Introduction
Microbiological contamination in table eggs is concerning to consumers and thus safety measures are required in the egg industry. In fact, Salmonella is the most common cause of food poisoning in the US. Because poultry is one of the major reservoirs of human Salmonella (Bae et al., 2013), control measures in this source become a critical issue in the domestic egg industry. Previous studies showed that the isolation rate of Salmonella spp. from chicken cecal contents was quite high at 21.3% (Cheong et al., 2007). Zoonotic bacteria present in the digestive tracts of poultry may contaminate the egg contents or eggshell during egg production. Consumption or handling of the contaminated eggs can then cause human infections.
Salmonellosis is one of the most important and widely distributed foodborne bacterial illness (CDC, 2008; WHO, 2013). Salmonella spp., particularly Salmonella Enteritidis (Salmonellaenterica subsp. enterica serovar Enteritidis; SE), have been attributed to many food-borne disease outbreaks in humans, which were traced back to eggshells (Braden, 2006; Chousalkar et al., 2010; ECDC, 2014; Morgan et al., 2007; Zielicka-Hardy et al., 2012) The Food and Drug Administration (FDA) estimates that 142,000 illnesses are caused by consuming eggs contaminated with SE each year (FDA, 2009). In the US, multi-state outbreaks of human SE infection associated with shell eggs occurred in 2010, resulting in approximately 1939 illnesses (CDC, 2010). In Europe, multi-country outbreaks of SE infection have been associated with the consumption of eggs produced in Germany. Outbreaks in Austria, France, and Germany were traced to the same egg packaging center in southern Germany (ECDC, 2014).
In the hatching egg industry, hygienic management is directly linked to the hatching rate. Bacterial diseases, such as fowl typhoid (FT) and pullorum disease (PD) result in large costs to the hatching egg industry by lowering the hatchout rate. These diseases are serious problems in the many countries where measures for controlling bacterial diseases are not sufficient.
FT and PD, caused by Salmonella Gallinarum (Salmonellaenterica subsp. enterica serovar Gallinarum; SG) and Salmonella Pullorum (Salmonellaenterica subsp. enterica serovar Pullorum; SP), respectively, are among the most significant diseases in poultry. In chicks and poults, SG and SP cause diarrhea, dehydration, anorexia, weakness, and high mortality. These diseases in mature fowl cause lower egg production and hatchout rates combined as well as anorexia and high mortality. Trans-ovarial infection, leading to infection of the egg and the chick, is one of the most significant infection routes related to these diseases (Shivaprasad, 2000). FT and PD have nearly disappeared from commercial poultry meats in developed nations, including the US, Canada, New Zealand, Australia, Japan, and many European countries. However, these diseases are common in countries in Central and South America, Asia, and Africa (CFSPH, 2010; Shivaprasad, 2000).
In Korea, outbreaks of FT and PD have been increasing since the 1990s, inflicting economic damage on domestic poultry farms. Thus, a live attenuated SG vaccine is used in broilers to prevent FT. However, the efficacy of this vaccine remains uncertain, and live vaccines cannot be used in layer hens because of the potential for egg-transmitted infections (Kim et al., 2006; Kim et al., 2007; Kwon et al., 2010). Therefore, new methods to prevent these two chronic diseases are required.
Chlorine, usually used as disinfectant, produces trihalomethanes and chlorophenols which are reported as a potentially mutagenic compounds or carcinogenic substances (Dunnick and Melnick, 1993; Owusuyaw et al., 1990). Researchers have focused on chlorine dioxide as chlorine’s alternative sanitizer. Chlorine dioxide (ClO2) produces less substance when it reacts with organic compounds. Furthermore, it is more stable and has 5 times higher sterilizing power than chlorine (Beuchat et al., 2004; Kim et al., 1998; Moore et al., 1980).
In this study, we chose chlorine dioxide (ClO2) as a disinfecting agent to control microorganism-contaminated eggshells. In addition, the disinfection efficacy of ClO2 solution in dentistry, for example, with dental materials and instruments and for oral hygiene, has been studied (Agnihotry et al., 2014; Drake and Villhauer, 2011; Watamoto et al., 2013).
In 1998, the FDA approved the use of ClO2 solution for washing fruits and vegetables. In turn, ClO2 was approved as an alternative sanitizer by the US Environmental Protection Agency and the US FDA for postharvest application to fruits and vegetables in 2006 (FDA, 1998; Sun et al., 2014). Recently, many studies have reported the advantages of gaseous ClO2. ClO2 gas is more diffusible than aqueous form, making it more efficient for eliminating microorganisms in food or on food processing surfaces (Du et al., 2002). Gaseous from has been shown to be more effective than aqueous form at the same concentration against Listeria monocytogenes on the surface of green peppers (Han et al., 2001). Additionally, the freshness and quality of food can be reduced through contact with water, and thus, disinfectants in gaseous forms are more appropriate than the aqueous form.
The objective of this study was to evaluate the effects of ClO2 gas on salmonellae inoculated onto eggshells under laboratory-controlled settings. Choi et al. (2015) reported that aqueous ClO2 has an efficacy of reducing Salmonella enterica on the surface of eggshells. This study is analogous to ours, but we used gaseous ClO2 and controlled environmental factors such as exposure time of wide range, varying concentrations and humidity.
Materials and Methods
Eggs were collected from a market or an egg-packing center. Very dirty eggs with visible fecal contamination were excluded from the experiment samples.
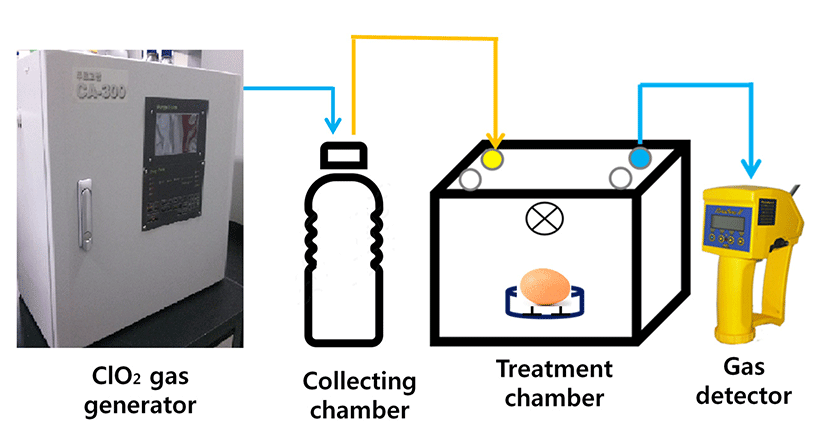
The ClO2 gas was generated by a ClO2 generator (CA- 300, PurgoFarm, Korea). Aqueous NaClO2 was passed through the patented multi-porous membrane electrode assembly, producing highly pure ClO2 gas (>98% ClO2). After a sequence of electrochemical reactions, ClO2 gas was released through a vent into a collecting chamber in the dark. The stream of ClO2 was controlled by a valve to maintain the desired ClO2 concentration in the treatment chamber. During treatment, the ClO2 concentration in the chamber was monitored using a PortaSens II gas leak detector (C-16, Analytic Technology, USA) (Fig. 1).
SE (ATCC 13076) and SG (ATCC9184) were selected as test bacteria. Each strain was incubated in nutrient broth (Oxoid, Hampshire, UK) for 24±2 h at 37℃. The inoculum density was found to be ≥ 0.01 absorbance at 600 nm by using a micro ELISA plate reader (VERSAmax, Molecular Devices, USA).
SE and SG were inoculated onto the eggshells of clean eggs. The inoculation was performed by spotting 100 μL of the test inoculum (>106 CFU/mL of the test organism) with sterilized micropipette tips onto the surface of the eggshell.
The government guidelines on disinfectants efficacy recommends that 5% (w/v) yeast extract is used as substitute for organic matter to determine the bacterial reduction (QIA, 2013). Therefore, to set up the environment containing organic matter, 96 mL of 5% or 10% yeast extract (Sigma, USA) solution and 4 mL of a test inoculum were vortexed together, and then 100 μL of this mixture was spotted onto each eggshell. All eggs used in the experiments were allowed to dry at room temperature for 40-60 min.
Following inoculation of the bacterial suspensions and drying, two eggs for each bacterial sample were exposed to ClO2 gas. Two untreated eggs were used as a control. All treatments were performed in triplicate.
Environmental factors, including concentrations (ppm), contact time (min), humidity (% RH), and percentage organic matter, were applied differently during gas exposure. The applications of these environmental factors are summarized in Table 1.
To measure bacteria on the eggshells, the egg was placed aseptically into a plastic bag containing 50 mL sterile distilled water and shaken for 10 min on an orbital shaker. One milliliter of the resulting water sample was serially diluted by 10-fold.
Xylose lysine deoxycholate agar (Oxoid, UK) for SE and the Salmonella Chromogenic Agar Base with Salmonella Selective Supplement SR0194 (Oxoid, UK) for SG were inoculated with 100 μL of each 10-fold serially diluted sample. Agars were incubated for 24±2 h at 37℃.
Results
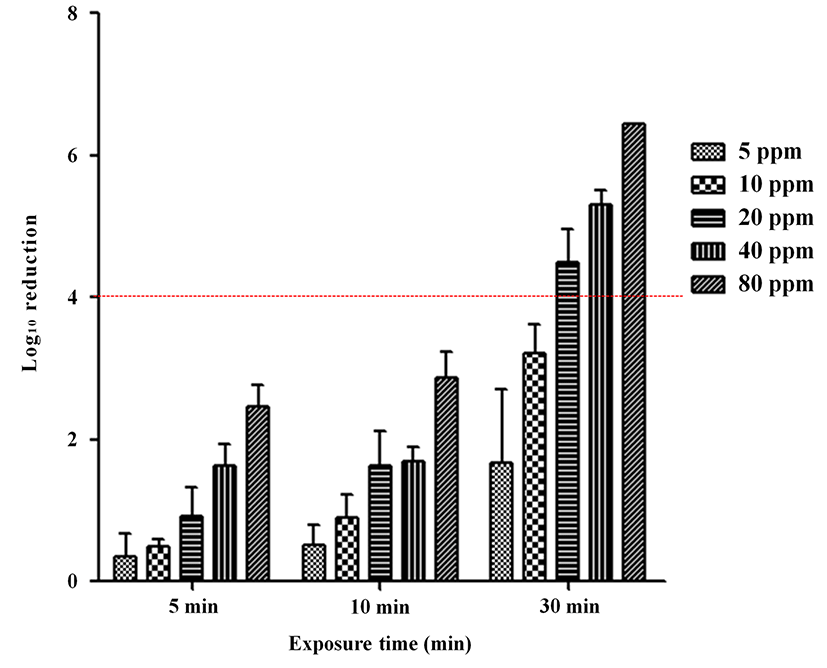
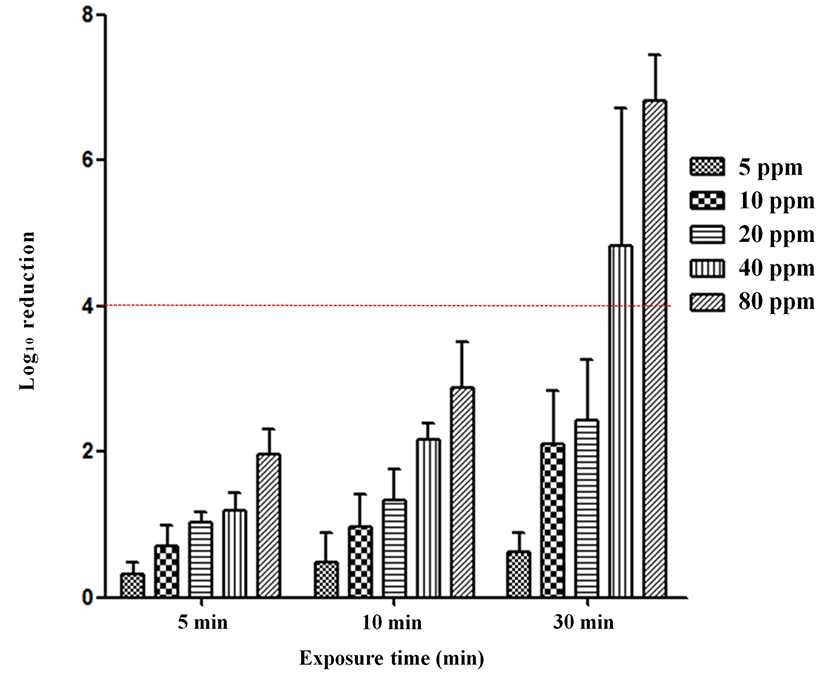
We evaluated the efficacy of ClO2 gas against SE under wet conditions (80±5% RH). The change in ClO2 concentrations (5, 10, 20, 40, 80 ppm) and exposure time (5, 10, 30 min) reduced SE contamination after inoculation (Fig. 2). Exposure to 5 ppm ClO2 gas reduced SE by <2 log (0.36, 0.51, 1.67 log) after 5, 10, and 30 min. With 10 ppm, 20 ppm, and 40 ppm ClO2 gas exposure, SE was reduced by <2 log (0.49, 0.90 log-10 ppm/0.92, 1.62 log-20 ppm/1.63, 1.69 log-40 ppm) after 5 and 10 min of exposure. A reduction of 2-4 log occurred at 10 ppm after 30 min (3.22 log), and at 80 ppm after 5 and 10 min (2.45, 2.87 log). A >4 log reduction was achieved by exposure to 20 ppm (4.49 log), 40 ppm (5.3 log), 80 ppm (6.45 log) for 30 min.
We evaluated the efficacy of ClO2 gas against SG under the wet condition (80±5% RH). The reduction in SG surface contamination changed with varying concentrations (5, 10, 20, 40, 80 ppm) and exposure times (5, 10, 30 min) (Fig. 6). SG contamination on the eggshells was reduced by <2 log at 5 ppm ClO2 gas exposure for 5, 10, and 30 min (0.33, 0.48, 0.63 log), 10 ppm for 5 and 10 min (0.70, 0.96 log), at 20 ppm for 5 and 10 min (1.02, 1.32 log), 40 ppm for 5 min (1.18 log), and 80 ppm for 5 min (1.97 log). SG was reduced by 2-4 log at 10 ppm and 20 ppm after 30 min (2.10, 2.43 log), and 40 ppm and 80 ppm after 10 min (2.16, 288 log). An exposure time of 30 min was required to achieve a >4 log reduction at 40 ppm (4.83 log) and 80 ppm (6.82 log).
ClO2 gas showed a relatively strong bactericidal effect against SE, regardless of the presence of organic matter (yeast extract, Sigma, USA) under wet as compared to dry conditions.
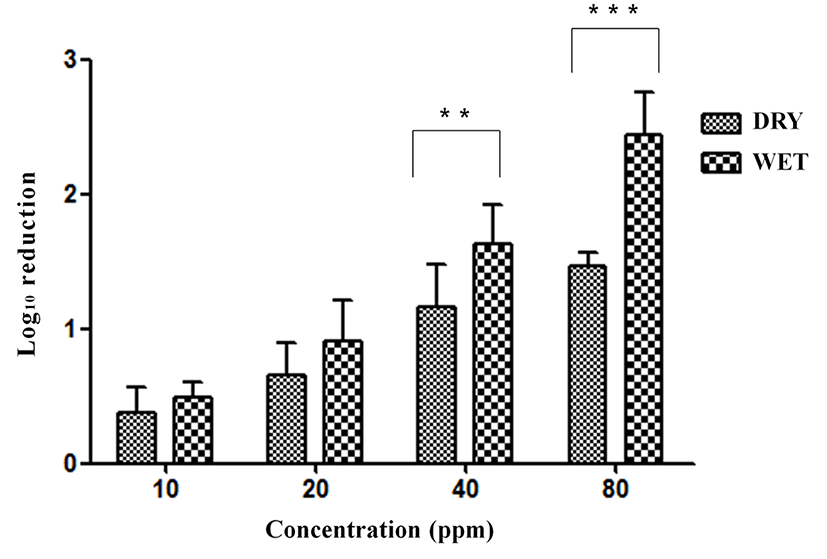
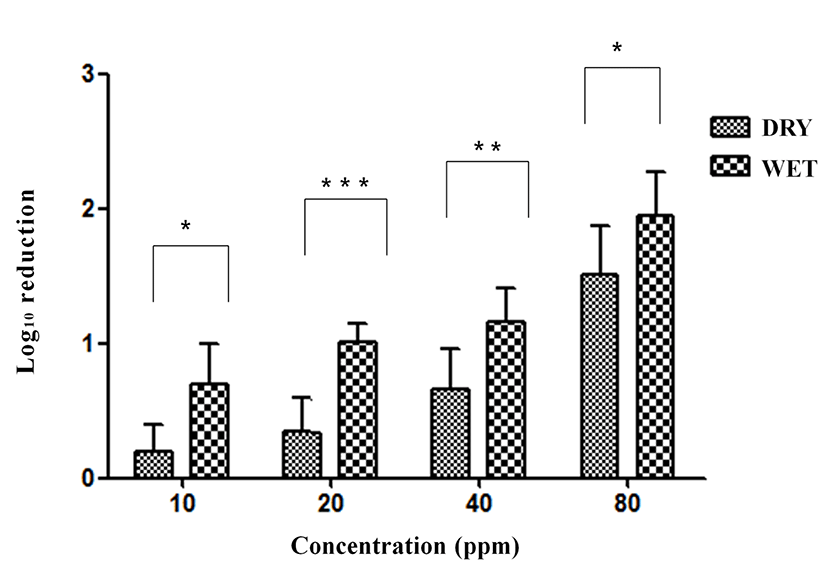
Exposure to ClO2 gas resulted in 0.37, 065, 1.16, and 1.47 log reductions at 10, 20, 40, and 80 ppm for 5 min under dry conditions without organic matter (Fig. 3). The reductions in SE were 0.49, 0.91, 1.63, and 2.45 log under wet conditions without organic matter. A significant difference (p<0.001) in SE reduction was found between dry conditions and wet conditions with exposure to 40 ppm (p<0.01) and 80 ppm of ClO2 gas for 5 min without organic matter. The SE contamination on eggshells was reduced by 0.23, 0.27, 1.071, and 1.27 log at 10, 20, 40, and 80 ppm, respectively, for 5 min under dry conditions with organic matter (yeast extract, Sigma, USA) (Fig. 4). The reductions in SE were 0.47, 0.51, 1.27, and 1.71 log at the same ClO2 exposures under wet conditions with organic matter. A significant difference (p<0.05) was found between the dry and wet conditions at 80 ppm for 5 min with organic matter.
The reduction in SE on the eggshells by an 80 ppm ClO2 gas treatment for 5 min without organic matter (0% yeast extract) ranged from 2.14 to 2.67 Log CFU/eggshell (25% to 75%) and from 2.03 to 2.99 Log CFU/eggshell (minimum to maximum) (Fig. 5). The median and mean of the log reduction in SE without yeast extract were 2.43 and 2.45, respectively. The reduction in SE on the eggshell by the same gas treatment with 5% yeast extract ranged from 1.48 to 2.34 log CFU/eggshell (25% to 75%) and from 1.37 to 2.43 log CFU/eggshell (minimum to maximum). The median and mean of the log reduction in SE with 5% yeast extract were 1.73 and 1.85, respectively. The reduction in SE on the eggshells by the same treatment with 10% yeast extract ranged from 1.56 to 1.84 Log CFU/eggshell (25% to 75%) and from 1.42 to 2.07 Log CFU/eggshell (minimum to maximum). The median and mean of the log reduction in SE with 10% yeast extract were 1.66 and 1.71, respectively. A significant difference (p<0.001) was observed between SE reductions with 0% yeast extract and 5-10% yeast extract.
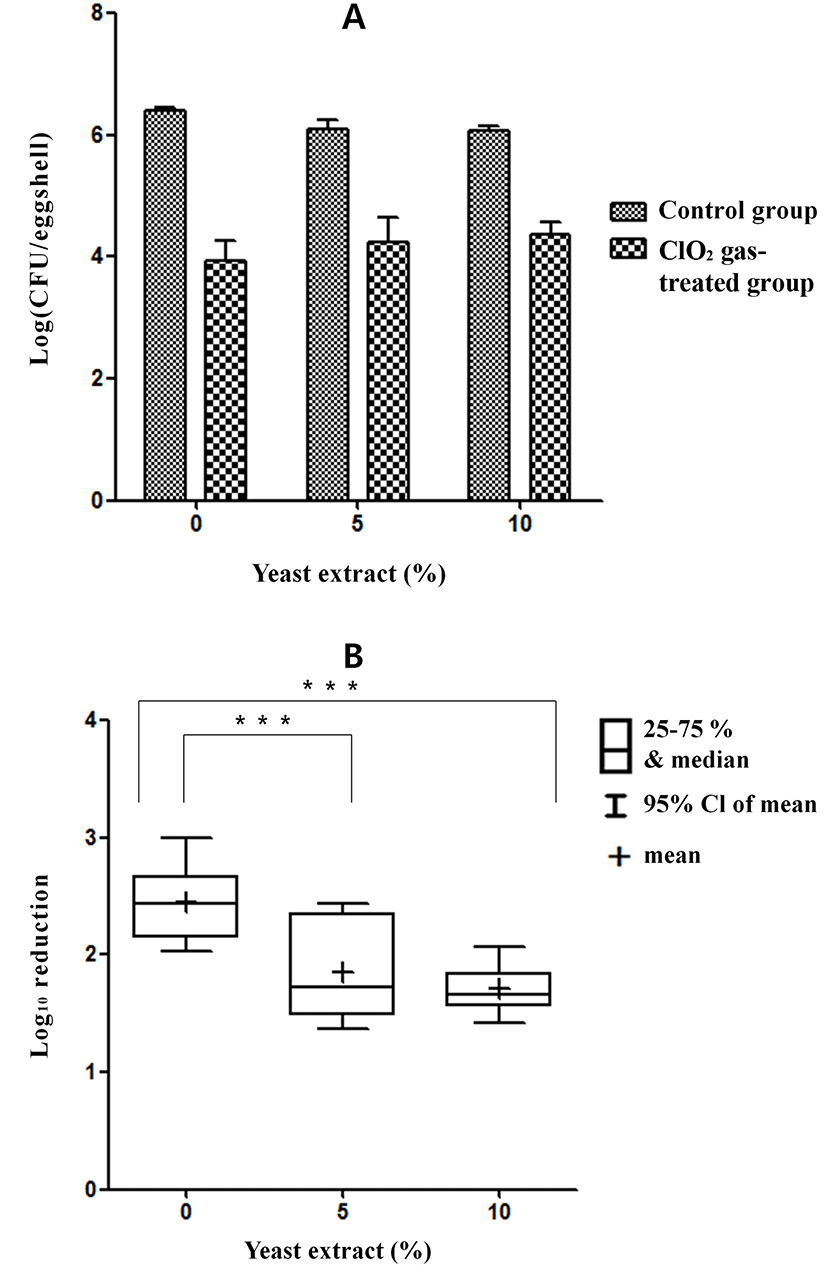
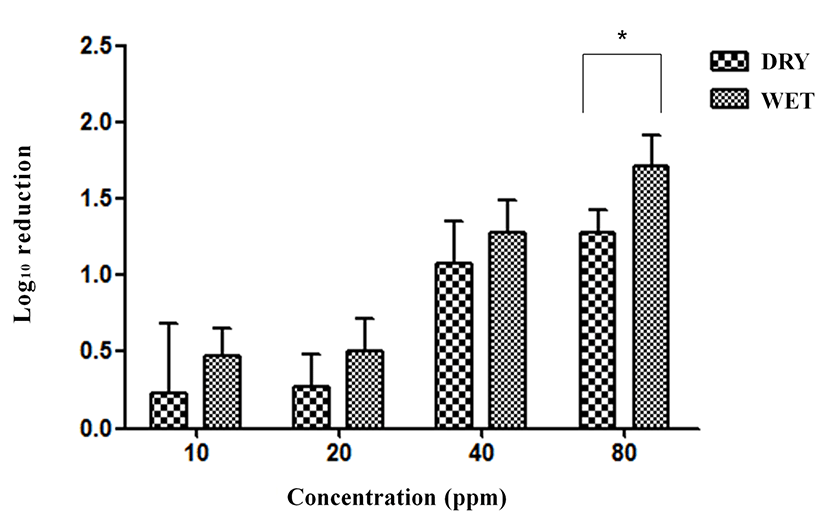
Exposure to ClO2 gas resulted in 0.20, 0.33, 0.66, and 1.54 log reductions in SG at 10, 20, 40, and 80 ppm, respectively, for 5 min under the dry condition without organic matter (Fig. 7). The reductions in SG were 0.70, 1.02, 1.18, and 1.97 log under the wet condition without organic matter. Significant differences were found between the dry condition and wet condition at 10 ppm (p<0.05), 20 ppm (p<0.001), 40 ppm (p<0.01), and 80 ppm (p<0.05).
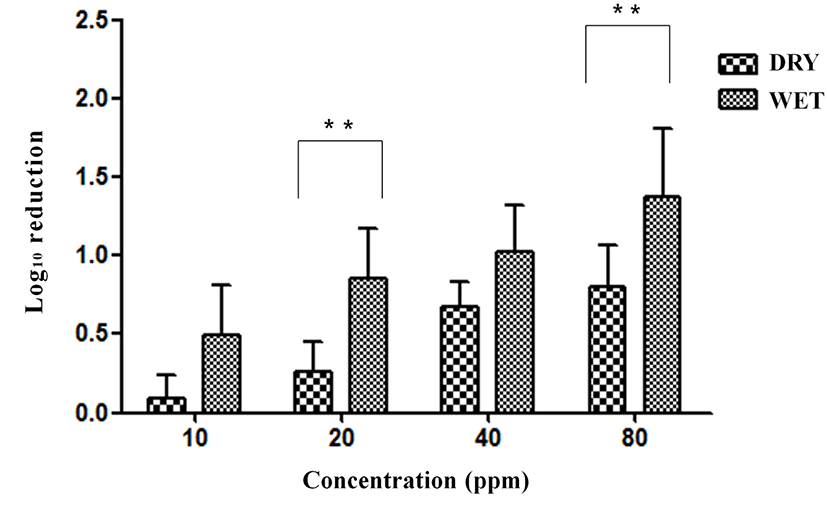
The SG contamination on eggshells was reduced by 0.10, 0.26, 0.66, and 0.80 log with exposure to 10, 20, 40, and 80 ppm ClO2 gas, respectively, for 5 min under the dry condition with organic matter (yeast extract, Sigma, USA) (Fig. 8). The reductions in SG were 0.49, 0.85, 1.03, and 1.38 log under the wet condition with organic matter. Significant differences were obtained between the dry and wet conditions at 20 ppm (p<0.01) and 80 ppm (p<0.01) ClO2 gas exposure for 5 min with organic matter.
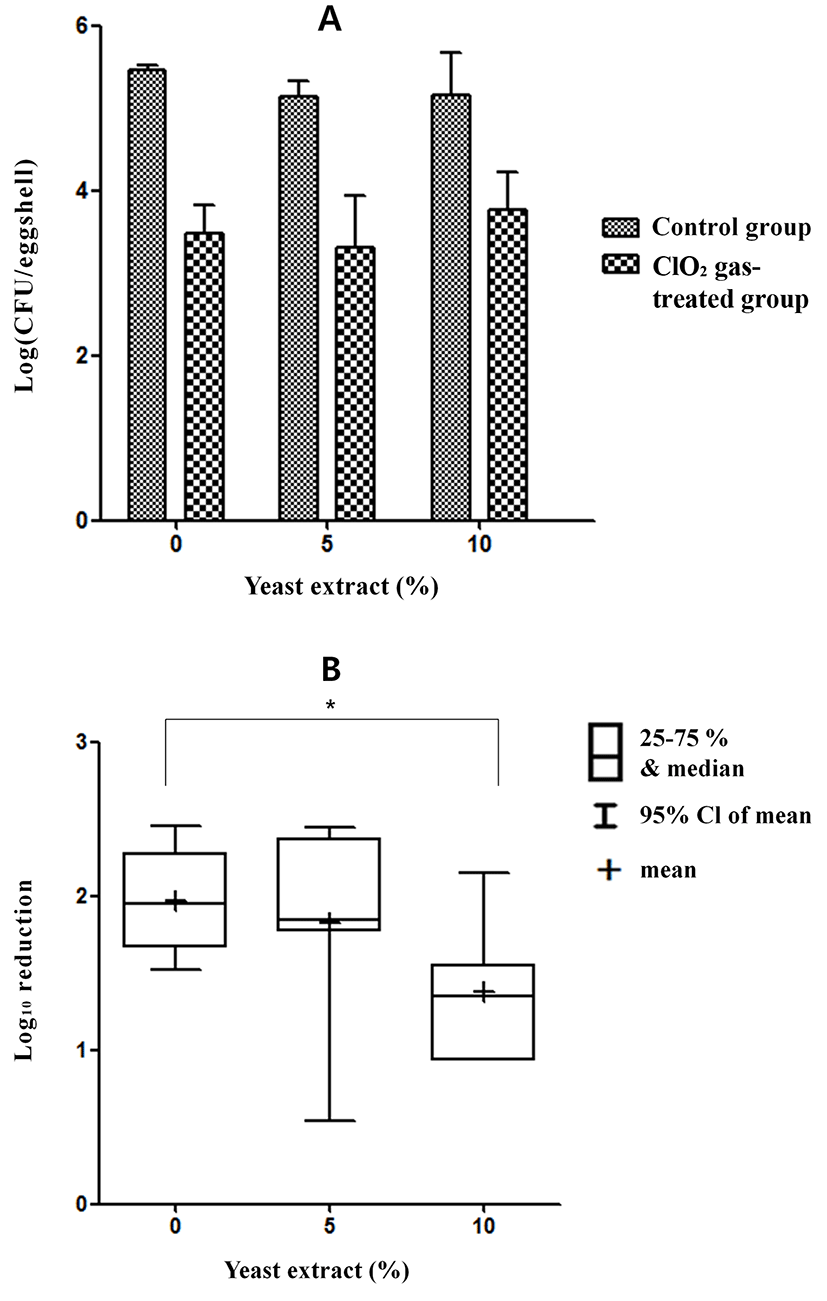
The reduction in SG on the eggshells by ClO2 gas treatment of 80 ppm for 5 min without organic matter (0% yeast extract) ranged from 1.66 to 2.28 Log CFU/eggshell (25% to 75%) and from 1.53 to 2.45 Log CFU/eggshell (minimum to maximum) (Fig. 9). The median and mean log reductions in SG with no added yeast extract were 1.96 and 1.97, respectively. The reduction in SG on the eggshells following the same treatment with 5% yeast extract ranged from 1.77 to 2.40 Log CFU/eggshell (25% to 75%) and from 0.54 to 2.45 Log CFU/eggshell (minimum to maximum). The median and mean log reductions in SG with 5% yeast extract were 1.84 and 1.8, respectively. The reductions in SG on the eggshells following the same treatment with 10% yeast extract ranged from 0.93 to 1.55 Log CFU/eggshell (25% to 75%) and from 0.93 to 2.15 Log CFU/eggshell (minimum to maximum). The median and mean log reductions in SG with 10% yeast extract were 1.35 and 1.38, respectively. A significant difference (p<0.05) in SG was observed between the results with 0% yeast extract and with 10% yeast extract.
Discussionc
ClO2 has been known to be a potent sanitizer/disinfectant for more than 40 years (Benarde et al., 1965). Recently, ClO2 in its gaseous form has been used to disinfect space, food, and food-related equipment (Hsu and Huang, 2013; Lee et al., 2004; Lowe et al., 2013; Prodduk et al., 2014; Sun et al., 2014; Trinetta et al., 2012; Trinetta et al., 2013a; Trinetta et al., 2013b). The use of ClO2 gas as an effective disinfectant is promising for the microbial reduction on fruits (Du et al., 2003). Sun et al. (2014) reported that a controlled-release ClO2 gas fumigation technology showed promise as an effective and practical antimicrobial strategy for the commercial clamshell packaging of blueberries and other fruits. Further, Prodduk et al. (2014) found that ClO2 gas treatment was capable of penetrating and inactivating cells attached to inaccessible sites and within biofilms on sprout surface. In this study, the disinfectant efficacy of ClO2 gas varied with environmental factors such as gas concentrations (ppm), exposure time (min), humidity (% RH), and presence of organic matter.
First, the antimicrobial effects of ClO2 gas against SE and SG were evaluated according to changes in gas concentrations (5, 10, 20, 40, 80 ppm) and exposure times (5, 10, 30 min) under the wet condition (80±5% RH). We considered a 4-log reduction to be an effective standard based on MFDS Regulations (Surface Tests of Sanitizers and Disinfectants) (MFDS, 2009).
In the case of SE, <4 log reduction was noted when each of the concentration and exposure time of ClO2 gas was 20, 40, or 80 ppm for 30 min. SE is a major pathogen related to food poisoning by contaminated eggs. Previous studies have reported the efficacy of commercial cleaning or sanitizing solutions on eggshells contaminated with SE. Sodium carbonate, sodium hypochlorite, and potassium hydroxide were evaluated for their bactericidal activity on eggshells contaminated with SE. The results indicated that the three chemicals applied at the concentrations recommended by the manufacturer (sodium carbonate, 36 ppm; other treatments, 200 ppm) could not eliminate 104 or 106 CFU/mL of SE from eggshells (Soljour et al., 2004). Iodine- based disinfectants (approximately 75 ppm) and chorine (200 ppm) spray reduced SE by 1.35 log and 1.45 log on the eggshell in one study (Knape et al., 2001). The antimicrobial effects of ClO2 gas against SE observed in this study were remarkable compared with commercial cleaning or sanitizing solutions. A few recent studies investigated the effects of electrolyzed oxidizing water on eggs. They showed a log reduction of ≥2.1 log in SE using electrolyzed oxidizing water, whereas a log reduction of 1.7 log in SE was observed for typical commercial detergent-sanitizer (100 ppm free chlorine solution) treatments. However, both electrolyzed oxidizing water and commercial detergent-sanitizers significantly affected the cuticle layer in eggs (Bialka et al., 2004). The cuticle layer of eggshells plays an important role as a natural barrier (Gole et al., 2014). Destruction of the cuticle layer may be a cause of food poisoning, as it is then becomes easy for microorganisms to penetrate the eggshell.
SG is the causative bacteria of FT, a severe systemic disease in chickens. FT has been eradicated from commercial poultry in many developed countries, including the US, Canada, and most countries in Western Europe (Shivaprasad, 2000). However, this disease remains common in some countries in Central and South America, Africa, and Asia, including Korea (Shivaprasad, 2000). It causes serious economic problems in the poultry industry in Korea, although SG has little public health significance because it has highly adapted to the chicken as a host species. Thus, the efficient control of SG in hatching eggs is particularly important. However, fewer studies on the antimicrobial effects of disinfectants against SG have been carried out than for SE. In this study, we achieved >4 log reduction of SG at 40 ppm and 80 ppm ClO2 gas treatment with a required exposure time of 30 min.
Humidity is one of the crucial factors in reducing microorganisms by ClO2 gas. Previous studies reported that inactivation of bacterial contaminants by ClO2 gas was negatively impacted by a low relative humidity (Lowe et al., 2013). This study also revealed that disinfection of eggshells by ClO2 gas was less effective under the dry condition (30±5%) than under the wet condition (RH 80±5%). Regardless of the bacterial species and organic matter present, the reduction in bacteria by ClO2 gas was greater under high RH conditions. A significant difference was found at a high concentration of ClO2 gas between dry (RH 30±5%) and wet conditions (RH 80±5%).
Most disinfectants, including chlorine and its derivatives, are inactivated by interactions with organic matter. Eggshell surfaces can be contaminated by organic matter such as feces or rice straws during the production process. Therefore, an agent with high efficacy in the presence of organic matter may be effective for disinfecting eggs. When ClO2 gas was used to inhibit SG inoculated onto eggshells under wet conditions, no significant difference was observed in the presence of 0% yeast extract vs. 5% yeast extract. However, the reduction in SE with 5% yeast extract was significantly different from the control. The percentage of organic matter had a greater influence on the effect of ClO2 gas on SE than on SG.
Numerous studies have evaluated the antimicrobial effects of ClO2 in various fields. ClO2 has been studied as an agent for water disinfection and various other purposes (Hsu and Huang, 2015). In this study, ClO2 gas is a considerable sanitizer against Salmonellaenterica on eggshells. Like this, industry related with poultry could use ClO2 gas treatment in order to reduce attached salmonellae on poultry skin. With the application of a proper concentration and exposure time, ClO2 gas exposure may be an excellent method for egg disinfection as an alternative to current methods used to sanitize eggs. Moreover, although it needs further study, ClO2 gas could be widely utilized in various food industries.