Introduction
The consumption of marinated meat has recently increased as consumer and retailer demand for ready-to-eat and convenience foods has increased. Marinade solutions commonly contain water, salt, and/or other functional ingredients with water-binding, tenderization, and flavor enhancement ability and/or antimicrobial or antioxidative activity (Alvarado and McKee, 2007). The quality characteristics of marinated meat can be affected by the type of solution, method, time, and temperature of marination. Injection and tumbling is a widely used approach to improve the quality properties of meat products; the marinade solution is uniformly dispersed into muscles for the effective extractability and solubility of myofibrillar proteins (Gamage et al., 2017; Kim et al., 2015; Toldra et al., 2010). As a major ingredient in marinade solutions, salt increases the solubility of myofibrillar proteins and ionic strength of myofibrils (Wu and Smith, 1987), thereby improving the water retention or holding ability and tenderness of final meat products (Aksu et al., 2003). Various additives, including inorganic salts, phosphates, and calcium chloride, are typically used in marination solutions (Lawrence et al., 2003). However, to meet consumer demand, ingredients derived from natural sources, such as kiwi, fig, pear, and ginger, have been used to improve the quality properties of marinated meat (Choe and Park, 1996; Park et al., 1999; Pawar et al., 2007).
Collagen contains approximately 30% protein and is widely used for the preparation of meat products owing to its functionality, including its effects on texture, water-binding ability, adhesion, and cohesion (Gomez-Guillen et al., 2011). Previous studies have shown that collagen or collagen mixtures with other functional ingredients improve the water holding capacity (WHC) in cured ham, reduced fat sausage, and chicken nuggets (Choe and Kim, 2019; Kim et al., 2015; Schilling et al., 2003). For these reasons, commercial collagen mixtures from various sources, especially those derived from pork, are widely sold as food additives. However, to our knowledge, studies of the effects of commercial collagen mixtures composed of collagen, carrageenan, isolated soy protein, whey protein, and other components on injected/tumbled meat are lacking. In this study, we compare the functional effects of three commercial collagen mixtures on quality characteristics, including the proximate composition, pH, cooking yield, WHC, cooking loss, shear force, and color, of marinated pork loin using injection and tumbling. In addition, changes in lipid oxidation in the meat samples injected with three commercial pork collagen mixtures were examined at days 0 and 7 of refrigerated storage.
Materials and Methods
Fresh pork loins were purchased from a local market (Seoul, Korea). After removing the subcutaneous and intramuscular fat and visible connective tissue, the loins were cut into 16 slices of equal weights (approximately 200 g) and sizes (height 15 cm). Three commercial collagen mixtures (A, 20% pork collagen, 30% isolated soy protein, 30% konjac, and 12% carrageenan, and 8% guar gum; B, 20% pork collagen, 30% L-lysine monohydrochloride, 20% maltodextrin, 20% whey protein, 5% inulin, and 5% tapioca starch; C, 40% pork collagen, 30% L-lysine monohydrochloride, 20% whey protein, 8% maltodextrin, and 2% tapioca starch) were purchased from different companies (Gyeonggi-do, Korea). The composition (w/w) of the marinade solution was 93.6% water and 6.4% nitrite pickled salt (salt:nitrite=99.4:0.6) for the control. For treatment groups, each commercial collagen mixture (A, B, or C; marinade solution:collagen mixture=25:1) was completely dissolved in the marinade solution at 45°C under mild stirring. The solution was injected into each slice of pork loin at a ratio of meat:solution of 10:2 (w/w) using an injector (PR8; RÜHLE GmbH, Grafenhausen, Germany). The optimal amounts for injection were determined in our preliminary study. The injected pork slices were placed in plastic bags and intermittently tumbled for 90 min (45 min on, 15 min off) at 1±1°C in a tumbler (MKR150; RÜHLE GmbH). After tumbling half of the raw sample was collected to determine the pH value, cooking yield, and color. The other part was dried at 60±1°C for 30 min, smoked at 65±1°C for 30 min, and cooked at 80±1°C for 30 min to reach an internal temperature of 72°C. For the lipid oxidation analysis, samples were stored at 3±1°C for 7 days.
The proximate composition of each sample was analyzed as described by Lee et al. (2018) following standard AOAC (2012) methods.
The pH values were measured in a homogenate prepared with 4 g of meat sample and distilled water (16 mL) using a pH meter (Model S220; Mettler-Toledo, Greifensee, Switzerland). All determinations were performed in triplicate.
The WHC of each sample was measured following the methods of Grau and Hamm (1953), with modifications. In brief, 300 mg of sample was placed on Whatman No. 2 filter paper and then pressed for 3 min with constant pressure using a binate plexiglass plate. Outer and inner sections were measured using a planimeter (Planix 7; Tamaya Technics Inc., Tokyo, Japan) to evaluate exuded moisture and meat, respectively. The ratio between the inner and outer section was defined as the WHC (%).
Cooking yield was determined for individual samples by calculating the weight before and after cooking as follows:
For the shear force values of the cooked samples were determined using a Warner-Bratzler attachment on a texture analyzer (TA-XT2i; Stable Micro Systems Ltd., Godalming, UK). Test speeds were set to 2 mm/s. Data were collected and the shear force values (N) were used to obtain the maximum force required to shear each sample.
The colors of raw and cooked meat samples were determined using a colorimeter (CR-10; Minolta, Tokyo, Japan; illuminate C, calibrated with a white plate, CIE L*=+97.83, CIE a*=−0.43, CIE b*=+1.98). Lightness (CIE L* value), redness (CIE a* value), and yellowness (CIE b* value) values were recorded.
Lipid oxidation was assessed using the direct-distillation method as described by Tarladgis et al. (1960), with minor modifications. Samples were analyzed at days 0 and 7 of storage at 4°C in triplicate. Each sample (10 g) was blended with 97 mL of distilled water prior to homogenization (AM-7; Nihon Seiki Kaisha Ltd., Tokyo, Japan) for 2 min and transferred to a distillation flask. Then, 2.5 mL of 4 N HCl and a few drops of an antifoaming agent, silicone o/w (KMK-73/ Shin-Etsu Silicone Co., Ltd., Seoul, Korea), were added. The mixture was distilled and 50 mL of distillate was collected. After filtration through Whatman No. 1 filter paper, 5 mL of extract was added to 5 mL of 0.005 mol L−1 2-thiobarbituric acid and heated at 100°C for 10 min. After cooling on ice, absorbance was measured at 532 nm and thiobarbituric acid reactive substances (TBARS) was calculated as mg of malonaldehyde per kg of sample.
The proximate composition, pH value, WHC, cooking yield, shear force, instrumental color, and TBARS were analyzed using two-way analysis of variance (ANOVA) and Duncan’s multiple range test implemented in SAS (Release 8.01, SAS Institute Inc., Cary, NC, USA). The results were considered significant if p<0.05 and values are expressed as means±SE. In addition, for pH values, instrumental color, and TBARS, the difference between raw and cooked samples or between the initial and final storage period within each group was tested with the independent samples t-test.
Results and Discussion
The addition of collagen mixtures significantly increased the moisture content of marinated samples, regardless of the mixture type (Table 1). This result was consistent with previous results indicating that collagen immobilizes water during cooking, thereby increasing the moisture content in meat products (Daigle et al., 2005; Schilling et al., 2003). In addition, the ingredients of each collagen mixture including konjac, carrageenan, and tapioca starch could help enhancement in water retention ability of each sample as exhibiting gel formation (Chin et al., 2009; Desmond et al., 1998; Hinrichs et al., 2003). Crude protein, fat, and ash contents of marinated samples were not affected (p>0.05) by the addition and type of collagen mixtures.
The pH value of raw meat is an important determinant of the water retention/holding capacity. Raw meat with pH values of <5.7 yield final products with low WHC due to reduced electrostatic repulsion between proteins (Aksu et al., 2003). In this study, raw meat supplemented with various collagen mixtures exhibited pH values of 5.73 to 5.91, which are acceptable values for meat manufacturing (Table 2). The addition and type of collagen mixtures did not influence (p>0.05) the pH values of both raw and cooked meat samples, except for the sample with collagen mixture B. The addition of collagen mixture B led to the highest (p<0.05) pH values for both raw and cooked meat samples among all groups. In this study, using collagen mixture B (Table 2), the cooking yield and WHC were not substantially affected by the pH value of raw pork. Regardless of the pH value of raw meat, the addition of the collagen mixture significantly increased the cooking yield and WHC compared to those of samples without the collagen mixture. The type of collagen mixture significantly influenced the WHC (p<0.05); in particular, the WHC was highest in meat samples containing collagen mixture C, which had an intermediate pH value. This observation probably reflects the high level of pork collagen in collagen mixture C, which can influence gel formation by the absorption of water during thermal treatment (Osburn and Mandiso, 1998). According to Sosulski and McCurdy (1987), protein enhances the gelation and swelling of muscle-based food products with high WHC. The different components (non-meat ingredients) of the three commercial collagen mixtures may affect the WHC. Previous studies have reported that collagen reduces syneresis in the final product as non-meat protein, including isolated soy protein and transglutaminase, promote water retention in interstitial spaces of the gel matrix (Prestes et al., 2013; Pietrasik et al., 2006). In addition, the combination of collagen and non-meat ingredients enhance stability during heat treatment and subsequent results in high cooking yields (Pietrasik et al., 2006).
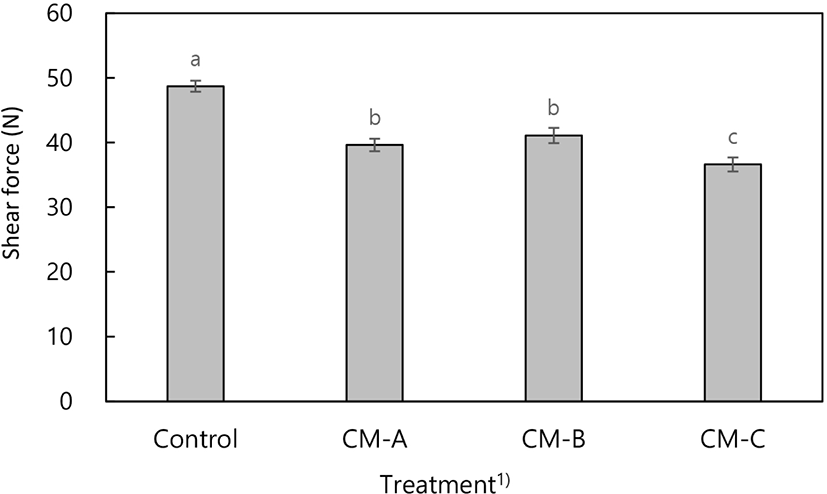
The instrumental tenderness of meat samples varied depending on the addition and type of collagen mixtures (Fig. 1). In detail, the highest (p<0.05) and lowest (p<0.05) values were observed in meat samples with collagen mixture C and without a collagen mixture, respectively. Collagen mixture C enhanced the tenderness (24.7%) of meat samples compared to that of collagen mixture-free samples. The improvement in tenderness might be explained by the increase in the moisture content of the meat samples based on of the WHC results (Table 2). Increased juiciness of meat products is associated with increased tenderness (Lee et al., 2018).
The color of meat and meat products is an important factor for consumer purchasing decisions. In this study, the addition and type of collagen mixture did not influence (p>0.05) L*, a*, and b* values of meat samples (Table 3). After cooking, L*, a*, and b* values increased depending on the type of collagen mixture (Table 3). The addition of collagen mixtures led to significant increases in the L* values of cooked samples, except in the group with collagen mixture A. The addition of non-meat ingredients to meat products can lead to increases in lightness (Choe and Kim, 2018). Control samples had higher (p<0.05) a* values than those of samples with collagen mixtures. This observation may be explained by the intrinsic color of the collagen mixture itself, as observed by Choe and Kim (2018). No significant difference in b* values among cooked groups was observed, except in the group with collagen mixture B.
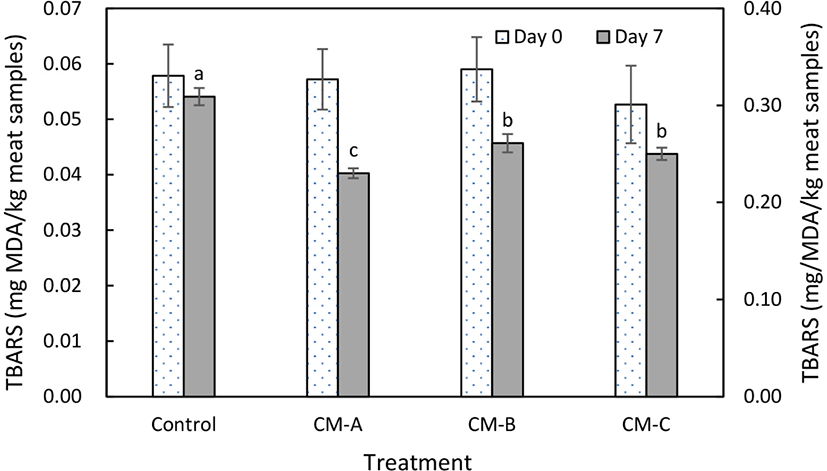
TBARS levels indicate the amount of secondary lipid oxidation products, including aldehydes, and carbonyls, which cause the development of a rancid flavor (Choe et al., 2019). In this study, there were no significant differences in TBARS at the initial day of storage among groups, ranged from 0.049 to 0.058 mg MDA/kg meat samples (Fig. 2). After 7 d of storage, TBARS levels increased (p<0.05) in all groups and the samples containing collagen mixtures showed the lower (p<0.05) lipid oxidation levels compared to sample with no collagen mixture. Especially, the samples injected with collagen mixture A had the lowest (p<0.05) values in TBARS. This result may be due to the presence of bioactive amino acids in collagen; this explanation is supported by previous results indicating that amino acids possessing antioxidant activity, including arginine, histidine, and methionine, are present in gelatin (collagen hydrolysate) (Aleman et al., 2011). Additionally, many researchers have attempted to retard lipid oxidation in injected meat products by adding natural ingredients. Jongberg et al. (2018) found that the incorporation of green tea and mate extracts into injection brine reduces lipid oxidation of injected chop samples during chilled storage for 7 days. Armenteros et al. (2016) reported that cooked loin ham injected with a mixture of garlic, cinnamon, cloves, and rosemary shows a dramatic increase in stability against lipid oxidation. The difference in TBARS levels among the groups was probably caused by antioxidant activity of various component of collagen mixtures.
Conclusion
In this study, we compared the functional effects of three commercial collagen mixtures on quality characteristics, including the proximate composition, pH, cooking yield, WHC, cooking loss, shear force, color, and lipid oxidative stability, of injected/tumbled loin ham. Our results indicated that collagen mixture C and collagen mixture A injected in brine could be used to improve quality characteristics and lipid oxidative stability, respectively, as possessing either greater WHC and tenderness or lipid oxidative stability. Further studies should evaluate the protein retardation effect of each commercial collagen mixture.













