Introduction
In recent years, researches and developments have focused more on films and coatings made from various agricultural proteins because of their nutritional qualities, film forming abilities and relative abundance. Utilization of agricultural proteins creates new outlets for agricultural products, by-products and waste streams, all of which can positively impact the economics of food processes (Dangaran et al., 2009). Mechanically deboned chicken meat (MDCM) is one of the important sources of agricultural proteins, and is produced from edible tissue on chicken bones by deboning or separation techniques. MDCM is commonly used all around the world in comminuted meat products, such as salami (Raphaelides et al., 1998), sausage (Pereira et al., 2011) and patty (Lyon et al., 1980), due to its fine consistency and relatively low cost. Against these advantages, the deboning or separation techniques cause cell breakage, protein denaturation and an increase in lipids and free heme groups, which imply several disadvantages such as color, flavor, palatability and microbial load, making MDCM a very easily perishable raw material (Pereira et al., 2011). For these reasons, with regulation issued by Republic of Turkey Ministry of Food, Agriculture and Livestock in 2012, the usage of MDCM in comminuted or emulsified meat products was prohibited (TGK, 2012) and thus, led to formation of a new waste product for meat processors. Based on this information, the usage of MDCM proteins (MDCM-P) in the production of edible films and coatings is a possible way of giving value to this waste product.
Protein polymers have film forming ability and the source of proteins is highly effective on their properties (Rostamzad et al., 2016). Most of protein films have good barrier properties against gas, organic vapor and oil as compared to synthetic films. However, the poor mechanical properties and the high permeability against water vaporof protein films limit their application as packaging material (Jiang et al., 2007; Jiang and Tang, 2013; Tang et al., 2011). For this reason, many researchers focused on the improvement of mechanical and water permeability properties of protein films. An effective approach to improve these properties is the cross-linking technique by chemical or enzymatic methods (Jiang et al., 2007). It was reported that the mechanical and permeability properties of protein films could be improved by transglutaminase (TGase) addition, which is regarded as safe and effective cross-linking agent (Jiang and Tang, 2013; Rostamzad et al., 2016; Weng and Zheng, 2015). Acyl-transfer reactions between λ-carboxyamide groups of glutamine residues (acyl donor) and ε-amino groups of lysine residues (acyl acceptor) can be catalyzed by TGase (E.C. 2.3.2.13), thus intra and intermolecular cross-linked biopolymers of ε-(λ-glutaminyl) lysine are formed (Chambi and Grosso, 2006; Tang et al., 2011). Covalent cross-linking of food proteins changes the protein functionality and therefore, protein based films could be modified (Tang et al., 2011).
There are enough studies about the effect of TGase on the properties of films made from various agricultural proteins such as gelatin (Chambi and Grosso, 2006; De Carvalho and Grosso, 2004; Jiang and Tang, 2013), soy proteins (Jiang et al., 2007; Tang et al., 2005), bean proteins (Tang et al., 2011), zein (Masamba et al., 2016) and fish proteins (Rostamzad et al., 2016). Tang et al. (2005) studied the effect of TGase treatment on the properties and microstructures of soy protein isolate films, and suggested the TGase treatment of film-forming solutions of soy protein isolate prior to casting could greatly modify the properties and microstructures of soy protein isolate films. Tang et al. (2011) reported that the tensile strength and elongation at break values of red bean protein films were greatly improved by the TGase addition at low concentrations (4-10 U/g), but considerably impaired at higher concentrations (20-40 U/g). Similarly, Weng and Zheng (2015) reported that the strength of gelatin-soy protein isolate composite films was increased by adding 1% TGase irrespective of soy protein isolate addition, but decreased when the TGase concentration was further increased.
As seen from the above results, the improvement in the properties of protein films by TGase depends on types of substrate protein and some processing parameters, such as enzyme concentration. To the best of our knowledge, there are no reports on the effect of TGase on properties of MDCM-P films. Therefore, the object of this research was to determine the effect of TGase addition, at different concentration (0, 1, 2, 3 and 4%), on the physicochemical properties of MDCM-P films.
Materials and Methods
Minced MDCM was obtained from Köytür Chicken Company (Turkey). The proximate composition of MDCM including moisture, protein and lipid was determined according to AOAC official methods 934.06, 960.52 and 960.39, respectively; of the Official Methods of Analysis of AOAC (1990) international procedure and they presented 64.19±4.05% moisture, 13.17±0.02% protein and 21.04±0.43% lipid. MDCM proteins (MDCM-P) were extracted using the method of Zavareze et al. (2014) with several modifications. Firstly, MDCM was defatted using diethyl ether overnight on a shaker, and then, defatted MDCM was mixed with water at a ratio of 1:9 (w/v) in commercial blender (Waring-80011 S, USA) for 2 min. The pH of the slurry was adjusted to 12 with 5 M NaOH under continuous agitation by a magnetic stirrer (MTO-PS, HS15-03P model, Korea) for 1 h. After protein solubilization, the dispersion was centrifuged at 9,000×g for 30 min at 4ºC. The supernatant was collected, acidified with 5 M HCl for protein precipitation at pH 4.70 by mixing with magnetic stirrer for 5 min and finally centrifuged again at 9,000×g for 10 min at 4ºC. The supernatant was then discarded, and the MDCM-P remained. The precipitate was dried in an air circulated oven for 24 h at 40ºC. Finally, the MDCM-P were milled, sieved from 212 µm and stored at 4ºC until film preparation. The protein content of MDCM-P was determined according to AOAC official methods 960.52 of the Official Methods of Analysis of AOAC (1990) international procedure and they presented a protein content of 71.89±0.28%. TGase in powder form with enzyme activity of 100 U/g, according to information provided by the industry was obtained from Ajinomoto Co. Inc., Japan. Glycerol (Merck, Germany) was used as film plasticizer. All the other reagents and chemicals were of analytical grade.
The MDCM-P films were prepared according to the methods of Limpan et al. (2010) and Masamba et al. (2016) with several modifications. 4.0 g of MDCM-P was added to 100 mL distilled water, pH was adjusted to 11.5 with 5 M NaOH, and 40% (w/w of MDCM-P) glycerol was then added as plasticizer. The solution was homogenized at 10,000 rpm for 5 min with an Ultraturrax (IKA, T25 model, Germany). The solution was later heated at 85°C for 60 min in a water bath on a magnetic stirrer to allow film formation, cooled to room temperature (~20°C), and the solution pH was adjusted to 6.7 with 2 M HCl for optimal enzyme activity. Different concentrations of TGase (0, 1, 2, 3 and 4% based on protein weight) were mixed with 1 mL distilled water (45°C) until completely dissolving the enzyme, and then the dissolved enzyme was added to film solution, stirred for 1-2 min on a magnetic stirrer before incubation at 45°C for 60 min. After incubation, the solutions subjected to centrifugation at 5,000 rpm for 5 min to remove air bubbles and undissolved debris and cooled to 402 C. Finally, the solutions (50 g) were poured into plastic petri dishes (15 cm diameter) and dried at 40°C for 24 h in air circulated oven (JSR, JSOF-50 model, Korea). Dried films were then peeled off from petri dishes and conditioned to 54% relative humidity (using a saturated Mg(NO3)2) within a desiccator at room temperature (~20 °C) for 3 d. All films were prepared in triplicate.
A digital micrometer (Insize, 3101-25A model, China) having a precision of 0.001 mm was used to determine film thickness and measurements were taken at ten different locations of each film. Three samples of each treatment were measured.
Moisture content of film pieces was determined by drying in an oven at 105°C until constant weight according to AOAC official methods 934.06 of the Official Methods of Analysis of AOAC (1990) international procedure.
Film solubility was determined according to the method of Gennadios et al. (1998) with a slight modification. The initial dry matter of the preconditioned films (20×20 mm) was determined by drying in an oven with air circulation at 105°C for 24 h. Dried film pieces were immersed in 50 mL distilled water containing sodium azide (0.1%, w/v) to prevent microbial growth and stored at room temperature for 24 h under periodic agitation. The insoluble matter was separated carefully and dried at 105°C for 24 h for determination of the final dry weight. Water solubility of films was calculated by subtracting the weight of undissolved dry matter from the initial weight of dry matter and expressed as a percentage of total weight. Three samples of each treatment were measured.
To determine WVP, the films (14 mm diameter) were sealed in glass cups containing silica gels (0% relative humidity) and then stored at 25°C in desiccators containing distilled water (100% relative humidity). The cups were weighed at 1 h interval over 8 h. Three samples of each treatment were measured, and WVP was calculated by:
where w/t is calculated by linear regression (R2>0.99) from the water absorbed by the system at the steady state was reached. A is the film area exposed to moisture transfer (1.539×10−4 m2), x is the mean sample thickness, and ΔP is the partial pressure difference through the film at 25°C (kPa) (ASTM, 2003).
The color of three film samples of each treatment was measured using a colorimeter (Chroma meter CR 400, Konica Minolta Camera Co., Ltd., Japan), and measurements were taken at five different locations of each film. The color of the films was expressed as L* (lightness/brightness), a* (redness/greenness) and b* (yellowness/blueness) values. The colorimeter was calibrated using white and black standard tiles, illuminate D 65, and a 10° standard observer and the color of the films were determined on a white standard plate.
The opacity of the film specimens was evaluated by measuring the absorbance at 600 nm using a UV-Visible spectrophotometer (Agilent Technologies, Cary 60 model, Australia) according to the method of Gomez-Estaca et al. (2009). An empty test cell was used as the reference and three samples of each treatment were measured. Opacity was calculated by:
where x is the mean film thickness (mm) and Abs600 is the value of absorbance at 600 nm. According to this equation, low values of opacity demonstrate higher transparency.
The mechanical properties of film samples such as tensile strength (TS) and elongation at break (EAB) were measured with a texture analyzer (TA-XT2 Texture Analyzer, Stable Micro Systems Co., Ltd., USA) based on the ASTM standard method (ASTM, 2001). For this purpose, the films were cut in strips (10×40 mm) and conditioned for 3 d (54% relative humidity). The thickness values of strips were randomly taken from ten different points before mechanical tests. The force and distance were measured during the extension of the strips mounted between the grips at 1.5 mm/s until break. TS was calculated by dividing the load at break by the original cross-sectional area (mm2) of the film, while EAB (%) was calculated by dividing the elongation at the moment of rupture by the initial gauge length and multiplying by 100. The results are given the average of five samples for each treatment.
The FTIR spectra of films were recorded on a spectrophotometer (Perkin Elmer, Model Spectrum Two, USA) fitted with a Miracle Single-Reflection Diamond ATR device in the wavelength range of 4,000-650 cm−1 with a spectral resolution of 4 cm−1. Measurements were performed at room temperature directly on films which were conditioned for 10 d in desiccators with silica gel. FTIR spectra of films were represented as the average of at least three measurements.
The dried film samples were mounted on aluminum stubs with double-sided adhesive tape, and coated with a thin layer of gold-palladium (Quorum SC7620, USA). Morphological observations of the surface and cross-section of the film were done with a scanning electron microscope (Jeol, model JSM-7001F, Japan) at 10 kV, and photographs were taken at 1500× magnification.
Experiments were performed as triplicates and values were expressed as mean ± standard deviation. Analysis of variance (ANOVA) was used to analyze the data and the means of the results were compared with Duncan’s multiple range tests. SPSS statistical package program (SPSS 17.0 for windows, SPSS Inc., USA) was used for the analysis with a significance level of 0.05.
Results and Discussion
Table 1 shows the effect of TGase addition on the physical properties of the MDCM-P films. As seen, thickness values were affected by the addition of TGase (p<0.05) and they increased from 0.164 to 0.191 mm as enzyme concentration increased from 0 to 4%. This could be explained by the increase of dry matter in the MDCM-P films by the addition of the enzyme. This explanation is also confirmed by the moisture content of films. Similar findings were also obtained by Tang et al. (2011) in TGase-treated red bean protein films. However, Masamba et al. (2016) reported that the enzyme addition did not significantly affect the thickness values of zein films incorporated with oleic acid.
In terms of food quality maintenance it is crucial for food packaging industry to know moisture retaining properties of packaging materials, and therefore the knowledge of moisture content of edible films is also important (Masamba et al., 2016). As seen in Table 1, the moisture content of MDCM-P films slightly decreased as TGase concentration increased, but this decrease was not significant. The similarities in moisture content suggested that the covalent linkages in protein introduced by TGase did not affect water uptake. Similar results were also reported in TGase-treated soy protein isolate films (Tang et al., 2005) and in TGase-modified gelatin films (Liu et al., 2016). However, various researchers reported that the moisture content of soy protein isolate films (Jiang et al., 2007), red bean protein films (Tang et al., 2011), gelatin films (Wangtueai et al., 2010) and zein films (Masamba et al., 2016) varied with the addition of TGase. These different results could be attributed to the type and concentration of solvent used, type of protein, and enzyme concentration.
Water solubility of film samples is regarded as an indicator of resistance to water, so it is significant for food packaging because of high water activity and the possibility of contamination in presence of water (Rostamzad et al., 2016). Therefore, low solubility is crucial for improving the overall integrity of films in wet environments (Jiang et al., 2016). The solubility of MDCM-P film without TGase was 38.14% (Table 1). This value is similar to that determined by Tang et al. (2005) and Jiang et al. (2007) in soy protein isolate films, but is lower than that determined by Rostamzad et al. (2016) in fish protein films. When TGase was added to the MDCM-P matrix, the solubility of the films decreased and the lowest value (32.14%) was obtained in MDCM-P films containing 3% TGase (Table 1). However, no significant differences in solubility were observed between films containing 2, 3 and 4% TGase (p>0.05). The lower solubility could be attributed to covalent cross-linking effect of TGase. This effect creates the new large molecular polymer in films, leading to the impaired solubility (Jiang et al., 2016). Similar results were found in whey protein-carboxymethylated chitosan composite films, gelatin films and fish protein films with nanoclay by Jiang et al. (2016), Liu et al. (2016) and Rostamzad et al. (2016), respectively, these authors observed that the solubility decreased with TGase addition.
Values are mean ± standard deviation.
Different letters (a-d) in the same column indicate significant differences (p<0.05).
Water vapor permeability (WVP) is one of the most important application properties of food packaging, and it should be as low as possible to reduce moisture loss of the packaged food (Jiang et al., 2016). As seen in Table 1, MDCM-P films with or without TGase exhibited high WVP values because of high hydrophilic properties of proteins and the significant amounts of glycerol added to films as plasticizer. High WVP values in protein-based films were also determined by many researchers (Jiang et al., 2016; Kolodziejska and Piotrowska, 2007; Yildirim and Hettiarachchy, 1998). The WVP values of films were affected by the addition of TGase (p<0.05) and in contrast to the solubility, the all MDCM-P films treated with TGase exhibited higher WVP values than that of control film without TGase (p<0.05). The increase in WVP of MDCM-P films might restrict their application in foods. Many factors such as the chemical nature of macromolecules, crystallinity, molecular mass, orientation and degree of cross-linking can affect WVP of films. Permeability is also influenced by membrane porosity, the surface absorption and desorption behavior of the permeant, relative humidity and the amount of plasticizer (Rostamzad et al., 2016; Yi et al., 2006). The increase in WVP could be attributed to additional and/or larger pores in MDCM-P films treated with TGase because, during the cross-linking reaction, the orientation of protein molecules around each other could produce additional pores or cause enlargement of existing pores (Yildirim and Hettiarachchy, 1998). The increase of WVP of TGase treated films could also be due to the increase in mobility of the chains. The increase of chain mobility causes to an increasing water diffusion coefficient, which results in more permeable film against water vapor (Chambi and Grosso, 2006). In addition to these, the formation of high molecular weight polymers with TGase weakened the WVP of films which subsequently led to higher WVP (Wang et al., 2015). Increase in WVP of protein-based films treated with TGase was also reported by Yildirim and Hettiarachchy (1998), Tang et al. (2005) and Chambi and Grosso (2006). However, various researchers (Jiang et al., 2016; Rostamzad et al., 2016; Weng and Zheng, 2015) reported that the WVP of protein-based edible films was improved by TGase addition. These different results could be attributed to the protein source and its proportion in the final film (Jiang et al., 2007; Tural and Turhan, 2017), enzyme (Jiang et al., 2007; Rostamzad et al., 2016) and plasticizer concentration (Tural and Turhan, 2017), degree of cross-linking (De Carvalho and Grosso, 2004; Yi et al., 2006), membrane porosity (Yi et al., 2006) as well as differences in test procedure (Tural and Turhan, 2017).
Optical properties of edible films using as a food packaging material directly affect the consumer preferences (Shojaee-Aliabadi et al., 2014). Table 2 shows the effect of TGase addition on the optical properties of the MDCM-P films. As seen, all color parameters of MDCM-P films were affected by the addition of TGase (p<0.05), and the highest L* and the lowest a* and b* values were observed in control film without TGase (p<0.05). All MDCM-P films containing TGase exhibited a slight yellowish-reddish tint compared with control film because of the higher a* and b* values. When TGase concentration increased, in general, the L* values of MDCM-P films decreased, but a* and b* values increased. These effects could be attributed to the covalent cross-linking induced by TGase between adjacent proteins, leading to a different response to light. Similar L* and b* values were reported by Uresti et al. (2003) for fish gels with TGase . Similarly, Rostamzad et al. (2016) found that a* and b* values of fish protein films containing nanoclay significantly increased by TGase addition. However, Wangtueai et al. (2010) found that TGase treatment had no effect on L*, a* and b* values of lizardfish scale gelatin films.
Opacity is a measurement of a film’s transparency and has a direct effect on the appearance of packaged products (Rostamzad et al., 2016). As seen in Table 2, the opacity values of MDCM-P films ranged from 1.20 to 2.56, and all films with or without TGase showed very good barrier properties against UV light. This result suggested that lipid oxidation in foods induced by UV light could be prevented by MDCM-P films. This property of protein films could be attributed to their high aromatic amino acid contents that absorb UV light. Similar opacity values in protein films were also reported in previous studies (Rostamzad et al., 2016; Shiku et al., 2003; Yi et al., 2006). The opacity values of MDCM-P films were affected by the addition of TGase, and the highest value was determined in films with 1% TGase (p<0.05). TGase treatment is probably cross-linked or aggregated the proteins in film matrix, which caused to more turbid film-forming dispersions and thus, opacity increased (Tang et al., 2005). The opacity values of films decreased as TGase concentration increased, but no significant differences in opacity values were observed between the control film and films with 2 and 3% TGase (p>0.05). Similar to our findings, various researchers reported that the opacity values of edible films were differently affected by the addition of TGase. For example, Tang et al. (2005) observed that the opacity value of soy protein isolate films increased after treated by TGase. In another study, the same researchers reported that the opacity value of red bean protein isolate films was increased with TGase incorporation; however, there were no significant differences between various TGase incorporated films (Tang et al., 2011). Similarly, Rostamzad et al. (2016) found that the opacity value of fish protein isolate films was increased by the TGase treatment, but TGase concentration did not significantly affect the opacity of films.
Values are mean ± standard deviation.
Different letters (a-d) in the same column indicate significant differences (p<0.05).
1)L*: Lightness/brightness (100: white, 0: black).
2)a*: Redness/greenness (+, red; −, green).
3)b*: Yellowness/blueness (+, yellow; −, blue).
4)TS: Tensile strength.
5)EAB: Elongation at break.
The use of edible films as packaging material depends on their tensile strength and elongation at break properties. The tensile strength provides a measure of film strength, while the elongation at break is an indicator of flexibility of the materials (Di Pierro et al., 2006). Table 2 shows the effect of TGase addition on the mechanical properties including tensile strength (TS) and elongation at break (EAB) values of MDCM-P films. As seen, the control film without TGase had TS and EAB of 2.39 MPa and 97.56%, respectively. Different results for TS and EAB were reported in soy protein isolate films (Jiang et al., 2007), red bean protein films (Tang et al., 2011) and zein films (Masamba et al., 2016). These differences could be attributed to the amount and source of protein used in production, the amount of plasticizer and the film thickness. The TS and EAB values of MDCM-P films were affected by the addition of TGase (p<0.05). With the TGase concentration increasing from 1 to 3%, both TS (except films with 2% TGase) and EAB increased, and the highest values were determined in films with 3% TGase (p<0.05). However, with the enzyme concentration increasing from 3 to 4%, TS and EAB values decreased (p<0.05). In general, protein molecules in film matrix are interconnected by cross-linking agents such as TGase, which causes to increase of molecular weight of proteins, and hence film strength increases. At low TGase concentration, mechanical properties of films cannot be improved due to insufficient cross-linking between protein molecules. On the contrary at high concentrations, the mobility of protein molecules are limited due to excess cross-linking, which causes to the lowest mechanical properties of films (Weng and Zheng, 2015). Similar results were reported by Jiang et al. (2007) in TGase-treated soy protein isolate films, by Tang et al. (2011) in red bean protein films cross-linked by TGase, and by Weng and Zheng (2015) in gelatin films treated by TGase. It is clear from these results that an optimal enzyme concentration could be used in order to improve mechanical properties of MDCM-P films, and in this study, the concentration was determined to be 3%.
The interactions between functional groups of protein and TGase could be better understood by fourier transform infrared (FTIR) spectra of MDCM-P based films, and spectroscopic patterns (4000-950 cm−1) of films containing 0, 1, 2, 3 and 4% TGase are shown in Fig. 1. Films prepared at different TGase concentrations showed similar major peaks, but the amplitudes of peaks varied significantly. Control sample produced without TGase showed the lowest absorbance values for all bands, while the highest absorbance values were observed from 2% TGase treated film, then decreased slightly for 3% and 4% TGase treated samples. This means that enzyme treatment of MDCM-P could change the chemical bonds such as N-H, C-H, C=O and C-N which are related with these peaks. The Amide A displays the stretching vibration of free hydroxyl and the asymmetric and symmetric stretching of N-H bonds in amino groups. Although the wavenumber of all films were almost similar, the absorbance of control film for amide A band was lower than those of TGase treated films. This could be attributed to higher hydrogen interactions between water, plasticizers, protein and TGase (Blanco-Pascual et al., 2014). The bands between 2750 cm−1 and 3000 cm−1 (Amide B) are related with stretching vibrations of C-H bonds in -CH2 (Paluszkiewicz et al., 2011).
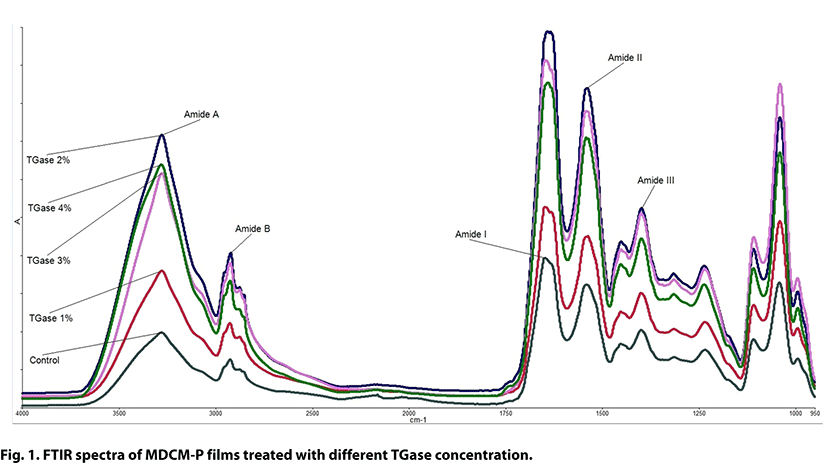
Generally, all film samples had the similar spectra in the range of 1700-950 cm−1, covering Amide-I, II and III bands, which represent C=O stretching/hydrogen bonding coupled with COO, the bending vibration of N-H groups and stretching vibrations of C-N groups and the vibrations in plane of C-N and N-H groups of bound amide or vibrations of CH2 groups of glycine, respectively (Aewsiri et al., 2009). As seen in Fig. 1, Amide-I band had a characteristic peak at around 1640 cm−1 wavenumber and the addition of TGase did not significantly affect the wavenumbers of Amide-I band. However, absorbance of the Amide-I peak increased as TGase level increased up to 2% concentration which illustrated that α-helix or random coil secondary structure proportion increased (Peng et al., 2017). Wang et al. (2015) reported that the increase of TGase concentration on the crosslinking network of gelatin films increased Amide I bands amplitude, because enzymatic treatment can affect the secondary structure, functional groups and interaction of gelatins in films. The increase of amide I band intensity was also reported before in gelatin films produced with TGase and glycerol (Liu et al., 2017), in fish protein films cross-linked with nano-clay using TGase (Rostamzad et al., 2016) and in gelatin films cross-linked with calcium carbonate using TGase (Wang et al., 2015). The peak located around 1100-1000 cm−1 corresponds to the glycerol content, and absorbance of this band was not significantly affected by TGase concentration due to same glycerol concentration used.
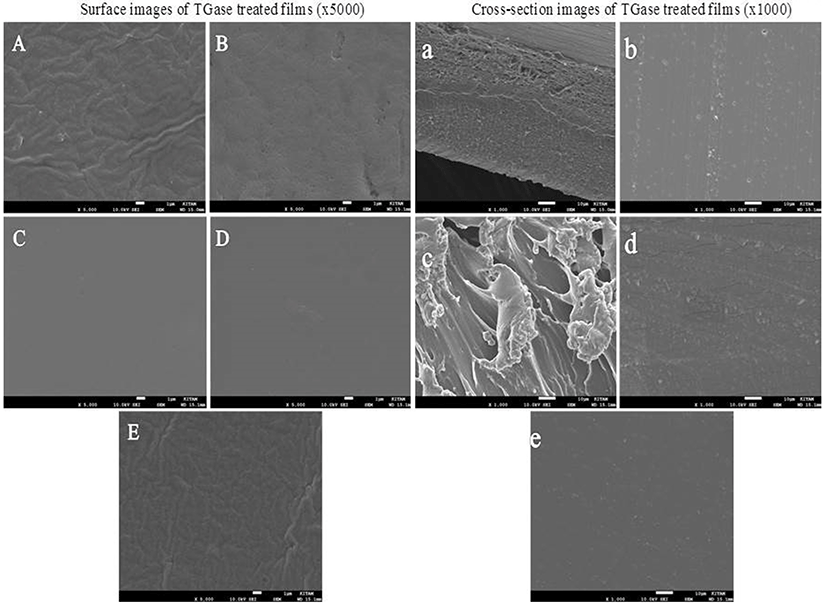
As a consequence of discussed results, we investigated the possibility of using TGase for producing MDCM-P edible films. The aim of our experiments, thus, was attempting to improve the morphological and functional properties of the edible films recently obtained by using only MDCM proteins. Surface (A-E) and cross-sectional (a-e) images of films are given in Fig. 2. As seen, TGase treated films showed more smooth and ordered surface structure than control film, except for 4% TGase treated sample which was rough like control probably due to high molecular weight aggregation of protein and enzyme, and hence increased hydrophobicity, leading to a rougher surface during drying of film (Cui et al., 2017). There were more ripples and protruding strips on the surface of control and 4% TGase treated films. However, TGase treatment at 1, 2 and 3% concentrations caused to smooth and homogeneous surface as reported by Mariniello et al. (2003), who showed that TGase-treated pectin-soy flour films had a smoother surface and higher homogeneity. On the contrary, TGase treated soy protein isolate films plasticized with glycerol, sorbitol and a mixture thereof showed more ripples and protruding strips than control film due to the aggregation of exposed hydrophobic groups of SPI induced by TGase (Tang et al., 2005).
The SEM micrographs of cross-section (at ×1000 magnification) of control and TGase treated films are also given in Fig. 2. Control film showed some mesh or knitting-like structure, while TGase treatment led to occur homogeneous and compact microstructure, except for films treated with 2% TGase (Fig. 2c). TGase treatment at 2% concentration caused to irregular cross-section which could be attributed to covalent and non-covalent bonding between protein chains induced by enzyme (Wang et al., 2015). This result is well supported by FTIR results (Fig. 1) at which 2% TGase treated film displayed the highest absorbance values for all peaks indicating increased α-helix or random coil secondary structure. However, the increase of TGase concentration from 2% to 4% resulted in more compact and smoother cross-section structure with less bumps (Fig. 2d-e), which is due to greater cohesion of protein matrices cross-linked by TGase (De Carvalho and Grosso, 2004; Liu et al., 2016). Moreover, cross-section micrographs also showed that treatment with TGase caused to the increasing porous structures of films, and hence, WVP of films was negatively affected by TGase treatment.
Conclusions
The effects of TGase addition on physical, water barrier, optical and mechanical properties of MDCM-P films were investigated in this study. The results demonstrated that the thickness, solubility, WVP, color, opacity, mechanical properties, molecular organization and intermolecular interaction, and microstructure of MDCM-P films were influenced by TGase addition. When TGase was added to the MDCM-P matrix, the solubility of the films decreased, but WVP values increased. The increase in WVP of MDCM-P films might restrict their application in foods. The all films exhibited a slight yellowish-reddish tint and showed very good barrier properties against UV light. The highest TS and EAB values were determined in films with 3% TGase. The addition of TGase altered molecular organization and intermolecular interaction in the film matrix. TGase treated films showed smoother and ordered surface structure than control film. Control film showed some mesh or knitting-like structure, while TGase treatment caused homogeneous and compact microstructure. These results showed that the use of 3%transglutaminase in MDCM-P films can provide a better alternative to improve the solubility and mechanical properties.













