Introduction
Chicken meat is considered a staple of the human diet, providing an important source of animal protein and other nutrients such as B vitamins, iron, zinc, phosphorous, and magnesium (Delgado et al., 2021; Kim et al., 2020; Promket et al., 2016). It is more acceptable than red meat due to low cholesterol, fat, and saturated fatty acid contents and high protein contents, which are important human health considerations (Jaturasitha et al., 2008a; Jaturasitha et al., 2008b; Tunim et al., 2021). According to recent statistics, poultry are more widely raised and consumed (Magdelaine et al., 2008), in particular, the market of indigenous chickens is rapidly growing (Tang et al., 2009). The majority of people prefer indigenous chicken meat over broiler chicken meat because of its distinct taste and flavor and the lack of potentially dangerous substances such as drug residues, intoxicants, allergenic components, and microbial contamination, all of which can contribute to health issues (Charoensin et al., 2021; Grashorn, 2007; Wattanachant et al., 2004).
As in other Sub-Saharan African and Asian countries, in Tanzania, the trend of indigenous chicken production and consumption continues to rise faster than that of broiler chickens. Furthermore, people in Tanzania prefer indigenous chicken meat over that of broiler chickens because of its unique taste and flavor, disease resistance, heat tolerance, and affordability (Safalaoh, 1997). According to Michael et al. (2018), Tanzania has 43.7 millions chickens, and 41.8 million of them (96%) are indigenous chickens. Among the popular indigenous chicken breeds are Ufipa, Kuchi, Ching’wekwe, Morogoro-medium, Pemba, Unguja, Tanga, Mbeya, Singamagazi, and Nzenzegere (Khondowe et al., 2018; Lwelamira, 2012; Lwelamira et al., 2008; Lyimo et al., 2013; Msoffe et al., 2005). Researchers have classified these chickens on the basis of phenotypic characteristics such as plumage color, body shape and size, production, and geographical origin (Msoffe et al., 2001; Msoffe et al., 2004; Msoffe et al., 2005; Msoffe et al., 2006).
Ufipa indigenous chickens are among the Tanzanian-bred chickens that originated in the Rukwa region of the Southern Highlands. Due to their distinct sensory characteristics (taste, flavor, and texture), consumers prefer them to broiler chickens, as they do other indigenous chickens. They are widely consumed as roasted meat in most urban locations, particularly in hotels and restaurants and at weddings (Manning et al., 2007; Temba et al., 2016).
Regardless of the phenotypic and geographical definition of Tanzanian indigenous chickens, researchers have speculated that physicochemical characteristics or properties might exist among these chickens (Msoffe et al., 2001; Msoffe et al., 2004; Msoffe et al., 2005; Msoffe et al., 2006). Physicochemical characteristics or properties of chicken meat (determinant of meat quality) such as color or appearance, texture, juiciness, tenderness, and flavor have been reported to be superior in other indigenous chickens, for example, those indigenous to Thailand and Korea (Choe et al., 2010; Promket et al., 2016; Wattanachant et al., 2004) and are generally considered to be influenced by genotype (breed/strain; Fletcher et al., 2000; Jayasena et al., 2013; Mir et al., 2017; Qamar et al., 2019; Tang et al., 2009). However, there is currently a scarcity of information regarding the physicochemical factors influencing the taste and texture of Tanzanian indigenous chicken meat. Therefore, the objective of this study was to characterize the meat quality traits that affect the texture and savory taste of Ufipa indigenous chickens by comparing the proximate composition, physical characteristics, collagen content (which is involved in tenderness/toughness and textural qulities), and nucleic acid content (which is involved in meat flavor and taste) with those of commercial broilers. The findings of this study provide a broad understanding of why most people prefer indigenous chicken meat to broiler chicken meat. Furthermore, this first report on Ufipa indigenous chickens represents the market and consumer impression of the healthy meat of other Tanzanian indigenous chickens.
Materials and Methods
This study was conducted in Sumbawanga Municipality in Rukwa Region, Tanzania. Sumbawanga Municipal is the administrative center of the Rukwa Region (province). The Rukwa Region is among the 30 regions in the United Republic of Tanzania found in the Southern Highlands, latitude 7°58S and longitude 31°37’.
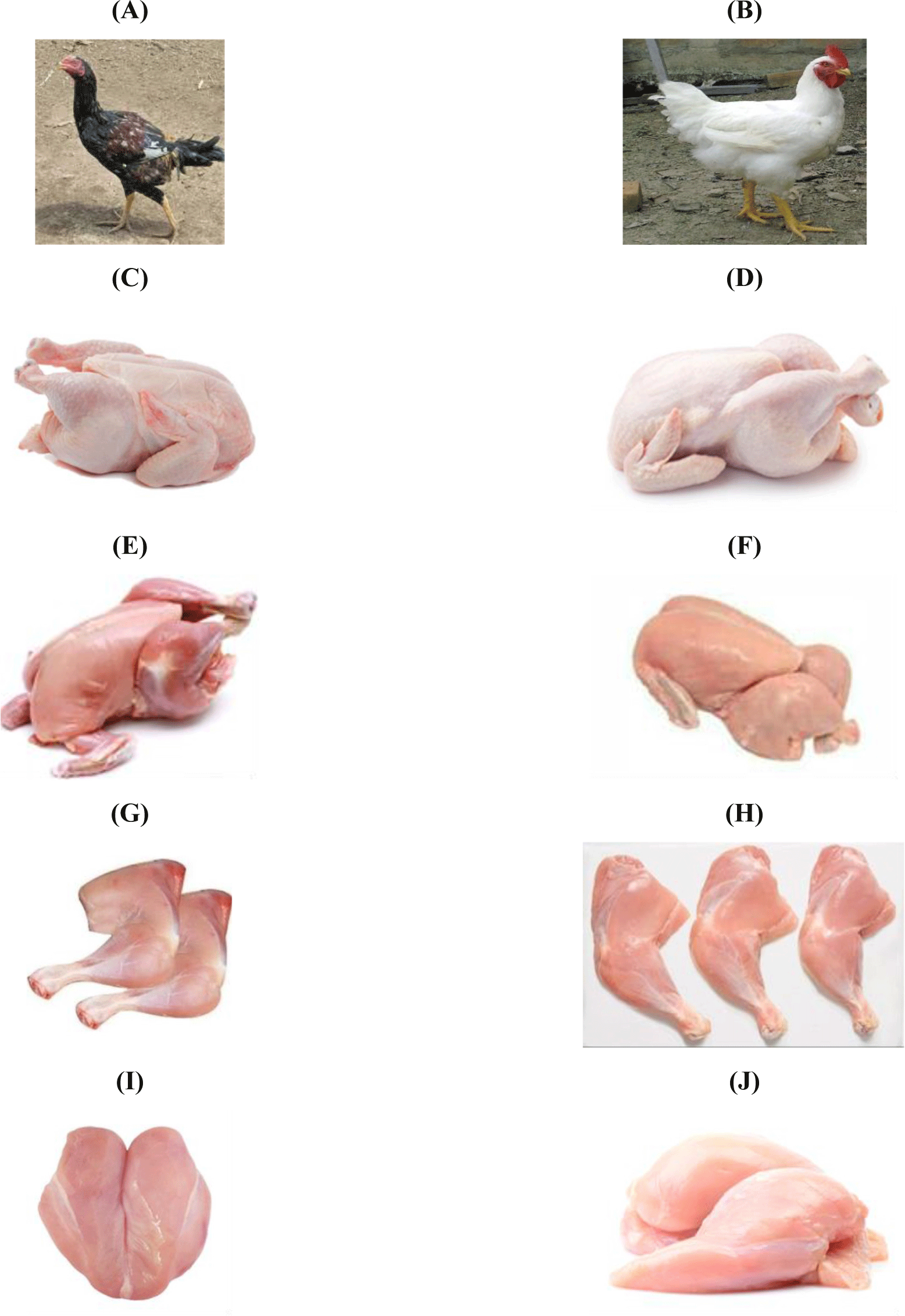
Forty-eight carcasses of chicken meat from two chicken breeds, Tanzanian indigenous chickens [Ufipa chickens (n=24)] and commercial broilers [Ross chickens (n=24)], which were raised under a homogeneity environment in terms of management, were obtained from local farms within Sumbawaga municipal. The animals were slaughtered using conventional neck cut, bled for 2 min, scalded at 60°C for 2 min, plucked in a rotary drum picker for 30 sec, and eviscerated at their market weight (1.5 kg) at 24 weeks for Ufipa chickens and at 12 weeks for commercial broilers. The carcasses were stored at 4°C for 24 h. After chilling, the breast and thigh muscles were dissected from the whole carcass sample of meat chicken. Fig. 1 shows different carcass traits of Ufipa and commercial Ross chicken breeds. Twelve samples from each breed were vacuum-packed and stored at 4°C until used to determine the proximate composition and physical characteristics [pH, color, water holding capacity (WHC), and shear force values] within 24 h. The others were minced, placed in plastic bags, and stored frozen (–20°C) until used for chemical analyses (collagen and nucleic acid contents).
All operations were approved by the Animal Ethics Committees of the Sokoine University of Agriculture, with the assistance of the Sumbawanga Municipal Veterinary Officer.
The proximate composition (moisture, ash, protein, and fat) of breast and thigh muscles were measured using the method of AOAC (1997) with little modifications in Intarapichet et al. (2008). Briefly, the moisture content was determined by oven-drying at 105°C for 24 h. The ash content was determined by ashing at 600°C in a muffle furnace (Carbolite, UK) for 6 h or until light gray or white ash was obtained. Total protein (n×6.25) content was determined by the Kjeldahl method. To determine fat content, 9 g of briefly ground breast and thigh meat meat and 4.5 g of skin were used for analysis. Percent fat content was read directly from the Paley bottle. The multiplication factor of 2 was used for the skin sample.
The pH of breast and thigh samples was measured after chilling for 24 h using a pH meter (HI99163, Hanna Instruments, Woonsocket, RI, USA) as described by Motsepe et al. (2016). Briefly, the glass electrode of the pH meter was inserted directly into the center of the breast and thigh meat, and the mean value from two replicates were recorded.
The color was measured using the method described by Mourão et al. (2008). Breast and thigh muscles were measured on the medial side of each muscle using a Minolta CR-200 Chroma Meter reflectance colorimeter (Konica Minolta, Osaka, Japan). Three color readings, each measured in 4 replicates, were taken from different locations on each muscle (breast and thigh). The color was measured using the L*, a*, and b* scale, where L* represents the degree of lightness (0=black to 100=white or bright), a* represents green (–a*) to dark or red (+a*), and b* represents blue (–b*) to yellow (+b*). Values for each color were averaged together.
The WHC was measured in terms of the drip loss, the thawing loss, and the cooking loss, according to the method described by Mueller et al. (2018). Briefly, drip loss was measured by positioning the whole left breast and thigh muscles freely hanging in a net placed in a sealed plastic bag at 2°C–4°C for 24 h and calculated as the percentage of weight loss during storage. For measurement of thawing and cooking losses, the right breast and thigh meat were weighed before freezing, after thawing overnight, and after cooking to a core temperature of 74°C in a water bath in sealed bags, respectively. The samples were then allowed to equilibrate to room temperature and reweighed, and cooking loss was measured as the weight loss percentage. The rest of the sample was kept for shear analysis.
Shear force was measured on a cooked meat samples using the method described by Wattanachant et al. (2004). Briefly cooked meat samples were cut to 1.0×2.0×0.5 cm for shear measurement using a texture analyzer equipped with a Warner-Bratzler shear apparatus. The operating parameters consisted of a 2 min/sec crosshead speed and a 50-kg load cell. The shear force perpendicular to the axis of muscle fibers was measured with four replicates for each of 4 birds for both breast and thigh muscles. The peak of the shear force profile was regarded as the shear force value.
The total collagen content was measured via alkaline hydrolysis, as described by Reddy and Enwemeka (1996). The samples were hydrolyzed with 7 M sodium hydroxide (NaOH) at 120°C for 40 min. The hydrolysate was neutralized with 3.5 M sulfuric acid (H2SO4), filtered, and reacted with chloramine T solution and Ehrlich’s reagent. The absorbance at 550 nm was measured using a spectrometer (Jenway, Bibby Scientific, Staffordshire, UK). The amount of hydroxyproline was measured, and the total collagen content was calculated using a factor of 7.25 while the content of insoluble collagen was measured following the method described by Liu et al. (1996). The meat samples were homogenized with 25% Ringer solution, and the homogenates were heated to 77°C for 70 min in a water bath and then centrifuged at 2,300×g for 30 min at 4°C. The extraction was repeated twice, and the residues were dried overnight at 105°C. The insoluble collagen content of the residues was measured and calculated.
Inosine-5’-monophosphate (IMP), adenosine-5’-phosphate (AMP), hypoxanthine, and inosine, were measured using the method described by Choe et al. (2010). Briefly, the meat samples (5 g) were mixed with 25 mL of 0.7 M perchloric acid and centrifuged for 1 min at 1,130×g to extract nucleic acids. The extracted nucleic acids were then centrifuged for 15 min at 2,090×g and filtered through Whatman No. 4 filter paper (Whatman, Clifton, NJ, USA). The supernatant was then adjusted to pH 7 with 5N KHO. The pH-adjusted supernatant was placed in a volumetric flask and the volume adjusted to 100 mL with 0.7 M perchloric acid (pH 7). After 30 min of cooling, the sample was centrifuged at 1,130×g (0°C), and the supernatant was filtered through a 0.2 um PVDF syringe filter (Whatman International, Maidstone, UK). The filtrate (5 mL) was measured using HPLC (ACME 9000, Young Lin Instruments, Seoul, Korea). With regard to the analytical conditions for HPLC, A waters-Atlantis Dc18RP column (4.6×250 mm, 5 um particles, Waters, Milford, MA, USA) was utilized, with a mobile phase of 0.1 M triethylamine in 0.15 M acetonitrile (pH 7.0). The flow rate of the mobile phase was 1.0 mL/min, and the injection volume was 10 μL. The column temperature was maintained at 35°C, and detection was monitored at a wavelength of 260 nm. The peaks of the individual nucleotides were identified using the retention times for standards: Hypoxanthine, inosine, IMP, AMP (Sigma-Aldrich, St. Louis, MO, USA), and the concentration was calculated using the area under each peak.
Statistical analyses were performed using SPSS v 16.0 software (SPSS, Chicago, IL, USA). The independent t-test was used to analyze the means differences of the chicken breeds between Ufipa indigenous, and the Ross broiler chickens on proximate composition (protein, fat, ash, and moisture content) and physicochemical characteristics/properties (pH, color, WHC, shear forces, collagen, and nucleic acid). Results were presented as the mean±SEM at a p<0.05, which differed significantly. Before determining the statistical difference, the population variance (equal or unequal variance) between the number of samples in each treatment group and each parameter was tested by Welch’s t-test. Principal component analysis (PCA) was performed with proximate composition, physical characteristics, collagen, and nucleic acid data in each chicken breed to demonstrate those relationships of meat quality characteristics and get more information about the variables that primarily influence meat quality.
Results and Discussion
The proximate composition of Ufipa indigenous chickens and Ross broiler chickens is shown in Fig. 2. The crude protein content was higher in the breast than in the thigh muscle, while the reverse was true for crude fat and moisture in both chicken species, but ash content was not different between the breeds (p>0.05). However, a comparison of species with similar muscle types demonstrated that the crude protein content of Ufipa indigenous chickens was significantly higher than that of Ross broiler chickens. By contrast, the crude fat content was lower (p<0.01). Furthermore, the moisture content of Ufipa indigenous chicken thigh meat was significantly lower than that of Ross broiler chicken thigh meat (p<0.05).
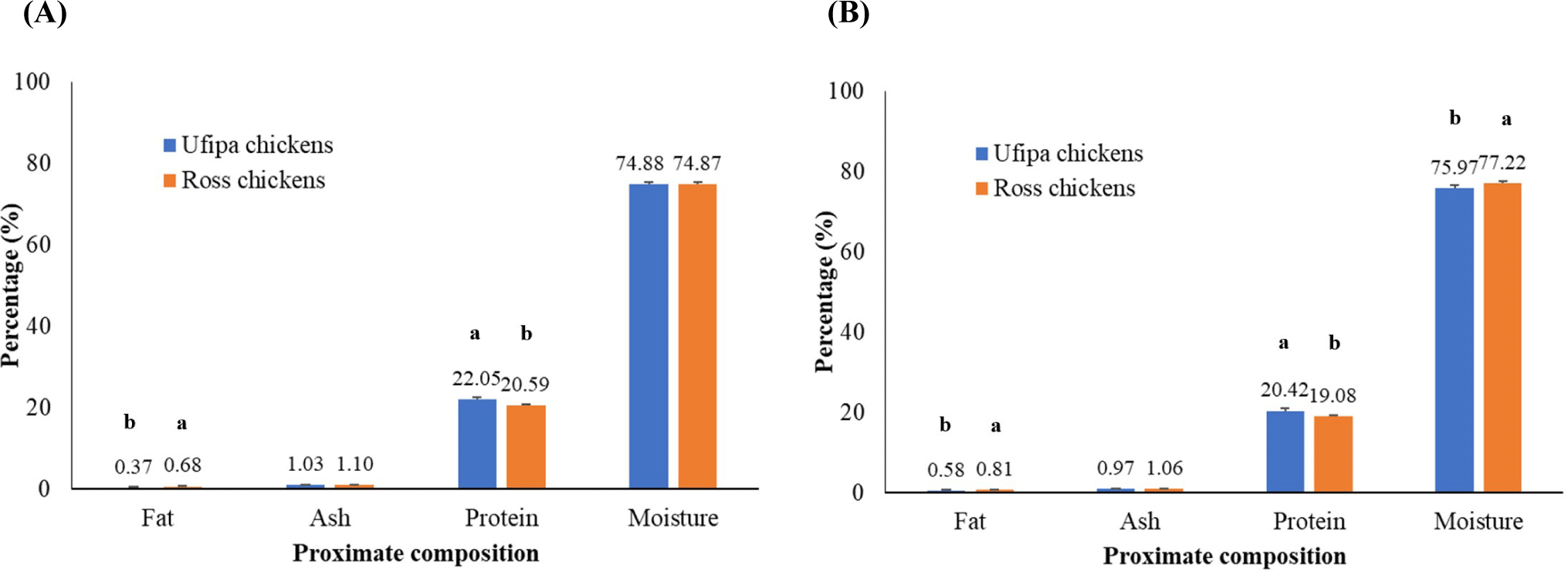
These findings are inconsistent with those previously published (Jung et al., 2014; Tang et al., 2009; Wattanachant et al., 2004) regarding the breast and thigh meat of Thai, Chinese, and Korean chickens versus broiler chickens (Zerehdaran et al., 2004). The higher fat content in broiler chickens was attributed to a higher supply of feeds than was required, resulting in increased muscle growth and maintenance. Because of that, excess energy was generated to boost body fat deposition to meet the market demand (Zerehdaran et al., 2004). By contrast, Choe et al. (2010) and Jung et al. (2010) showed that the lower fat content in Korean indigenous chickens compared to broilers was due to their unique characteristics and genotype (breed/strain; Tang et al., 2009). Moreover, Jung et al. (2014) showed that the difference in crude protein was attributed to the activity of chickens during growth since indigenous chickens are more active than broilers. Therefore, they are likely to contain higher crude protein amounts, confirming the genetic difference between the breeds. This might be a reason for the significantly higher crude protein in the breast and thigh meat of Ufipa indigenous compared to broiler chickens. A lower moisture content in thigh meat of Ufipa indigenous chickens has been reported previously in Thai and Korean indigenous chickens compared to broiler chickens (Jayasena et al., 2013; Wattanachant et al., 2004).
Table 1 shows the physicochemical characteristics of pH, color, and WHC. There was no statistically significant difference in the pH parameter between the Ufipa indigenous chicken and the Ross broiler chicken. Regarding color values, Ross broiler chickens had considerably higher L* and b* in both breast and thigh meat than Ufipa indigenous chickens (p<0.01), except for a*, which was significantly greater in Ufipa indigenous chickens (p<0.01). The color values demonstrate that Ross broiler chicken breast and thigh meat was brighter and yellower than that of Ufipa indigenous chickens, but the breast and thigh meat of Ufipa indigenous chickens were darker than those of broilers. Fletcher et al. (2000) and Wattanachant et al. (2004) found that the color difference between Thai indigenous and broiler chickens was due to the meat pH, with a higher pH resulting in darker meat. However, this thesis contradicts our findings because the pH of both meat types was not significantly different between the breeds, implying that the meat did not experience oxidative stress during processing. However, Mir et al. (2017) and Qamar et al. (2019) found that differences in meat color are likely attributable to the impact of genotype (breed/strain) and myoglobin content, which tends to increase with the animal’s age (Hoffman et al., 2009). This thesis is consistent with our findings. The darker breast and thigh meats of Ufipa indigenous chickens were mainly related to breeding; they had an increased myoglobin concentration because they were slaughtered at a later age than Ross broiler chickens.
The WHC in breast and thigh meat differed significantly (p<0.05) between breeds as measured in terms of drip loss, thawing loss, and cooking loss (Jaturasitha et al., 2016). Ufipa indigenous chicken breast had significantly higher (p<0.01) drip loss but lower thawing and cooking losses than that of Ross broiler chicken. In contrast, its thigh meat had significantly lower (p<0.01) drip and thawing losses but a higher cooking loss than Ross broiler chicken. Our findings are consistent with previous research. WHC is defined as the ability of raw meat to retain water or moisture under normal storage conditions and during thermal processing. It is vital in the carcass and other processed-meat products because it contributes to the juiciness and tenderness of the meat (Mehaffey et al., 2006; Wang et al., 2009). The higher drip loss reduces the WHC and shelf-life of meat and thus, meat tenderness (Mir et al., 2017), whereas the lower drip loss in ducks with increasing age at slaughter reduces the water content of breast meat (Baeza et al., 2002). As a result, increased drip loss in the breast meat of Ufipa indigenous chickens is likely due to their lower freshness. This also is consistent with Fanatico et al. (2007), who reported that slow-growing chickens have higher drip losses than fast-growing ones. As a result, it was hypothesized that because slow-growing chicken fillets are smaller and thinner, they had a greater surface area exposed to the air, relative to the meat mass, resulting in increased drip loss.
Furthermore, decreased thawing losses in Ufipa indigenous chicken breast and thigh meat have been reported (Abdullah and Matarneh, 2010). Regarding thawing loss (Fanatico et al., 2007), found that the freezing rate caused greater thawing losses in fast-growing chickens than in slow-growing ones. It was believed that because fast-growing chicken fillets were heavier and larger in size or thickness, the freezing rate was slower, resulting in the formation of large ice crystals and greater membrane damage. Although we did not measure the size of the fillets in our study, the above-described phenomenon has been widely accepted in previous studies, so we suggest that it will hold true in ours. Furthermore, thawing loss in chickens decreases with age (Abdullah and Matarneh, 2010). This argument may also hold true based on our findings that Ufipa indigenous chickens were older and had reduced thawing loss relative to Ross broiler chickens.
Table 2 shows the shear force values and collagen content. Shear force values were significantly higher in the breast and thigh meat of Ufipa indigenous chickens than in those of Ross broiler chickens (p<0.05).
Our findings are consistent with those previously published in Jaturasitha et al. (2008b), Tang et al. (2009), and Wattanachant et al. (2004) regarding Thai chickens versus broiler chickens and Chinese breeds (Wenchang and Xiang) versus broilers (Avian and Lingnanhuang). Furthermore, shear force values were higher in thigh meat than in breast meat, as previously reported (Chen et al., 2016). Shear force in Korat Thai chicken meat increased with age, resulting in tougher or less tender meat with a chewier texture (Cavitt et al., 2004; Katemala et al., 2021). This thesis is consistent with our findings that higher shear force values in Ufipa indigenous breast and thigh meat are likely related to older age at slaughter, which means less tender or tougher meat than broiler meat. In contrast (Kaewkot et al., 2019) found that despite the higher shear force values of Thai indigenous chicken breast and thigh meat when compared to that of Ross 308 chickens, its meat was more tender, a finding attributable to its textural uniqueness.
Regarding collagen content, the total collagen concentration of Ufipa indigenous thigh meat was substantially higher than that of Ross broiler chickens (p<0.05) but not statistically different in breast meat (p>0.05). These findings are consistent with those previously published by Ding et al. (1999) and Jayasena et al. (2013) on indigenous Chinese and Korean chickens versus broiler chickens.
Tenderness/toughness and textural qualities or characteristics of meat are influenced by collagen content, particularly insoluble and total collagen (Dawson et al., 1991; Jeon et al., 2010). The important role of collagen is to integrate the intramuscular connective tissues (endomysium, perimysium, and epimysium), resulting in meat with a tough or rough texture (Purslow, 2005). The difference in collagen concentration in breast and thigh meat between Korean and broiler chickens was related to the impact of age (Jayasena et al., 2013). According to Petracci and Cavani (2012), when animals age, the collagen cross-links between collagen molecules strengthen, resulting in increased strength of intramuscular connective tissues to join together, resulting in tough meat. This thesis is consistent with our findings. It suggests that the higher total collagen content in the thigh meat of Ufipa indigenous chickens was attributable to age differences, as they were older at slaughter than broiler chickens. It also implies that their thigh meat is tougher or harder and less tender than broiler thigh meat (Intarapichet et al., 2008).
The content of nucleic acids involved in meat flavor is shown in Fig. 3. Ufipa indigenous chicken breast and thigh meat had considerably higher nucleotide levels in IMP (190.30±2.38 and 59.10±2.86, respectively) than those of Ross broilers (155.00±2.38 and 24.70±2.86, respectively; p<0.05). However, there were no significant differences in AMP and inosine levels between breeds (p>0.05).
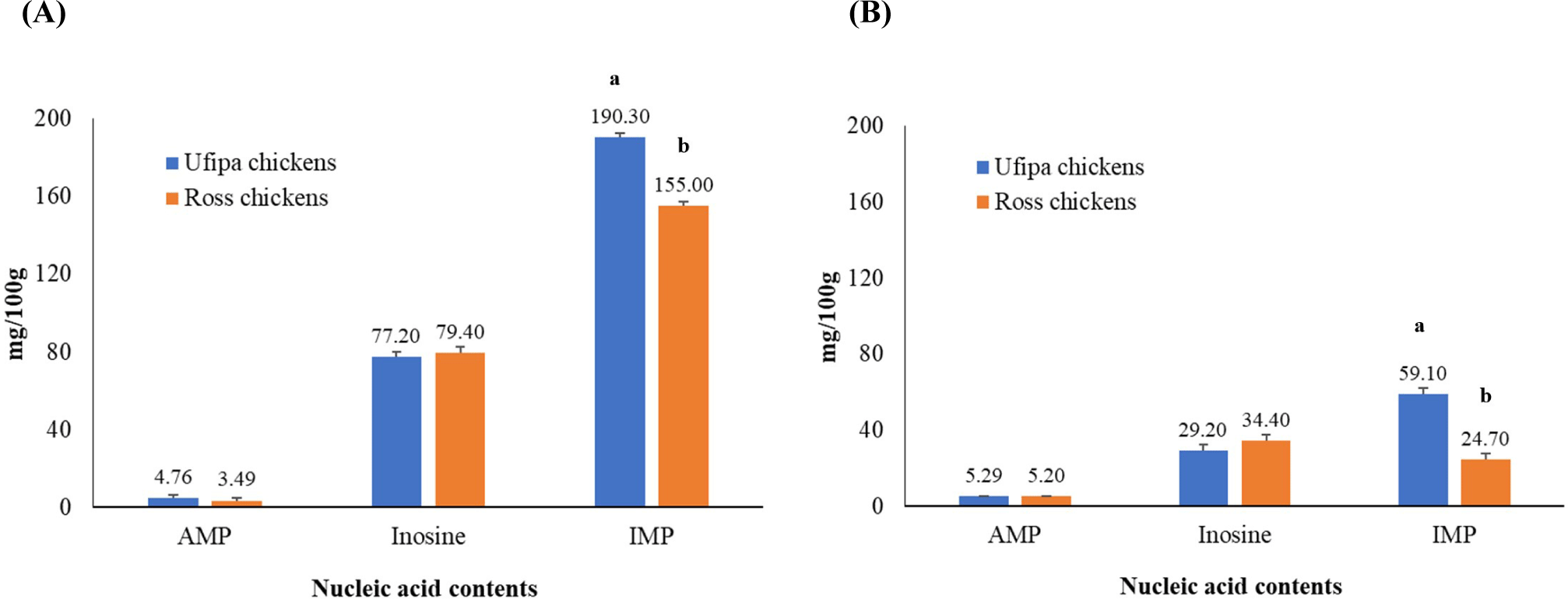
IMP, AMP, and inosine are byproducts of the breakdown of adenosine triphosphate (ATP), which occurs in muscle during the slaughtering and postmortem aging phases (Aliani et al., 2013). IMP degradation is one of these byproducts that contributes to the production (transformation) of ribose in meat, which imparts the flavor and umami character of chicken meat (Kawai et al., 2002; Manabe et al., 1991). Our findings are consistent with those published in Escobedo del Bosque et al. (2020). Furthermore, consistent with previous findings, IMP was more concentrated in breast meat than in thigh meat (Aliani et al., 2013; Katemala et al., 2021). Tang et al. (2009) found that the higher IMP content in Wenchang and Xiang Chinese indigenous chickens than in Avian and Lingnanhuang broiler chickens was attributable to the impact of genotype (breed/strain) and age or other interactions. This observation is consistent with our findings and with reports published by others (Ahn and Park, 2002; Kaewkot et al., 2019; Rikimaru and Takahashi, 2010) regarding Korean, Japanese (Hinai-jidori), and Thai indigenous chickens compared to broiler chickens, in which the higher IMP content was influenced by breed and age, implying a more umami taste and flavor. As a result, we believe that Ufipa indigenous breast and thigh meat has a more umami taste and greater flavor than Ross chicken.
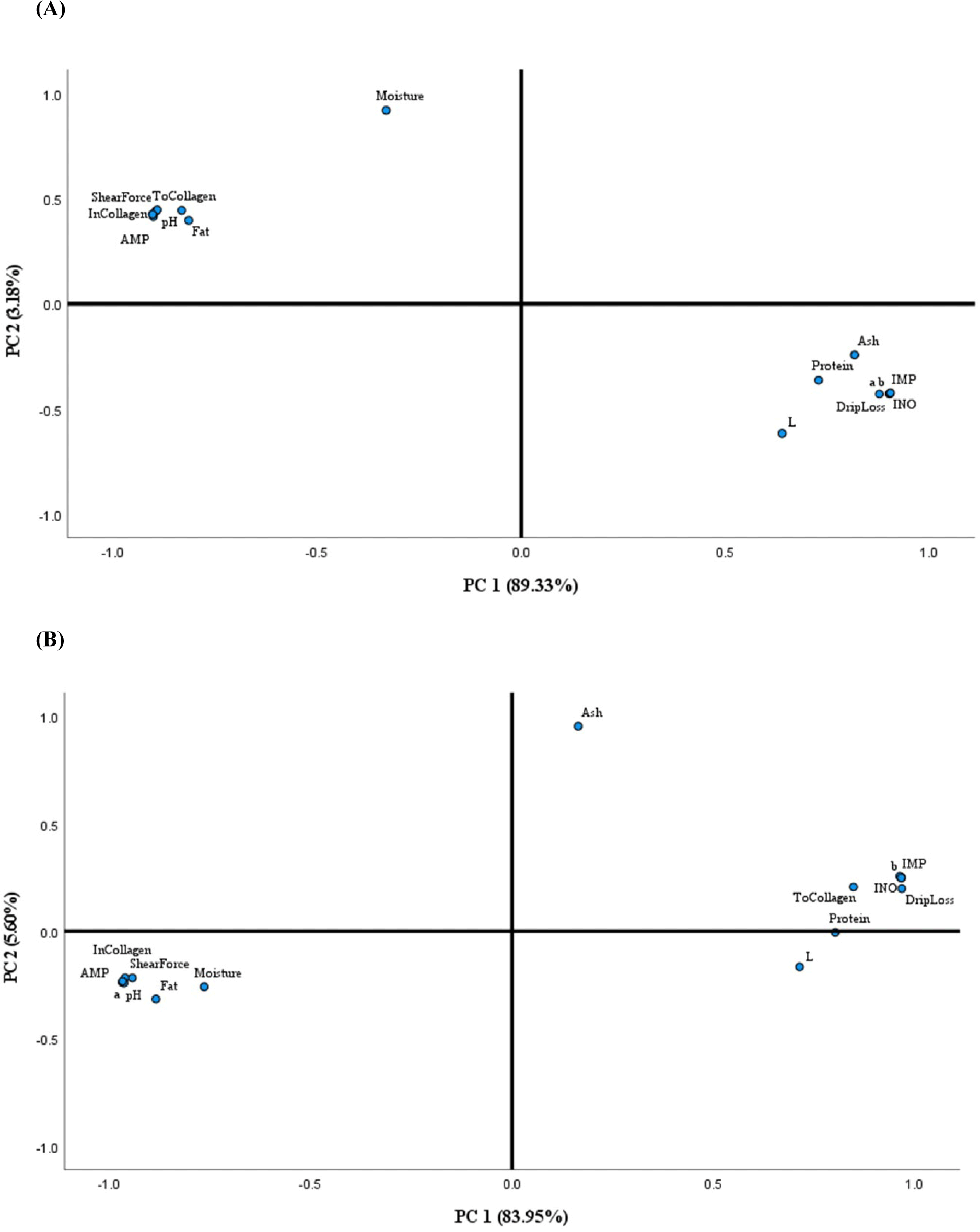
The PCA was conducted to provide easy visualization of the relationships among proximate composition (protein, moisture, fat, and ash), meat physical characteristics (pH, color in terms of L*, a*, and b*, drip loss, and shear force), collagen content (total and insoluble collagen), and nucleic acid content (AMP, IMP, and inosine) of Ufipa and commercial chicken meat. The loadings in the PCA loading plot expressed how the same PC explained correlated variables, and less correlated variables were explained by different PC (Belhaj et al., 2021; Chen et al., 2016; Michalczuk et al., 2018). Results for PCA applied to parameter values are summarized in Fig. 4 for Ufipa and commercial Ross chickens. The statistical analysis extracted highlighted two principal components (PC 1 and PC 2), explaining 92.51% and 89.55% of the total information in Ufipa and commercial Ross chickens, respectively. Both breeds’ protein content, IMP, drip loss, b*, and inosine loaded on PC 1 were positively correlated. Jung et al. (2014) described the correlation between protein and IMP that high protein content in indigenous chicken is due to contractile activities of muscles which on the other side results in the production of IMP, which is a derivative of ATP breakdown. However, both protein and IMP are influenced by genotype (breed/strain) and age of chicken breeds (Katemala et al., 2021; Tang et al., 2009). Also, shear force, insoluble collagen, AMP, and fat content of both breeds were loaded on PC 2, and it was found that, in Ufipa indigenous chicken, these parameters were positively correlated. However, in contrast, in the commercial, Ross was negatively correlated. Intarapichet et al. (2008), Jung et al. (2014), and Katemala et al. (2021) reported on the correlation between shear force and collagen content in chicken. These two parameters contribute to meat’s textural characteristics (Jeon et al., 2010), and they are affected by genotype (breed/strain) and increase in age at slaughter (Jaturasitha et al., 2008a; Wattanachant et al., 2004). This thesis supports our results that Ufipa indigenous chicken was slaughtered at an older age than Ross chicken. Negative correction shown among collagen, shear force, and fat in Ross chicken might be attributed to breed effects, as also has been described in Jaturasitha et al. (2008a) and Jung et al. (2014). Based on our results, PCA could be recommended to provide helpful information on the relationships between proximate composition and the physicochemical characteristics of chicken meat.
Conclusion
The present study compared different physicochemical properties between Tanzanian indigenous (Ufipa breed) and Broiler chicken meat. The results confirmed the substantial differences in proximate composition such as protein, fat, and ash contents and the physicochemical properties including color, texture, flavor, and shear force. Distinct features of Ufipa indigenous chicken meat in terms of nutrients (higher protein and lower fat contents), texture (higher shear force and collagen contents), and flavor (higher IMP) make them superior to the commercial Ross chicken meat. In addition, the relationship between proximate composition and the physicochemical characteristics of chicken meat in each breed has been analyzed and reported using PCA with some contrast. Therefore, the present study provides information on healthy food with good-tasting Ufipa indigenous chickens, which offer a promising market due to consumers’ preferences.













