Introduction
An eggshell is a biological protective barrier for egg contents. The quality of the eggshell is a very important factor for food safety and egg marketing. Cracked and broken eggs cause economic loss, thus making eggshell quality an important concern for layer farms.
Eggshells are formed in the uterus, in which they are mineralized rapidly 10 to 22 h after ovulation. The uterus or shell gland histologically consists of pseudo-stratified ciliated columnar epithelial cells, which include ciliated and non-ciliated cells, basal cells, and goblet cells (Mohammadpour et al., 2012). Structural and functional changes of the shell gland directly affect the quality of the eggshell (Huntley and Holder, 1978). The eggshell consists of the eggshell membrane, mammillary layer, palisade layer and cuticle (Hincke et al., 2012; Nys et al., 1999). The eggshell membranes are fibrous membranes that consist of the egg-enveloped portion of the inner membrane layer and the calcified portion of the outer membrane layer. The mammillary layer is composed of basal parts of calcified columns and knobs that penetrate the outer eggshell membranes (Athanasiadou et al., 2018; Parsons, 1982). The mammillary bodies are even in size and distribution and rounded so that there can be maximum attachment to the fibers of the outer membrane. Low quality eggshells tend to have an irregular, uneven, or incompletely formed mammillary core arrangement. The mammillary knob abnormalities often are manifested as reduced thickness or increased porosity (Dunn et al., 2012; Radwan, 2016). The palisade layer is made of calcite crystals in which an organic matrix is embedded, which accounts for the most of thickness of the eggshell (Fathi et al., 2007; Nys and Gautron, 2007). These palisade layers are the columnar shape with the spongy structure. The narrow columnar shape provides high strength against breakage to the eggshell and shell thickness (Ahmed et al., 2005; Fathi et al., 2016). The cuticle is the last layer to be deposited on the shell before egg laying. The cuticle limits movement of particles, water, and bacteria through the shell pores. Thus, the cuticles along with the mineralized shell and shell membranes play a role as the physical barrier against microorganism invasion and contamination to the egg contents (Gantois et al., 2009; Rose-Martel and Hincke, 2015). Minerals are essential for normal growth and development of chickens, especially eggshells and bone formation in laying hens (Richard et al., 2010). The minerals are involved in the synthesis of calcium crystals during shell formation as a function of the catalytic properties of the enzymes (Cusack et al., 2003; Fernandes et al., 2008; Stefanello et al., 2014). It is well known that some minerals are the main factor contributing to the mechanical properties of eggshell thickness (Cusack et al., 2003; Dunn et al., 2009).
In a previous study, egg weight, specific gravity and eggshell surface area increased but eggshell contents did not change with increasing hen age (Tůmová and Gous, 2012). Egg weight increased with increasing hen age, but the quality of the eggshells, such as shell thickness and specific gravity, decreased because the eggshell deposition did not increase proportionally (Roberts et al., 2013). According to Molnár et al. (2016), the shell index and shell thickness were decreased, and shell deformation was increased at the end of the laying cycle. Rodríguez-Navarro et al. (2002) also reported that eggshells from aged hens had lower strength against breakage and showed a greater variability in their structural properties such as thickness, grain morphology and crystallographic texture. Cuticle thickness and degree of glycosylation was also decreased in aged hens (Kulshreshtha et al., 2018; Rodríguez-Navarro et al., 2013).
Extensive studies were conducted to evaluate the ultrastructural variations in eggshell quality among breeds and species of poultry (Fathi et al., 2007; Radwan, 2016). It is well known that the material properties of the eggshell depend on its microstructure and chemical composition, both of which vary through the thickness of the eggshell. Therefore, this study was performed to investigate the ultrastructural and ionic component variations of eggshell with increasing hen age, using scanning electron microscopy (SEM) and inductively coupled plasma (ICP) spectrometry.
Materials and Methods
Hyline Brown commercial layers were raised at the Sangol Farm, Sancheong, Korea under the same environmental and management conditions. All hens were housed in windowless and environmentally controlled rooms and in battery cages with five hens per cage (420 cm2/hen) equipped with nipple drinkers, automatic chain trough feeders and a wire egg collector. The hens were fed a compound feed (15%–18% CP and 2,800–2,830 kcal ME/kg) and were given water ad libitum. The room temperature was kept at 21℃–22℃ and artificial light consisted of 15 h of light and 9 h of dark. To analyze eggshell quality, each 100 eggs were collected randomly from hens at 30, 60, and 78 wk old.
Each egg was weighed and broken, and the height of the thick albumen, egg shell color and yolk color were measured by a QCM+ system (TSS Ltd. York, England). The wet eggshell was weighed before drying. The thickness of the eggshell with the intact membrane was measured using a dial gauged micrometer (Series 293-IP65, Mitutoyo Corp. Kanagawa, Japan). Eggshell density (mg/cm2) was calculated using QCM Eggware software (TSS Ltd. York, England).
Five hens, aged at 30, 60, and 78 wk, were randomly collected and slaughtered to obtain uterine tissues. The hens used in the experiment were handled in accordance with the regulations of the Institutional Animal Care and Use Committee (IACUC) of this organization. The collected tissues were washed twice with a D-PBS solution and fixed with a 10% formalin solution (Sigma, St. Louis, MO, USA). The specimens were processed for paraffin section preparation with automated instruments called Tissue Processors (Model Microrn STP 120, Thermo Fisher scientific, Walldorf, Germany). The specimens were washed and passed through multiple changes of dehydrating and clearing solvents using ethanol and xylene. The specimens were placed in an embedding desk after processing. Formalin-fixation and paraffin embedding (FFPE) was performed on the tissue using an FFPE workstation (Model Histostar, Thermo Fisher scientific, Walldorf, Germany). Sections were cut at a thickness of 3–5 µm using a rotary microtome (Model Jung Histocut 820, Leica, Nussloch, Germany). Sections were placed on the surface of warm water in a flotation bath to flatten them, and they then were picked up onto microscope slides. The specimens were stained with a hematoxylin-eosin (H&E) solution (Muto, Tokyo, Japan) after thorough drying. The stained sections were covered with a glass coverslip, using Permount solution.
Blood samples were taken from the wing veins of the hens and were separated by centrifuge at 800×g for 20 min to obtain serum. The concentrations of mineral contents of the serum samples were measured by plasma spectrometric methods for four essential minerals, such as calcium (Ca2+), sodium (Na+), potassium (K+) and chloride (Cl–). The spectrophotometric analysis was conducted using Olympus AU2700 (Olympus Medical Engineering Company, Japan).
Each ten samples of eggshell were randomly taken from hens at 30, 60, and 78 wk old to investigate ultrastructural variations. The specimens were prepared by cutting a piece (1 cm2) of shell from the middle short circumference area of the egg. The shell membranes were removed by immersing in distilled water for 24 h. To remove the remaining membrane fibers, each sample then was immersed and boiled for 10 min in 2% sodium hydroxide (Liao et al., 2013). Thereafter, the samples were washed with distilled water and were dried at room temperature for 72 h. After the dehydrating process, the samples were coated with platinum for 3 min in Sputter Coater. These samples were observed using a field emission scanning electron microscope (FE-SEM) at 15 Kv (JSM-6701F, JEOL, Tokyo, Japan). The cross-sectional lengths of palisade and mammillary layers were measured in μm using scaling software provided with the SEM at a magnification of ×100. The number of mammillary knobs distributed within a 1 mm2 area of the shell surface was counted to therefore determine the density of mammillary knobs.
Sections from the center-region of 10 eggshells from hens were collected at 30, 60, and 78 wk old. The samples were washed with distilled water, and the membranes then were removed. Thereafter, the eggshells were dried at room temperature for 72 h and powdered. Next, 500 mg of the dried sample was placed in the pretreatment container, and 5 mL of 65% nitric acid then was added. The samples were placed in the high-pressure microwave sample preparation system, and the sample digestion was performed in accordance with the program (Ultrawave 2.0, Milestone, Italy). The pre-treated samples were transferred into a 50 mL flask and filled with deionized water. Analyzing samples were completed by filtration with a 0.45 µm filter. An ICP-optical emission spectrometer (ICP-OES) was used to measure the concentration of mineral elements in the eggshells (Optima 8300DV, Perkin Elmer, U.S.A). The calibration of the spectrometer was performed using aqueous calibration standards (MERCK ICP-OES standard of elements). Three standards were prepared with a dilution containing 1 ppm, 5 ppm, and 10 ppm of the 11 elements. Standard solutions were prepared from 1,000 mg/L stock solution (Merck, Damstadt, Germany) by dilution in 5% (v/v) HNO3.
The results were expressed as the mean values with standard deviation. The statistically significant differences between observations were tested by ANOVA procedure and followed by Tukey’s HSD using the SAS statistical package (SAS Institute Inc., Cary, NC, USA). For all analyses, the significant differences were considered at p<0.05.
Results
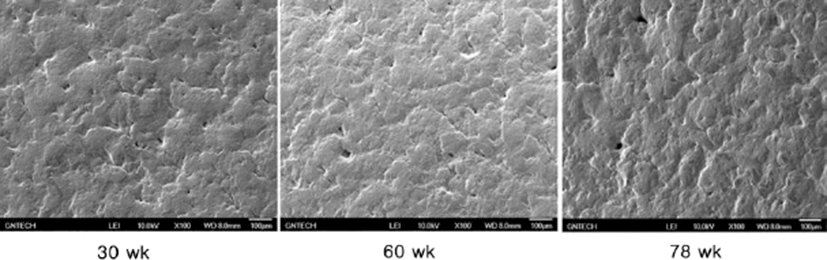
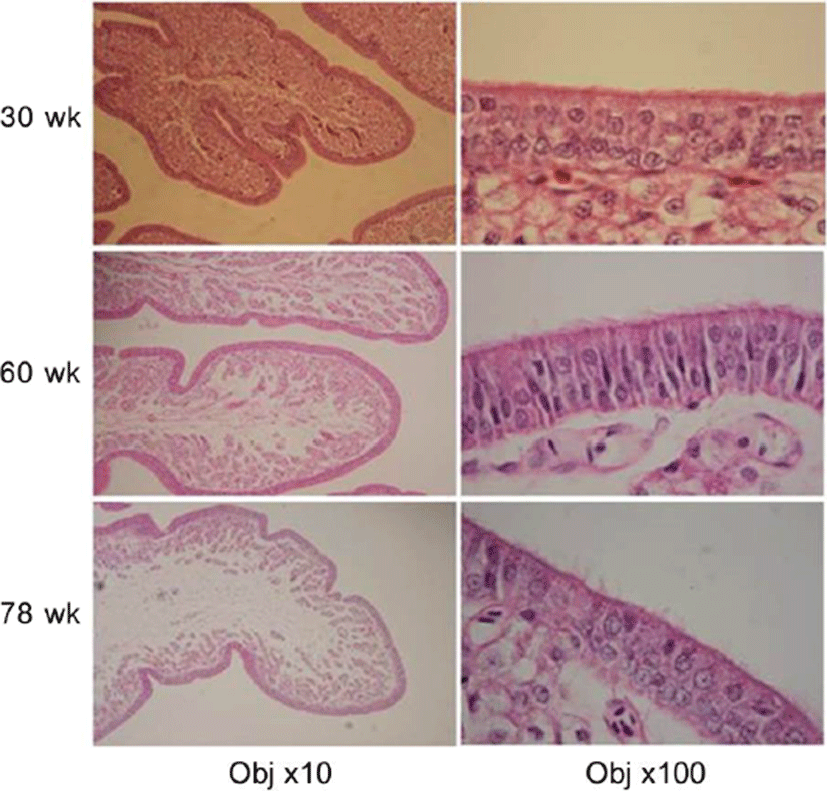
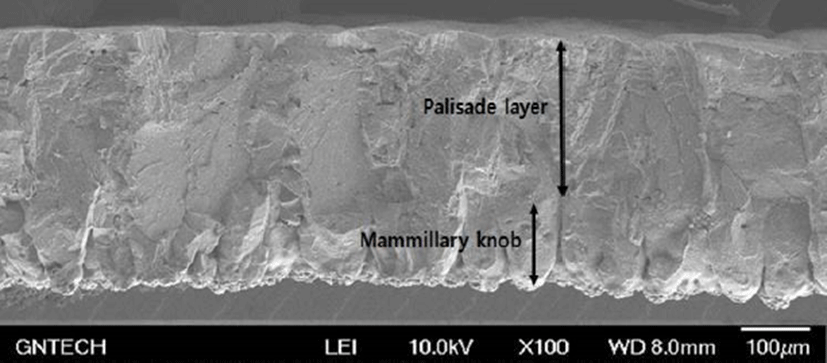
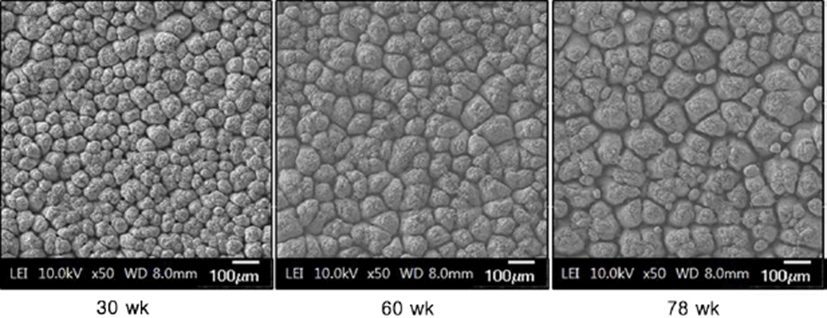
Fig. 1 shows the microscopic observations of the uterine tissues of the hens according to their age. In this figure, the changes of endometrial tissues were observed according to hen age. Histological deformation, fibrosis, atrophy and elimination of micro-villi were found with increasing hen age in the chicken uterine endometrium. The uterine tissue from 30-wk-old hens showed little damage and loss of the ciliated epithelium cells, and the shape of the ciliated cells was also short and uniform. However, the endometrial tissues of 60- and 78-wk-old hens showed increased length of uterine cilia and decreased cell density compared to the 30-wk-old hen tissues. Additionally, some of the endometrial tissues from aged hens were atrophied with progressive fibrosis. Table 1 shows the lengths of cilia in uterus ciliated cells according to hen age. As shown in Table 1, the length of the uterine cilia was significantly increased in older hens. Table 2 shows the concentrations of blood-ion components such as Ca2+, Na+, K+, and Cl– ions did not change significantly with hen age. In the effect of hen age on eggshell quality, Table 3 shows that the age of the hen did not significantly affect almost all parameters of the eggshell quality, except the measurement of eggshell weight. The eggs obtained from 60- and 78-wk-old hens showed significantly higher shell weight than values from 30-wk-old hens. Cross-section and surface analysis of the eggshells were performed using FE-SEM to observe the ultrastructure of eggshell according to hen age. Fig. 2 shows the cross-sectional structure of the eggshell, which consisted of cone-shaped mammillary knobs in contact with the inner eggshell and a columnar shaped basal layer. Fig. 3 shows the ultrastructure of the inner surface of eggshells obtained from 30-, 60-, and 78-wk-old hens. In this figure, the size of mammillary knobs increased with increasing hen age, but the number of knobs decreased with increasing hen age. The type B mammillary knobs, a type of fragmented knob, were observed frequently as age increased (Fig. 4). The physical parameters of eggshell structure were also measured according to hen age. As shown in Table 4, the ratio of the palisade layer decreased by 7.85% with increasing hen age from 30 to 78 wk, but the ratio of the mammillary layer increased by 21.63% in the same period. In addition, the mammillary knob density decreased by 38.9% with increasing hen age from 30 to 78 wk (p<0.05). Fig. 5 shows the ultrastructure of cuticle layer according to hen age. As shown in Fig. 5, there was no morphological change in the cuticle layer according to age. Table 5 shows the results of the concentrations of eggshell ion components such as Ca2+, Mg2+, P–, S2–, Na+, K+, V2+, Co2+, Ni2+, Mn2+, and Be2+ according to hen age by ICP analysis. The result showed that Ca2+, S2–, and Co2+ significantly decreased, whereas Na+, K+, and V2+ significantly increased with increasing hen age.

| Hen age | 30 wk | 60 wk | 78 wk |
|---|---|---|---|
| Length of uterine cilia (Lm) | 0.96±0.22b | 1.41±0.18a | 1.52±0.17a |
| Hen age (wk) | Na+ (mEq/L) | K+ (mEq/L) | Cl– (mEq/L) | Ca2+ (mg/dL) |
|---|---|---|---|---|
| 30 | 163.6±3.29 | 4.44±0.11 | 131.4±1.52 | 18.60±1.57 |
| 60 | 159.0±1.83 | 4.48±0.10 | 127.5±2.38 | 20.13±1.19 |
| 78 | 161.0±3.65 | 4.35±0.13 | 128.0±4.08 | 18.98±0.77 |
Discussion
Various factors such as strain, age of hen, egg storage condition and feeding affect eggshell quality (Lee et al., 2016b; Wilson, 2017). Among these factors, hen age is one of the main components affecting eggshell quality. Many researchers have reported that eggshell thickness decreased with increasing hen age (Lee et al., 2016a; Molnár et al., 2016; Rodríguez-Navarro et al., 2002). This was considered to be the most important factor in reducing eggshell quality.
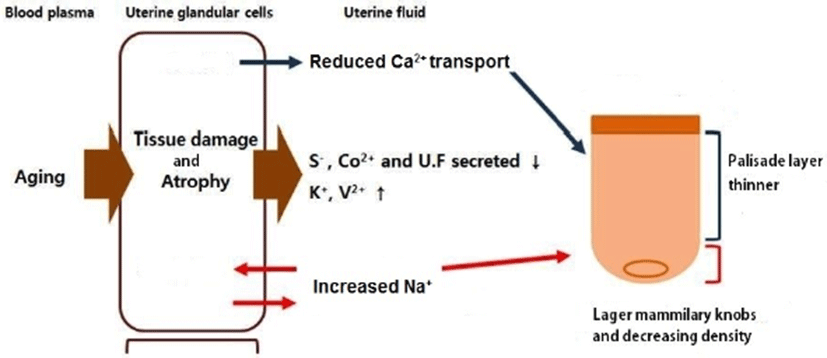
This study investigated the cause and the process of eggshell quality degradation according to hen aging. As hen age increased, histological deformation, fibrosis, atrophy and ciliated cell loss in the endometrium gradually increased. In our previous study, the amount of telomeric DNA in the uterine endometrial cells also decreased with increasing hen age (Park, 2018). Chronic stimuli such as sustained laying behavior caused fibrosis and atrophy of the endometrium, resulting in a functional decline in the uterus (Bahr and Palmer, 1989; Park et al., 2017). The increase in the length of uterine cilia due to aging implies that the regenerative capacity of the cells degraded (Woudstra and Thomson, 2002). Eggshell constituent ions that participate in eggshell mineralization are transported through uterine fluid. Many studies have reported that the lack of uterine fluid secretion has a negative effect on eggshell formation (Abe, 1996; Huntley and Holder, 1978). Hen aging promoted the damage and atrophy of the uterine tissue, which resulted in the lack of secretion of uterine fluid. Therefore, the condition of endometrial cells is considered to be one of the main factors affecting eggshell quality. Blood ion concentrations such as Ca2+, Na+, K+, and Cl– were not found to differ with hen age. This result seems to be similar that the contents of serum ions are constant despite the feeding with different dietary levels. An et al. (2010) reported the contents of serum Ca and phosphorus were not affected by dietary Ca levels. In addition, serum ions such as Ca2+ and HCO3– maintain homeostasis by internal regulatory mechanisms (Jiang et al., 2013; Jonchère et al., 2012). This suggests that the concentration of ionic constituents in the blood is not a direct factor affecting eggshell quality. In the eggshell ultrastructure, the palisade layer ratio and the density of mammillary knobs significantly decreased with increasing hen age. The mammillary layer is the innermost of the calcified portions of the eggshell. The degree of binding between the mammillary knob and the eggshell membrane is related closely to the strength of the eggshell (Athanasiadou et al., 2018). Low density of the mammillary knobs reduces the binding force between the mammillary knob and the eggshell membrane and weakens eggshell strength. In general, the constant size and shape of the mammillary knob and the strong bond between the knobs and the shell membrane resulted in high eggshell strength (Dunn et al., 2012; Radwan, 2016). In the classification of mammillary types, Fathi et al. (2007) reported that type A mammillary bodies were even in size and distribution and rounded so that there can be maximum attachment to the fibers of the outer membrane. The type A mammillary body contact with the membrane fibers was minimal, and the bodies had a conical appearance at the level of the mammillary layer (Rayan et al., 2010). However, the rounded type B bodies appeared as a small piece of debris between the mammillary knobs. They made no contribution to palisade layer formation but affected the weakening of the base of the palisade layer, thus resulted in the formation of a weaker shell and were more subject to breakage (Solomon, 1991). Our results showed that type B mammillary knobs increased with increasing hen age. The palisade layer is made of calcite crystals in which an organic matrix of 2%–3% is embedded and represents approximately 70% of the total thickness of the eggshell (Nys and Gautron, 2007). These palisade layers consist of the columnar shape of the spongy structure, which provides high strength against breakage to the eggshell (Ahmed et al., 2005; van Toledo et al., 1982). As hen age increases, the palisade layer becomes thinner, which causes the strength of eggshell to weaken (Rayan et al., 2010; Rodríguez-Navarro et al., 2002). In the elementary composition of the eggshell, Ca2+, S2–, and Co2+ ions were significantly decreased, while Na+, K+ and V2+ ions were significantly increased in older hens. Trace minerals are components of enzymes that synthesize calcium crystallization in eggshell formation. Thus, the trace minerals affected the eggshell quality either independently or interactively (Fernandes et al., 2008; Stefanello et al., 2014). Ca2+ is closely related to the calcification of the eggshell, and the concentration of Ca increases with increasing reproductive hormone secretion during the laying period (Bar, 2008; Gongruttananun, 2011). S2– is a major component of dermatan sulfate proteoglycan, which is secreted in uterine epithelial cells and plays an important role in the formation of fibrous structures of collagen molecules (Arias et al., 1991; Carrino et al., 1996). Co2+ is involved in bone formation and mineral deposition with ions such as Ca2+ and F– (Birgani et al., 2016). Decrease in the Ca concentration during aging reduces the thickness of the basal layer and the density of the mammillary knobs in eggshell formation (Chen et al., 2015). In addition, decreasing concentrations of Ca and S not only induce crystallization of abnormal mammillary knobs but also negatively affect the binding to the eggshell membrane (An et al., 2016). Na+ in uterine fluid is involved in the formation of early eggshell crystals and regulates the size of the crystals (Fernandez et al., 1997). The cells of the uterine gland actively transport Na+ through the Na channel of the cell membrane and secrete a basic anion that is a signal molecule that regulates the secretion rate of HCO3– in the cell. Hen aging reduces the transmission of anions, while the decreased anions result in a reduction in eggshell thickness (Vetter and O'Grady, 2004). K+ is involved in the regulation of uterine fluid secretion, and the concentration of K increases at the early stage of eggshell formation and at the late stage of calcification. V2+ is mainly involved in glucose and lipid metabolism and mineralization of bone, cartilage, and tooth formation (Panchal et al., 2017; Ścibior, 2016). V2+ is also known to be involved in Ca2+ homeostasis and osteopontin expression (Sanchez-Gonzalez et al., 2017). Therefore, the aging of the chicken causes endometrial cell damage or decreases the regulation of uterine fluid secretion, which increases the release of Na+ and K+ ions in endometrial cells. The V2+ ion also increased with the migration of Na+ and K+ in relation to Ca2+ homeostasis with increasing age.
In conclusion, hen aging damages the uterine tissues and decreases the secretion of gonadal hormones, which reduces the levels of Ca2+, S2–, and Co2+ in the uterus. Decreasing concentrations of Ca2+ and S2– ions inhibit crystallization of the basal layer and the formation of mammillary knobs, reducing the density of the mammillary knobs and the rate of the palisade layer. The increase of Na+ in the late laying stage increases the incidence of type B mammillary knobs and the size of crystals and widens the space between palisade layers, which causes poor eggshell formation.
Based on the conclusion, the mechanism of degradation of eggshell formation related to hen aging was summarized as shown in Fig. 6. Therefore, due to the physiological characteristics of the chicken and the physiochemical properties of the eggshell, it is posited that hen aging reduces the strength of the eggshell and degrades eggshell quality.