Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease that occurs more frequently in females than in males, being predominantly observed in the elderly (Guo et al., 2018). RA has various symptoms such as articular pain, cartilage degradation, bone destruction, and functional disability (Rudbane et al., 2018). In particular, RA treatment includes the use of both conventional and biological therapeutics such as tumor necrosis factor (TNF) inhibitors (certolizumab, golimumab, adalimumab, and infliximab) and biosimilars and small oral molecules (tofacitinib, upadacitinib, and baricitinib). However, anti-TNF-α treatment is discontinued in approximately 30%–40% of patients owing to primary failure problem, secondary loss of response, or intolerance (Rubbert-Roth et al., 2019). Meanwhile, there is a growing awareness that the gut microbiome (GM) plays an essential role in the RA improvement and advancement. The gut-associated lymphoid tissue (GALT) works with the GM on maintaining immune system homeostasis and perform as a marker of the health condition of host (Achi et al., 2019).
Probiotics are living microorganisms, which upon consumption in adequate amounts can improve the health of an individual. Metabolites as vitamins and short chain fatty acids can be produced by probiotic strains and are energy sources for the intestinal cells. In addition, these may improve the GM and intestinal immune system to maintain gut health. Recently, probiotics such as Lactobacillus rhamnosus, L. casei, L. reuteri, L. acidophilus, Bacillus coagulans, and Bifidobacterium bifidum have been studied for their potential to treat RA in randomized controlled trials. These probiotics have been shown to be safe and effective in patients with RA (Bodkhe et al., 2019). In these reasons, we used L. casei 3260 as probiotic strain which has been previously reported to have anti-inflammatory effect by inhibiting both cyclooxygenase-2, and nuclear factor-kappaB (NF-κB) in other institute (Lee et al., 2008).
Gleditsia sinensis Lam. thorn (GST) has long been used in traditional medicine for the treatment in early stage of carbuncle, unbroken ulceration, swelling, and skin diseases (Wang et al., 2018). The chemical compounds of GST possess a wide spectrum of therapeutic activities such as anti-inflammatory, anti-oxidant, anti-microbial, and anti-tumor effects (Kim et al., 2015).
The aim of this study was to assess the therapeutic effects of Lactobacillus casei 3260 (LC), as a probiotic, and those of herbal chemical compounds from LC-fermented GST (fGST) by measuring NO and TNF-α expression levels in LPS-stimulated murine macrophage RAW 264.7 cells. In addition, we evaluated the therapeutic effects of LC and fGST by analyzing the bone metabolism- and pro-inflammatory cytokine-related mRNA expression in a mouse model of RA induced by injecting bovine type II collagen in DBA/1 mice.
Materials and Methods
The GST was purchased from Kyung-dong market (Seoul, Korea); it was extracted using methanol as the medium. The protocol of extraction was obtained from the Natural Product and Metabolomics Lab (Seoul, Korea University). The extracted sample was then directly used for the experiments.
L. casei 3260 was obtained from Korean Collection for Type Culture (KCTC, Jeongeup, Korea). The strain was delivered in frozen state; therefore, the samples were activated by inoculating 1% (v/v) strain thrice in De Man Rogosa Sharpe (MRS) medium (Difco, Detroit, MI, USA) at 37°C for 24 h prior to initiating each cell and animal studies.
L. casei 3260 and GST were used to prepare fGST. L. casei 3260 was inoculated in 1% (v/v) GST extract, and the samples were then incubated at 37°C for 18 h. After fermentation, fGST was centrifuged to obtain a supernatant. The supernatant was then freeze-dried, powdered, and stored at −80°C for future use.
The Korea University Institutional Animal Care & Use Committee, Korea (KUIACUC-2017-39) approved this experiment. Six-week-old normal male DBA/1 mice (n=70) were provided by Orient Bio (Seongnam, Korea). The mice were then caged in a cage for 1 week. Standard diet, controlled environmental background, and water ad libitum (24±0.5°C, 50%–60% relative humidity, 12-h light/dark cycle) were provided.
Following protocol from Bendele (2001) and Stasiuk et al. (1996), the RA was induced by injecting bovine type II collagen (CII) to mice, CII was dissolved in 0.1 M acetic acid at 2 mg/mL overnight at 4°C, after which the solution was emulsified in an equal volume of complete Freund’s adjuvant (CFA) (Chondrex, Washington, DC, USA) containing 2 mg/mL of freeze-dried powder form of Mycobacterium tuberculosis. This emulsion containing 100 mg of CII was injected into the base of the tail (day 7), and mice were boosted using the same schedule 14 days later (day 21). To assess the severity of arthritis in mice, all paws were scored from 0 to 4 (0, normal; 1, mild swelling confined to the tarsals; 2, swelling of two or more toes or joints, or increased swelling; 3, moderate swelling extending from the ankle to the metatarsal joints; and 4, severe swelling encompassing the ankle, foot, and digits). The data were then represented by the sum of the scores of all four paws (Jung et al., 2017).
DBA/1 mice were allocated into six groups (n=10): G1=Normal control (Con); G2=RAs; G3=RA mice treated with FK 506 (5 mg/kg) (FK506); G4=RA mice treated with L. casei 3260 (1×108 CFU/g/day) (LC); G5=RA mice treated with GST (20 mg/kg) (GST); and G6=RA mice treated with fGST (20 mg/kg) (fGST). Each treatment was orally gavaged once daily for 2 weeks. Meanwhile, CII treatment was initiated with intraperitoneal (i.p.) injection after the adjustment period. Weight of all mice was measured twice a week. Each mice were then sacrificed by cervical sprain after placing them into a CO2 chamber for 2 min at the end of experiment (Fig. 1).
The expression of the mRNA in the serum and samples of femur were analyzed using qRT-PCR. Shortly, using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) with following the protocol, the RNA was extracted. Using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Wilmington, MA, USA), the concentration of RNA was then evaluated. And using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA), the 20 μL of reverse transcription was reacted with the mixture. The PCR mixtures were then mixed with MG 2X qPCR MasterMix (SYBR green) (MGmed, Seoul, Korea) following manufacturer’s protocol. After gene amplifying, the samples were analyzed with CFX96™ RT PCR machine (Bio-Rad, Hercules, CA, USA). The β-actin as the housekeeping gene. The expression of mRNA levels each genes of analyzed samples were analyzed with calculation using Bio-Rad CFX manager software (Bio-Rad) (Eor et al., 2020).
Mice blood samples syringed in BD VacutainerTM SST tube (BD, Franklin Lakes, NJ, USA) through cardiac puncture after CO2 inhalation. Following the collection, the samples were incubated at 25°C for 25 min and centrifuged at 12,000×g for 15 min at 25°C. The supernatant of the samples were collected and indicated as the serum sample. All serum samples were stored at −80°C before using for biochemical analysis. Serum NO was measured using the nitrate/nitrite colorimetric assay kit (Cayman Chemical, Ann Arbor, MI, USA). Assays were performed as per the manufacturers’ protocols. Serum TNF-α was measured using the mice TNF-α ELISA kit (Koma Biotech, Seoul, Korea) referring the added protocol. The levels of total triglyceride (TG) and total cholesterol (TC) in serum samples were analyzed with colorimetric enzymatic kits (Embiel, Gunpo, Korea) following manufacturer’s procedure. Absorbance of samples in each analysis was then measured using VersaMaxTM microplate spectrophotometer (Molecular Device, San Jose, CA, USA).
The evaluation of RA progression and severity was analyzed twice a week. The each group of mouse paw was observed by more than three researchers and scored in some criteria from 0 to 4 with graded scale following method from previous research (Baharav et al., 2004). Each paw phenotypic changes were assessed. The result from one mice may be maximum score of 16. Clinical severity score was also assessed in a same way in the ankle thickness with calipers of each paw. Total clinical assessments were evaluated in a blinded manner.
All hind paw samples were disected after sacrifice. The samples were then fixed with formalin 10% (v/v) after put into embedding cassettes (Simport Scientific, Québec, QU, Canada) for 24 h at 25°C. The synovium analysis with immunohistochemical staining was performed. The 5-μm thickness samples embedded with paraffin blocks was put in formalin. The sections were mounted in glass slides. The samples were then de-paraffinized in xylene. In the next step, the samples were re-hydrated in a series of ethanol. And the samples took microwave antigen (Ag) retrieval. The activity of endogenous peroxidase was inhibited with 3% hydrogen peroxide. After inhibition of the binding with putting the samples with 10% goat serum at 37°C for 60 min, the samples were then stored at 4°C with a 10−2 dilution of mouse anti-human calcineurin antibody (Ab) (Sigma-Aldrich, St. Louis, MO, USA). The slides were diluted and stored with secondary Ab known as biotinylated goat anti-mouse IgG (Agilent, Santa Clara, CA, USA). After all steps, the samples were put with peroxidase-conjugated streptavidin at 25°C for 30 min. To reveal Ag, 3,3-diaminobenzidine was added. The samples were counter-stained with Mayer hematoxylin. After that, cleaned, dehydrated, and mounted. In the same manner as described above, the negative control was also prepared without primary Ab was eliminated or changed with isotype control Ab (IgG1; R&D Systems, Minneapolis, MN, USA). All dyed and captured sections were then assessed using scoring method following grades as 0: no abnormality, 1: minimum (<1%), 2: medium (1%–25%), 3: moderate (26%–50%), 4: marked (51%–75%), and 5: severe (76%–100%) detection following method from Balkrishna et al. (2019) and cartilage score from Mauri et al. (2000).
Data were analyzed statistically using IBM SPSS Statistics software version 24.0 (IBM, New York, NY, USA). One-way analysis of variance was used to analyze the statistical difference between sample means. The statistical significance level was defined as α=0.05. The multiple comparisons of means were assessed by Tukey’s tests.
Results and Discussion
The mouse serum was analyzed as demonstrated in Fig. 2. The results indicate the effect of each treatment toward four serum profiles, which act as downstream response of RA. TG levels were constant after RA induction compared to those in the control group (Fig. 2A). Although TC levels were not significantly different after RA induction compared to those in the control group, three different treatments including either probiotics or GST, especially in fGST group, significantly reduced TC levels (Fig. 2B). In humans, TC levels increase in RA; therefore, RA can be predicted before the critical symptoms are initiated (Turesson et al., 2015). The serum NO level is a biomarker of rheumatoid NO and increase in inflammatory conditions (Garg et al., 2017). All treatments severely elevated serum NO levels in RA-induced mice; the levels were most dramatically declined in the GST group among the four different treatments (Fig. 2C). Moreover, myeloperoxidase (MPO) levels were significantly low after RA induction, but each treatment restored MPO activity against RA induction (Fig. 2D); restoration of MPO activity was previously indicated as the anti-oxidative and anti-inflammatory activity (Eor et al., 2020).
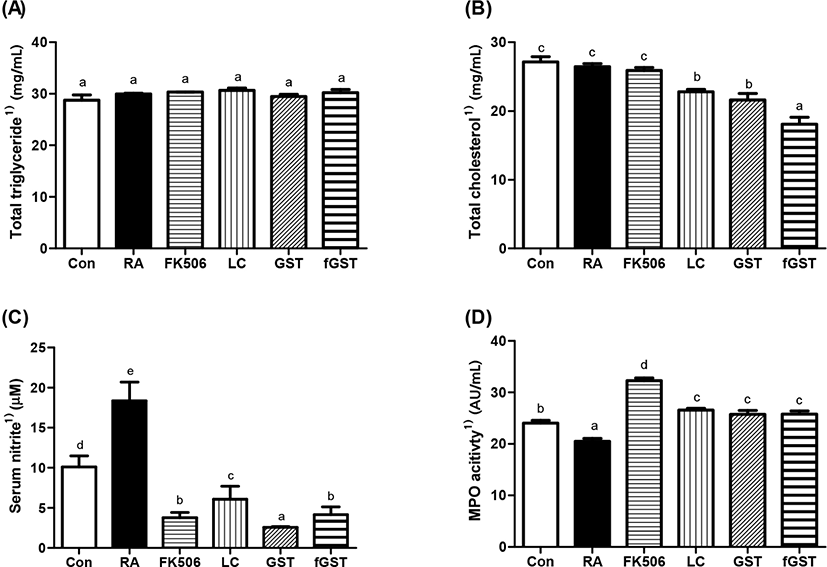
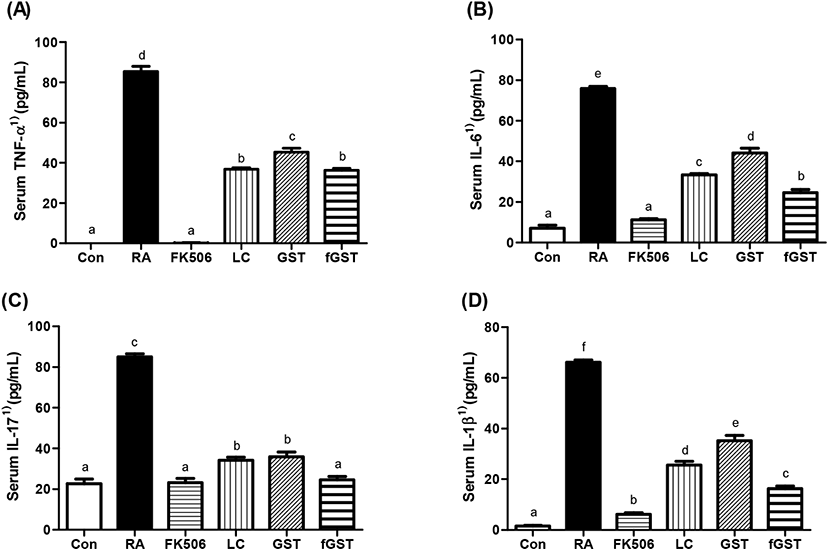
Effect of treatments on serum inflammatory cytokines level are shown in Fig. 3. TNF-α is an inflammatory cytokine in the serum and may also be the indicator of severity of inflammation (Bautista-Herrera et al., 2018). In our experiments, TNF-α level was dramatically increased by RA induction, and these elevations were recovered by either FK506 medication or each treatment (Fig. 3A). Previous reports suggest that interleukin (IL)-6 blockade may be the clinical target of patients with RA (Narazaki et al., 2017). IL-17 is also closely related to RA as it mediates mitochondrial functions (Kim et al., 2017). Therefore, we measured IL-6, IL-17, and IL-1β levels in the serum. Similar to the increase in TNF-α concentration levels, the levels of IL-6, IL-17, and IL-1β rapidly increased in the RA group, and then were attenuated by the four different treatments (Fig. 3B, 3C, and 3D). Interestingly, fGST group showed significant improvement in both IL-6 and IL-1β. This amelioration can be compared to sole treatment of GST by mitigating concentrations comparable to control group, respectively.
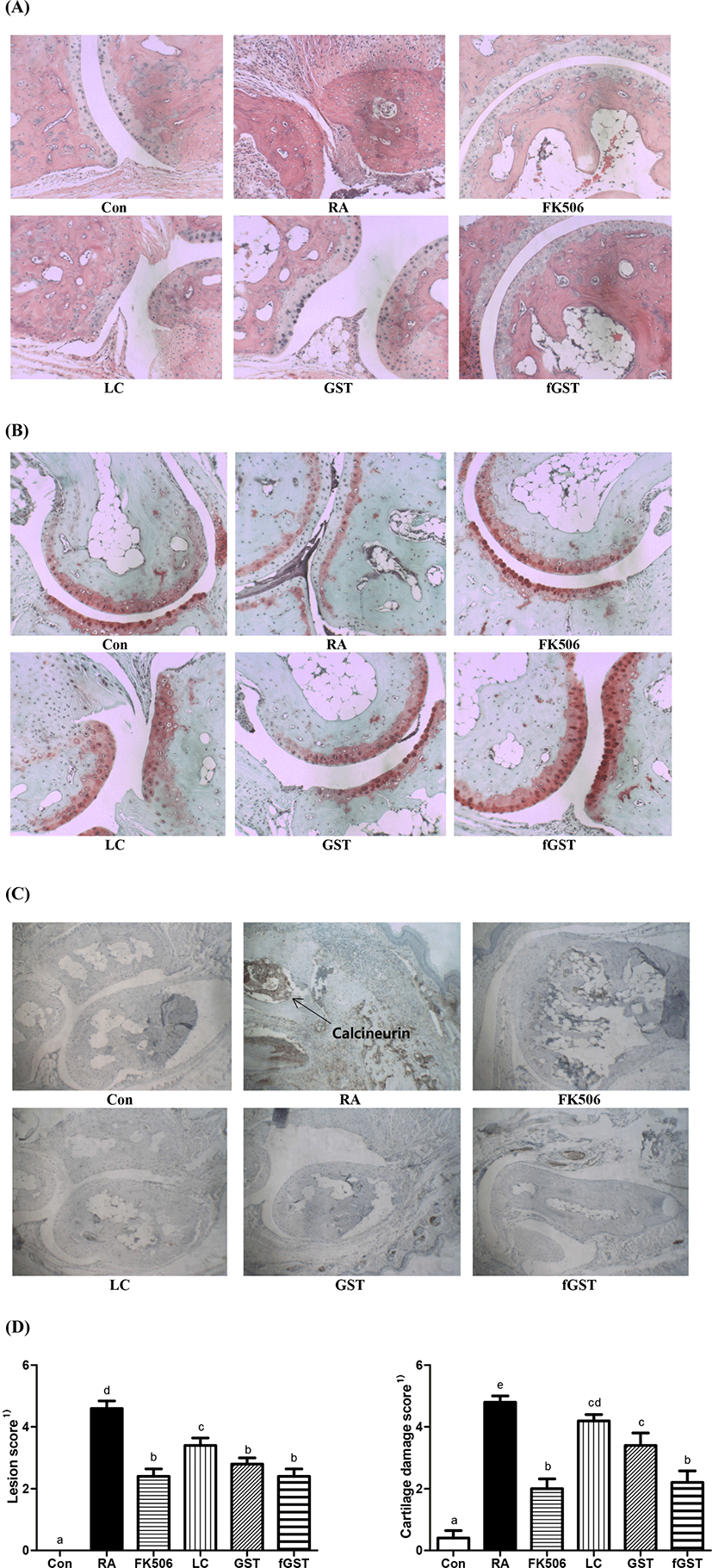
To analyze the protective effect of each treatments, three different kinds of staining were performed on the hind paw joints in mice (Fig. 4). First, H&E staining was performed to assess whether each treatment showed joint morphological differences. Severe disruption of cartilage in was observed in the RA group compared to that in the control group, and the disruption was recovered in FK506, LC, GST, and fGST groups. The fGST group showed a similar state as the FK506 group (Fig. 4A). A previous study reported that a neutrophil extracellular trap may enhance the cartilage component in an RA model, and thus, the cartilage was analyzed using safranin-O staining (Carmona-Rivera, 2020). Similarly, we used safranin-O staining to further investigate whether each treatment had any effects on cartilage integrity. The results indicated that cartilage integrity was weakened in the RA group, represented by the diminished red area compared to that in the control group (Fig. 4B). However, this disorganization was reversed after the four different treatments which restored the red areas in the cartilage (Fig. 4B). Calcineurin is increased in the patient joints with RA with inflammation (Yoo et al., 2006). Therefore, calcineurin staining was performed in the joints of the mice. The RA group showed the highest expression of calcineurin after the stained samples were incubated with calcineurin antibodies, whereas the elevated level of calcineurin was reduced by FK506 medication, and especially attenuated by LC, GST, and fGST (Fig. 4D). From the histologic scoring analysis, both lesion score and cartilage damge were restored after both GST and fGST group compared to RA group (Mauri et al., 2000).
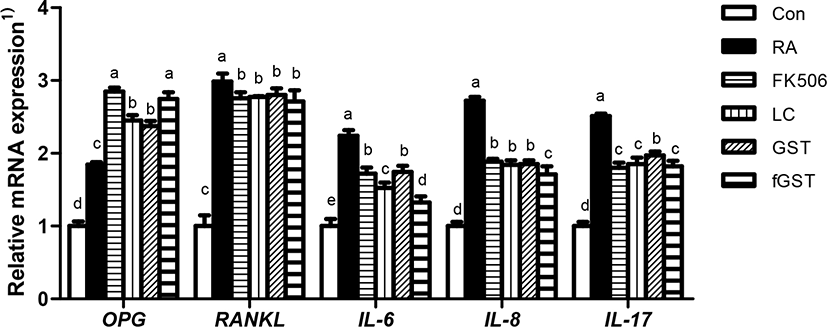
Osteoporosis and RA are related to the OPG and RANKL balance, which is responsible for bone formation and absorption through OPG/RANKL/NF-κB signaling pathways (Zhao et al., 2021). Fig. 5 shows the effect of each treatment on mRNA expression in hind paw joint of RA-induced mice. mRNA expression of OPG was increased in the RA group, and the treatments failed to mitigate the increase. RANKL expression also worsened in the RA group. Unlike the expression of OPG, all treatments diminished the mRNA expression of RANKL compared to that in the RA group without treatment. Similar to the serum level of inflammatory cytokines, the mRNA expression of IL-6 was elevated by RA induction. This is the parallel to the study by Pandolfi et al. (2020). The increase was attenuated by each treatment; it was especially declined in the fGST group. The mRNA expression of IL-8 and IL-17 showed a tendency similar to that of IL-6, which was increased by RA induction and most significantly attenuated in the fGST group (Lin et al., 2019).
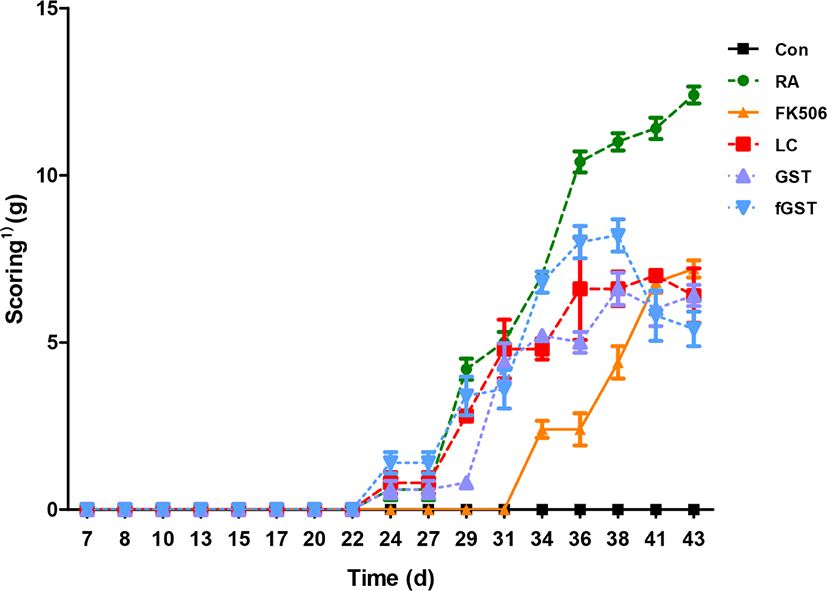
The arthritis score after each treatment is shown on Fig. 6. While the control group showed constant zero score of arthritis, RA induction initiated the symptoms at day 22 and they increased constantly. Although every treatment showed initiation at day 22 and continually increasing clinical score, each elevation of severity was hindered compared to RA group. Interestingly, the clinical score declined at day 41 of fGST treatment, whereas other treatments demonstrated gradually increasing severity.
Conclusion
This study was designed to assess the protective effect of either Lactobacillus strain or fGST in a mouse model of RA. Serum biochemical profiles and anti-inflammatory cytokine levels were improved by administrating each treatment (p<0.05). Moreover, three different morphological analyses of hind paw joints of mice suggested that each treatment may have joint-protective effect by lowering cartilage damage against RA induction. In parallel to morphologic analysis, qPCR results also showed the protective effect of the three treatments by normalizing OPG and RANKL balance and alleviating IL-6, IL-7, and IL-8 levels (p<0.05). Finally, the daily arthritis scoring further confirmed the protective effect of the treatments against RA (p<0.05), and this may be related to the alleviation of serum IL-6 and IL-17 (Haleagrahara et al., 2017). According to all implications and suggestions from the results, the administration of L. casei 3260, GST, and fGST may have the therapeutic potential to cure RA.