein H1W5B7 in parent protein, trH1W5B7CH063_00691 AMP-binding enzyme and peptide Ala-Asp-Ala-Ser-Val-Leu-Pro-Lye (ADASVLPK), of the parent protein trA0A011VSV8|RASY3_15265 Thiamine-phosphate pyrophosphorylase. It was evident that the amino acids Proline (P) and Valine (V) were present in all antioxidant peptides from the EBH. The molecular weight of antioxidant peptides of EBH is ranging from 799.38-1,447.82 Da (Table 5). The molecular weight of the fractions decreased after purification; ultrafiltration (<3 kDa)>gel filtration (0.5–3 kDa)>RP-HPLC (<1 kDa). These results confirmed that functional antioxidative peptides are highly affected by molecular structure and molecular mass, which is also the case in other studies involving other sources of peptides from marine and casein (Jeon et al., 1999; Suetsuna and Chen, 2002). The results in this study also show that antioxidative peptides of EBH have between 8-13 amino acid residues, which is supported by Pihlanto-Leppala (2000), where bioactive peptides are found to have typically between 2–20 amino acid residues.
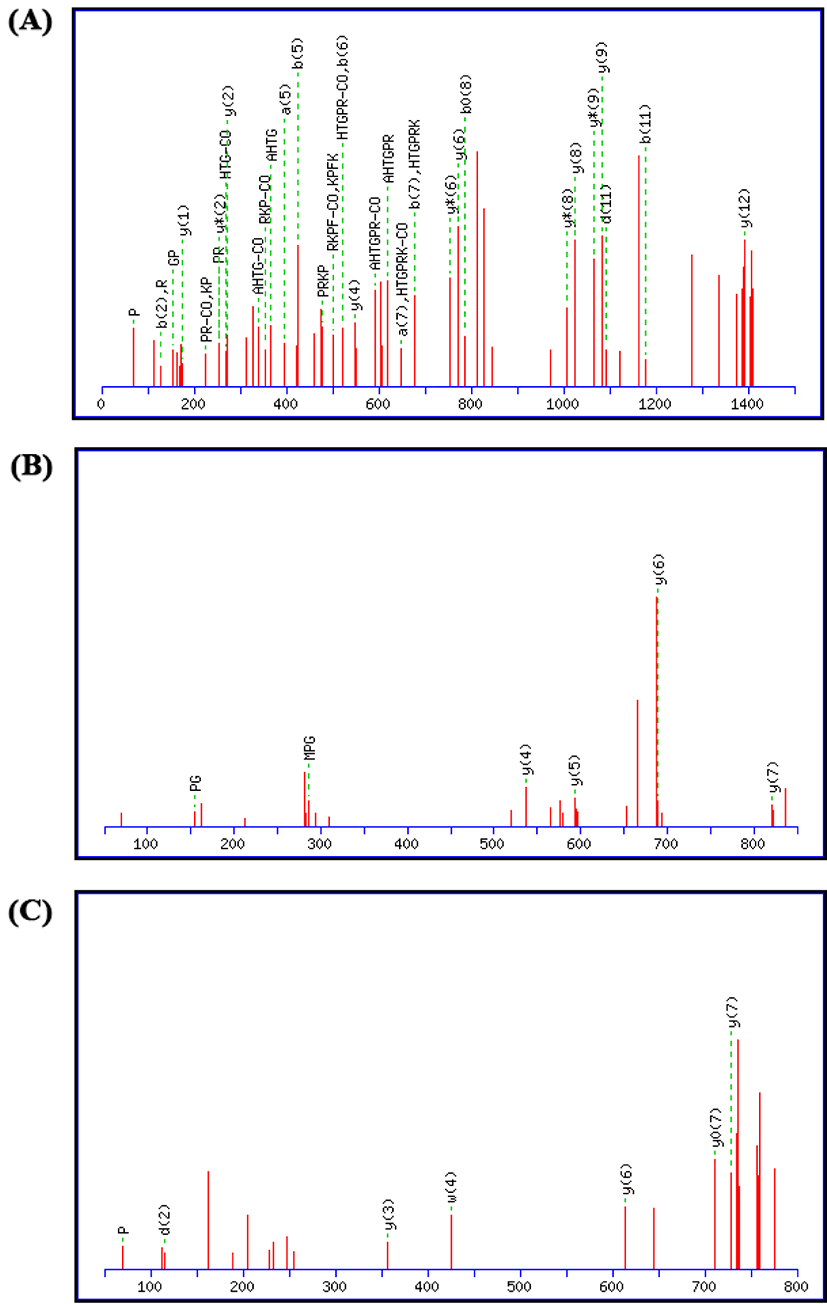
Overall, peptides of elastin extracted from EBH that have antioxidant activity contained relatively lower molecular weights and relatively hydrophobic amino acids, namely Glycine (G), Alanine (A), valine (V), and Phenylalanine (F). The results demonstrate the antioxidant capacity of elastin extracted from broiler, where the antioxidant peptides contain amino acid sequences involving aspartic acid, hydrophobic amino acids (valine and alanine), and hydrophilic amino acids (histidine and proline). Furthermore, the antioxidant property of EBH was reflected by the high ABTS radical scavenging activity thus proving the fact that free radical scavenging is the main antioxidant mechanism of EBH antioxidant peptides. The evidence that elastin hydrolysates from broiler (EBH) can be a novel source of antioxidants is also supported by a study on elastin isolated from neck ligaments (Rajapakse et al., 2005). These pepsin-solubilized and acid-solubilized elastin peptides possessed a low molecular weight and also confirmed having high antioxidant activity (Hattori et al., 1998).
Conclusion
In this study, elastin hydrolysate has been successfully extracted from poultry skin by using the enzyme elastase. Antioxidant peptides were separated using ultrafiltration, gel filtration chromatography and high-performance liquid chromatography (RP-HPLC). It was evident that the antioxidative activities were found in all the fractions. Further studies on elastin hydrolysate should include the use of a cell culture system to examine antioxidant properties of target peptides in vivo. The antioxidative protein hydrolysates obtained from the present study could potentially be a stepping stone on future animal and clinical studies.













