Introduction
Duck production has increased steadily over the past 20 years, and a total number of 1,132 million ducks was produced globally in 2014 (FAOSTAT, 2016). With increasing the production scale, the disposal of duck by-products as a biological waste, including feet, head, and intestine etc., results in environmental pollution. This has emerged one of major issues in the duck industry (Huda et al., 2013a). As a practically possible alternative, duck feet has received a great attention as a novel source for collagen and gelatin. Duck feet, which are webbed and a palmate bird feet structure, are composed of complex parts of bones and tendons. Due to such complicating structure, probably, previous studies on the application of duck feet as a collagen/gelatin source have commonly determined whole ground duck feet without deboning or separation of each part (Huda et al., 2013a; Huda et al., 2013b).
A total of twenty nine variants of collagen (type I-XXIX), which is abundant structural protein in mammal tissues, has been identified up to now (Wang et al., 2014). It has been reported that functional properties of collagen, such as gelling and film-forming properties, surface behavior, and microencapsulation, differs from collagen types (Gómez-Guillén et al., 2011). Thus, numerous previous studies have been conducted to characterize the collagen type of novel sources and to evaluate its functionality with various extraction methods (Lin and Liu, 2006a; Lin and Liu, 2006b; Wang et al., 2014). In particular, enzymatic digestion, using pepsin or papain, could improve the extraction yield of collagen and its functionality, such as thermal stability (Hashim et al., 2014; Matmaroh et al., 2011; Wang et al., 2008). In this regard, acid extraction with pepsin digestion has been generally considered as an effective collagen extraction method (Hashim et al., 2014). Thus, it would be reasonable to anticipate that high quality collagen can be extracted from duck feet through acidic extraction with pepsin digestion. However, there has been no literature on the isolation and characterization of collagen extracted from each part of duck feet. Therefore, the objectives of this study were to characterize pepsinsoluble collagen (PSC) extracted from bones, skins, and tendons of duck feet and to determine their thermal and structural properties. The results of this current study will provide detailed information on appropriate application of duck feet collagen to the food, pharmaceutic and cosmetic industries.
Materials and Methods
Frozen feet from Pekin ducks (Anas platyrhynchos, 6 wk) were obtained from a local poultry processor and thawed in a 4℃ refrigerator for 24 h. The thawed duck feet were washed several times with tap water, and the claws and external yellow skin were removed completely. The duck feet were manually deboned and visually assigned to three parts, such as bones, skin (including intermediate interdigital web, lateral interdigital web, and metatarsal pad), and tendons. Each part of duck feet was individually ground using a meat grinder equipped with 8 mm plate (MN-22S, Hankook Fujee Industries Co., Ltd., Korea), and the ground raw samples were used for PSC extraction.
PSCs were individually isolated from bones, skins, and tendons of duck feet according to the optimal condition suggested by Lin and Liu (2006a, 2006b) with slight modification. Each ground part of duck feet (100 g) was washed with 10 volumes (v/w) of 20% ethanol for 24 h to discard fat and pigments. The ethanolic-washed sample was centrifuged twice at 10,000×g for 20 min at 4℃. The residues obtained were treated with 10 volumes of 0.2 N NaOH for 24 h to remove proteinous substances, except for collagen. After washing with tap water, the residual part was treated with 10 volume of 0.5 M acetic acid containing 5% (w/w) pepsin (EC 3.4.23.1; P-7000, Sigma, USA) for 24 h (12℃). The acetic acid solution containing solubilized PSC was centrifuged at 20,000 g for 40 min at 4℃, and the supernatant was collected. The pH of supernatant was adjusted with 1 N NaOH to reach 7.0 and stirred to inactivate pepsin for 24 h. The pH was adjusted again with 0.5 M acetic acid to reach 3.5. Subsequently, PSC was precipitated with 0.9 M NaCl (final concentration) and was centrifuged at 20,000 g for 40 min at 4℃. The PSC precipitate was dissolved in 10 volume of 0.5 M acetic acid, and the PSC solution was dialyzed against 0.05 M acetic acid for 24 h. Finally, PSC powders were obtained through lyophilization and pulverizing.
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method of Laemmli (1970), using 7.5% running gels and 5% stacking gels (20 μL loaded). PSC (5 mg/mL of protein concentration) was dissolved in a buffer solution (0.02 M sodium phosphate, pH 7.2, containing 1% SDS and 3.5 M urea, Nagai and Suzuki, 2000) with and without 10% β-mercaptoethanol to identify disulphide bonds in PSCs. The loaded gel was stained with Coomassie Brilliant Blue R 250 (B7920, Sigma Chemical Co., USA), and was destained in methanol:distilled water:acetic acid solution (50:40:10). The separated protein bands were identified by comparison with those of standard protein marker (Precision Plus Protein Standards, Bio-Rad Lab., Hercules, USA).
Thermal stability of PSCs from duck feet was determined in triplicates by using differential scanning calorimetry (DSC, thermal analyzer, TGA 2050, Mettler Toledo, USA). The heat rate and range were 5℃/min and 5-150℃ (Lin and Liu, 2006b).
Fourier transform infrared spectroscopy (FT-IR) was conducted in triplicates by using a FT-IR system (Nicolet 6700, Thermo, USA). The spectra ranged from 400-4,000 cm−1, and beam splitter and infrared spectra source were potassium bromide (KBr) and Ever-GLo light source, respectively.
Surface morphology of PSC from duck feet was observed using a cold field emission scanning electron microscopy system (FE-SEM, S-4800, Hitachi, Japan). The observations of SEM were conducted at 10 kV accelerating voltage, and the gold-coated samples were scanned at ×100 and ×400 magnifications.
Results and Discussion
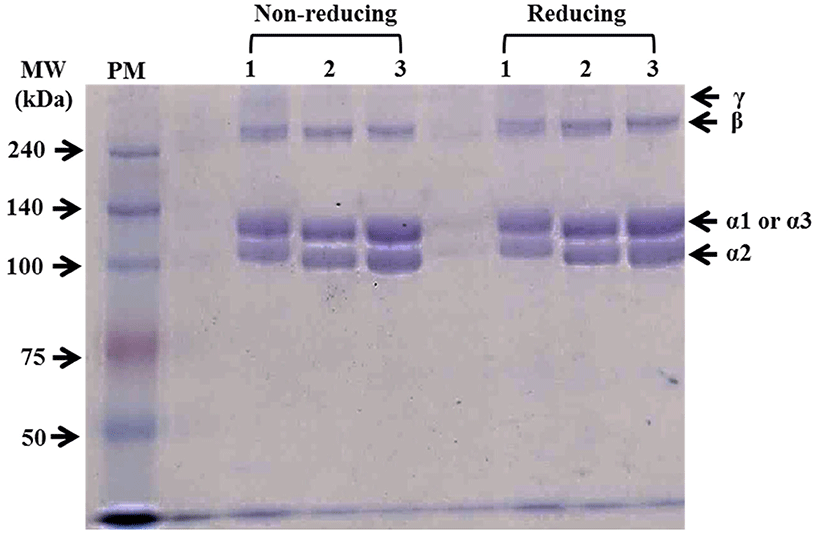
The protein patterns of PSCs extracted from duck feet using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) with reducing (with β-mercaptoethanol) and non-reducing (without β-mercaptoethanol) conditions are shown in Fig. 1. Two main monomers (α1 and α2 chains) between 100 and 140 kDa and β chain (dimer) were observed for all PSCs. Only PSC from duck feet bones (PSC-B) contained a small amount of γ chain (trimer). Previously, the ratio between α1 and α2 chains (2:1) has been regarded as one of characteristics of collagen type I extracted from various species (Lin and Liu, 2006a; Matmaroh et al., 2011). In this current study, the ratio between α1 and α2 of PSCs was similar, approximately 1:1. Cheng et al. (2009) reported that the protein band pattern of PSC from silky fowl feet differed from that of typical collagen type I and suggested that silky fowl feet might include more than two types of collagens, such as collagen type I and II. Thus, our finding suggests that PSCs from duck feet might be a mixture of collagen type I and II. In comparison with each PSC from duck feet, PSC-B had slightly higher molecular weights for α1 and α2 chains than PSC-S and PSC-T. According to Matmaroh et al. (2011), pepsin digestion could cleave telopeptide region, which contributes to the degradation of β and γ chains to α chains. The slight differences in the molecular weight of α chains might be related to the different cleavage sites on the collagen molecules from each part of duck feet. No differences in protein patterns between reducing and non-reducing conditions were found, indicating no disulphide bonds in PSCs from duck feed (Matmaroh et al., 2011).
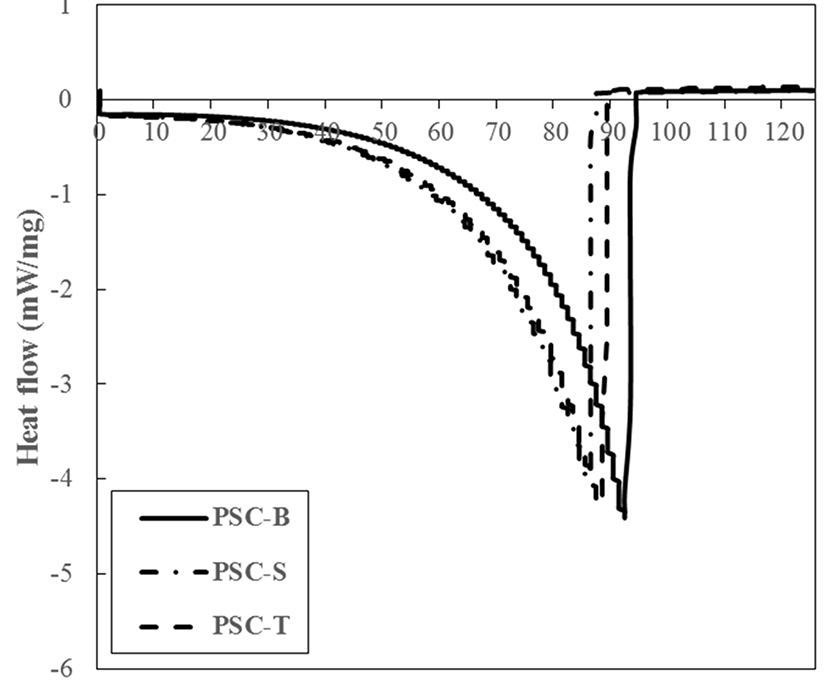
DSC patterns of PSCs from duck feet were measured to determine thermal properties (Fig. 2). PSC-B showed more resistant to thermal impact, when compared to PSC-S and PSC-T. The onset temperature (To, beginning point of melting) of PSC-B, PSC-S, and PSC-T was 77.83, 65.72, and 68.58℃, respectively (p<0.05). In addition, the peak temperature (Tp, maximum point of curve) of PSC-B (92.48℃) was higher than those of PSC-S (86.22℃) and PSC-T (88.46℃) (p<0.05). According to Lin and Liu (2006a), Tp of PSC from chicken broiler feet was 88.77℃, which was similar to our result. The thermal stability of collagen is generally affected by amino acid composition, growing temperature of collagen source, and extracting methods (Lin and Liu, 2006a; Lin and Liu, 2006b). In particular, pepsin digestion could lead to a decrease in denaturation temperature due to decreased molecular weight of collagen subunits by the removal of telopeptides region (Nalinanon et al., 2007). Thus, it was likely that the presence of γ chain and the high molecular weight of α chains might be responsible for high thermal stability of PSC-B.
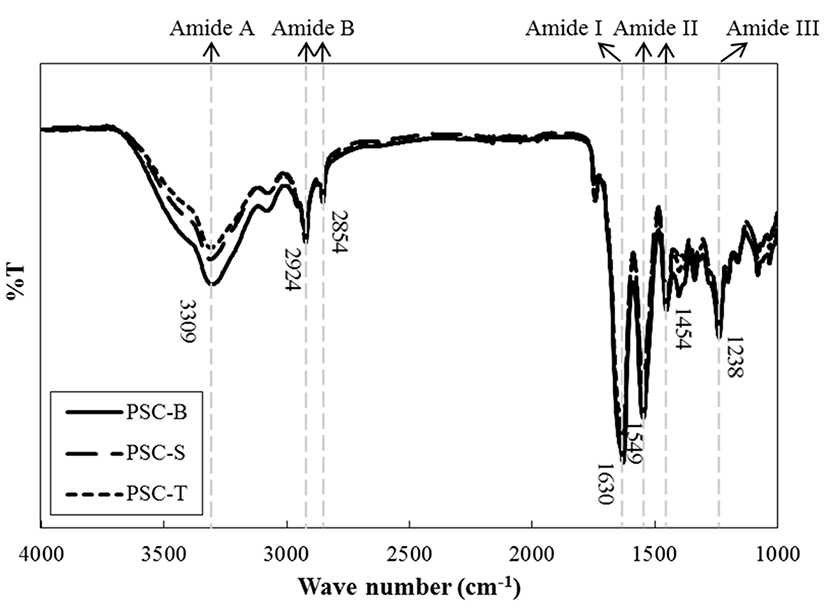
FT-IR, which is an analytical technique based on vibration and stretching in covalent bonds with IR radiation, has been extensively used as a powerful method to determine the characteristics of collagen and to identify the types of collagen (Belbachir et al., 2009). Amide A (~3,309 cm−1), amide B (~2,854 and 2,924 cm−1), amide I (~1,630 cm−1), amide II (~1,454 and 1,549 cm−1), and amide III (~1,238 cm−1) spectra were mainly observed for all PSCs from duck feet (Fig. 3). However, there were no differences in the regions for major spectra among PSC-B, PSC-S, and PSC-T. In general, the regions for amide A (N-H stretching vibration indicating hydrogen bonds) and B (CN stretching and asymmetrical stretching of CH2) peaks are found at 3,400-3,440 cm−1 and 2,925-2,935 cm−1, respectively. In this current study, the wavenumber of amide A was slightly shifted to lower regions, which could imply the presence of hydrogen bonds in PSCs from duck feet. The %T of PSC-B at amide A peak was lower than those of PSC-S and PSC-T, which indicates the presence of large numbers of hydrogen bond (Li et al., 2013). Amide I (C=O stretching indicating stretching vibrations of carbonyl groups in peptides), II (NH bending and CH stretching), and III (CN stretching) spectra are typically detected at 1,600-1,700 cm−1, 1,550-1,600 cm−1, and 1,454 cm−1 regions, respectively. Those peaks in PSCs from duck feet were found at lower wavenumbers compared to regular spectra reported. It has been suggested that the shifting of wavenumber at amide I, II, and III peaks is associated with the presence of secondary protein structure, hydrogen bonds, and intact triple helical structure in collagen, respectively (Liu et al., 2012; Muyonga et al., 2004). The ratios between amide III peak and the peak at 1,450 cm−1 were approximately 1.07. Matmaroh et al. (2011) reported that the ratio close to 1.0 could signify the existence of alpha triple helix structure in collagen molecules. Thus, our results indicate that PSC-S and PSC-T have similar intermolecular cross-links and chemical bonds and suggest that PSC-B had slightly different intermolecular bindings, which might be affected by the presence of more hydrogen bonds compared to PSC-S and PSC-T.
In all PSCs from duck feet, white sponge with porous surface structure was visible to the naked eye. As seen in Fig. 4, irregular structures including porous sheets and coiled filaments were observed. This morphological characteristics was similar to the PSCs from Amur sturgeon (Wang et al., 2014). Particularly, a loose and porous structure consisting of numerous random-coiled filaments was found at PSB-B. The structure of lyophilized collagen is an important factor determining application of collagen as a functional material to the food and pharmaceutical industries, and it is affected by the freeze-drying condition, collagen concentration, water content, collagen type with the proportions of α1, α2, β, and γ chains, and chemical treatments (Li et al., 2013; Zhang et al., 2007). In this current study, the increased irregularity and porosity in the structure of PSC-B was probably due to the different degree of pepsin digestion and cleavage sites at the regions of telopeptide.

In conclusion, our findings indicate that PSCs of duck feet might be similarly characterized as a mixture of collagen type I and II. PSC from duck feet bones showed slightly higher molecular weight of α chains compared to PSCs from duck feet skin and tendons, which could affect intermolecular crosslinks, thermal stability, and surface morphology. Therefore, this study shows that PSCs from duck feet have almost equivalent quality and suggests that duck feet could be used for collagen extraction without deboning and/or separation processes. Further, the efficacy of pepsin digestion on extractability and chemical composition of PSCs from duck feet would be warranted.













