Introduction
Lipid oxidation is the primary reason for the decrease in the quality of meat products, including aquatic products, livestock, and poultry. Lipid oxidation in meat results a rancid odor, deteriorates product color, and leads to oxide accumulation and loss of nutritional quality (Wu et al., 2022c). Therefore, controlling lipid oxidation is vital for maintaining meat and meat product quality.
Hemoglobin (Hb) is a pro-oxidant that can mediate lipid oxidation. Hb is a tetrameric protein comprising two α-chains and β-chains (α2β2), each with an iron protoporphyrin moiety (heme) located in the cavity of each globin chain. At post-mortem pH, iron protoporphyrin readily dissociates from globin and can get embedded within the phospholipid bilayer of the cell membrane; this facilitates the breakdown of preformed lipid hydroperoxides to generate alkoxyl and peroxyl groups for lipid oxidation (Wu et al., 2022b).
Black chokeberry is a shrub belonging to the Rosaceae family that is rich in polyphenols. Owing to its astringent taste, black chokeberry is generally not eaten raw; however, it is widely processed in the food industry for juices, syrups, jams, fruit teas, and dietary supplements (Sidor et al., 2019). Black chokeberry press cake (BCPC) is the residue or by-product and it is generally discarded directly during its processing, this treatment not only causes environmental pollution, but also leads to the waste of active polyphenols in BCPC. In fact, the cake pressing process is more conducive to the extraction of polyphenols (Witczak et al., 2021).
The literature has reported the antioxidant properties of black chokeberry both in vivo and in vitro (Gao et al., 2023; Hwang et al., 2014). However, only a few studies have reported the antioxidant effect of black chokeberry in meat products. Based on the promoting effect of Hb on lipid oxidation of meat, we hypothesize that BCPC has the ability to inhibit Hb oxidation and thus decrease lipid oxidation. Therefore, the objectives of this study were (i) to investigate the effect of BCPC on lipid oxidation in the Hb-mediated oxidative model using washed chicken muscle (WCM); (ii) to determine key antioxidant ingredients in BCPC and their effect on Hb autoxidation; and (iii) to elucidate which antioxidant ingredients of BCPC were most relevant to the reduction of lipid oxidation mediated by Hb.
Materials and Methods
Heparin, streptomycin sulfate, sodium chloride, hydrogen chloride (HCl), sodium hydroxide (NaCl), sodium phosphate, sodium acetate, glacial acetic acid, 2,4,6-tripyridyl-S-triazine, potassium persulfate, ferrous chloride (III), ammonium ferrous sulfate [NH4Fe(SO4)2], sodium carbonate, butanol, Folin-Ciocalteu reagent (1N), ethanol, chloroform, ammonium thiocyanate, ferrous chloride (II), methanol, potassium ferricyanide, trifluoroacetic acid, trichloroacetic acid (TFA), and thiobarbituric acid were purchased from Sinopharm Chemical Reagent (Shanghai, China). Tris, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), quercetin standard (purity>98%), chlorogenic acid (CA) standard (purity>98%), neochlorogenic acid (NCA) standard (purity>98%), anthocyanin 3-O-glucoside standard (purity>98%), proanthocyanidin (PCA) standard (purity>95%), and tetraethoxypropane were purchased from Shanghai Macklin Biochemical (Shanghai, China). Water was purified by an ultra-pure water system from Shanghai Canrex Analytic Instrument (Shanghai, China). All other chemical reagents were of analytical grade or higher purity.
The chicken breasts were obtained from fresh chicken (Gallus gallus) from Dadong Agricultural Market in Shenyang, Liaoning province, China. They were prepared and processed to obtain WCM according to Richards’ method (Richards et al., 2002). After chicken breast was ground in a mincer, the mince was mixed with distilled deionized water at a ratio of 1:3. The mixture was stirred for 2 min and then incubated on ice for 15 min. After drained with gauze, the mince was washed twice with 50 mmol/L sodium phosphate buffer (pH 6.3) and homogenized with a T 25 high-speed disperser (IKA-Werke, Staufen, Germany) at 4,025×g. The mixture was incubated for 15 min after washing and centrifugated at 12,000×g for 20 min at 4°C. The precipitation was collected as WCM, and every 20 g of WCM was divided into sealed bags and frozen at –80°C before analysis. All steps were performed at 4°C.
The chicken hemolysate was performed by the method of Lei et al. (2022a). The heparinized chicken blood was mixed with four volumes of ice cold 0.9% NaCl in 1 mmol/L Tris (pH 8.0) and centrifuged at 700×g for 10 min. The plasma (supernatant) was removed, the precipitate (red cell) was washed with 10 volumes of ice cold 1 mmol/L Tris (pH 8.0) for three times. Then red cells were lysed in 3 volumes of 1 mmol/L Tris (pH 8.0) for 1 h on ice. One-tenth volume of 1 mol/L NaCl was added to remove stroma, and then centrifuged at 12,000×g for 15 min. All the steps were conducted at 4°C and the hemolysate was stored at –80°C before analysis.
Black chokeberrys were purchased from Huadian City, Jilin Province, China. They were squeezed by the juicer (H-300 Series, Hurom, Seoul, Korea), which could separate juice and peel. The BCPC is the by-product from juice production. BCPC was frozen dry at –40°C for 48 h, and then was grinded to powder by a high-speed grinder (Hangzhou Baijie Technology, Hangzhou, China) at a speed of 7,154×g. Afterwards, the ground BCPC was sieved through a 300 mesh sieve, the coarse particles remaining on the sieve were discarded and the sieved BCPC powder was stored in sealed bag at 4°C until subsequent analysis.
The sample of oxidation model was prepared according to the method described by Lee et al. (2006) with minor revisions. The thawed 25 g WCM was adjusted to pH 6.3 in a beaker by using 1.0 mol/L NaOH solution, and if needed 1.0 mol/L HCl. The original moisture content of the WCM was 86.7±2.2%, and Milli-Q water was added to reach 90%. Streptomycin sulfate stock solution (2%, w/v) was added to give a final concentration of 0.02%. BCPC powder was added to the WCM oxidation models at 0%, 5%, 10%, and 15% by weight of WCM dry matter, respectively. Chicken hemolysate was thereafter added to a final concentration 25 μmol Hb/kg of WCM and the sample was transferred to the bottom of a 250 mL E-flask wrapped in aluminum foil (in dark). All steps were performed on ice and the final samples were stored on an ice bed in cooler bags. Sub-samples were taken from E-flasks every 2 days. Samples with the same concentration of BCPC added are grouped together, every group was conducted triplicate.
Primary lipid oxidation was studied by measuring peroxide value (PV) according to the ferric thiocyanate method as described by Sajib et al. (2022). The sample (1 g) was mixed with 10 mL ice-cold chloroform:methanol (2:1, v/v) containing 0.05% BHT (w/v) and homogenized at 7,104 g for 30 s, then sample was added into 4 mL ice-cold 0.5% NaCl and vortexed for 30 s. The mixture was centrifugated at 3,000×g for 6 min at 4°C and the bottom phase (chloroform phase) was further analyzed for PV. The chloroform phase of 2 mL was mixed with 1.33 mL new ice-cold chloroform-methanol (1:1, v/v), 33.4 μL ammonium thiocyanate and 33.4 μL freshly iron (II) chloride, and the mixture was vortexed for 2–4 s and incubated for 20 min at room temperature. The mixture was finally analyzed at 500 nm by UV spectrometer. The cumene hydroperoxide was used as standard curve at concentrations from 0 to 0.02 mmol/L. The PV was calculated and expressed as μmol cumene hydroperoxide/kg sample. Sub-samples were taken from E-flasks every 2 days. Every group was conducted triplicate.
Anisidine value (AnV) was determined in triplicate for each of the samples based on American Oil Chemists’ Society (AOCS) Official Method Cd 18-90 (1) (AOCS, 1992).
Procedures used have been described previously by Thiansilakul et al. (2012) with modifications. CIE a* loss was determined by Minolta CR-300 Chroma Meter (Minolta Camera, Osaka, Japan). The aperture size was 1 cm. A white calibration plate was used to calibrate the instrument. Every group was conducted triplicate.
Total phenolic content (TPC) was evaluated by the Folin–Ciocalteu method with slight modifications as reported by Zhang et al. (2022). BCPC powder (0.1 g) was added into 10 mL extraction solution (methanol:H2O+TFA, 70:30+1%) and vortexed for 15 s. The mixture was sonicated for 10 min, which was shaken after 5 and 10 min. The sample was incubated in a water bath (60°C) for 30 min and vortexed for 15 s. After centrifugating (5,000×g, 5 min, 4°C), the supernatant was collected. The precipitation was redissolved in 5 mL extraction solution and vortexed for 15 s. Afterwards, the sonication and centrifugation were repeated as above. The dilute sample (500 μL) was mixed with 50 μL Folin-Ciocalteu reagent, 200 μL distilled water and 250 μL saturated Na2CO3. After incubation at room temperature for 10 min at dark, the samples were centrifugetd at 15,000×g for 5 min at 4°C. The absorbance was recorded at 765 nm by UV spectrophotometer (Infinite M200 Pro Nano-quant, TECAN, Grödig, Austria). The gallic acid was used as standard curve at concentrations from 0 to 0.71 mmol/L. The TPC was calculated and expressed as milligrams of gallic acid equivalent per gram of dry weight (mg GAE/g DW). Every group was conducted triplicate.
The PCA content was evaluated by butanol-HCl assay with slight modifications as reported by Gao et al. (2023). Briefly, a 100 μL sample was mixed with 80 μL of ultra-pure water, 220 μL of MeOH, 2 mL of butanol-HCl solution (95:5, v/v), and 67 μL of NH4Fe(SO4)2-HCl solution. The mixture was heated at 95°C for 40 min and cooled for 20 min, the unheated solution was used as control. The absorbance was recorded at 550 nm. The level of PCAs was represented as milligram PCA B2 equivalents proportion in TPC (%). All trials were carried out in triplicate.
The qualitative analysis of polyphenols in BCPCs was carried out as described by Gao et al. (2023). Analysis of polyphenolic compounds in lingonberry was performed using ultra-high performance liquid chromatography with photodiode array (UPLC-PDA; LC-8050, Shimadzu, Kyoto, Japan). Briefly, the chromatographic column used was a Waters-C18 column (4.6 mm×250 mm, 5 μm). Different polyphenols in BCPCs were separated by gradient elution. The mobile phase was composed of A (0.1% formic acid-water) and B (0.1% formic acid-acetonitrile). The gradient elution was as follows: (0–1) min 10% B, (1–3) min 10%–11% B, (3–5) min 11%–12% B, (5–28) min 12%–15% B, (28–54) min 15%–25% B, (54–63) min 25%–30% B, (63–68) min 30%–100% B, and (68–72) min 100%–10% B. The injection volume was 10 μL and the flow rate was 0.3 mL/min. The detection wavelengths for PDA were successively 350 nm. CA, quercetin, and cyanidin-3-O-glucoside were used as standards for phenolic acids, flavanols, and anthocyanins, respectively. The content of polyphenolic compounds was determined by the corresponding peak area and calibration curves of the standards. Every group was performed in triplicate.
The DPPH test was conducted as Bujor et al. (2018) with a little modification. Briefly, a 50 μL of 0.01 g/mL BCPC solution was mixed with 1.5 mL of 0.06 mmol/L DPPH solution in methanol and vortexed for 2–3 s. After incubation for 20 min, the mixture was recorded at 515 nm. The DPPHo concentration was calculated from a calibration curve analyzed by linear regression. The percentage of inhibition was calculated as follows:
Where AB is the absorbance of blank; AE is the absorbance of samples.
The ferric ion reducing antioxidant power (FRAP) of BCPCs was measured according to the method as described by Chen et al. (2019). FRAP reagent was freshly prepared by mixing of sodium acetate in glacial acetic acid (300 mmol/L, pH 3.6), 10 mmol/L TPTZ in 40 mmol/L HCl and 20 mmol/L FeCl3 at the ratio of 10:1:1 (v/v/v). the sample was mixed with 300 μL of FRAP reagent and incubated at 37°C for 15 min. The sample was measured at 593 nm using a UV spectrophotometer (Infinite M200 Pro Nano-quant, TECAN, Männedorf, Switzerland). A standard calibration curve from 0–2 μmol/L of FeSO4o7H2O was plotted. Results should be expressed as equivalent μmoles of Fe2+/mL of the samples.
The ABTS was performed by the method of Dudonné et al. (2009) with minor revisions. The stock ABTS solution was mixed with 1 mL of 7 mM ABTS and 10.1 μL of 245 mM potassium persulfate, and incubated overnight (12–16 h) at room temperature at dark. The sample and 100-fold diluted stock ABTS solutions were mixed at a ratio of 1:19 and the absorbance was recorded at 734 nm.
Measurements were performed according to the method of Wu et al. (2022a) with slight modifications. Briefly, the standards of antioxidants were dissolved in ethanol to obtain a 5 mmol/L stock solution. The methemoglobin (metHb) was mixed with antioxidant standards and diluted 200-fold by 10 mmol/L Tris (pH 8.0). The mixture was stored at 2°C and determined every other day. The samples were analyzed by using a UV spectrophotometer from 510–700 nm.
The structures of the docked compounds CA, NCA, and procyanidin-B2 come from the PubChem database (https://pubchem.ncbi.nlm.nih.gov), and then were imported into the Chem3D software. The MM2 module was used for optimization, energy minimization, and preservation. The file is used as the ligand molecule for molecular docking. The protein crystal structure of Hb (PDB ID: 1HBR) was obtained from the RCSB database (https://www.rcsb.org/). The protein was processed on the Maestro11.9 platform and process with Schrodinger’s Protein Preparation Wizard, remove crystal water, add missing hydrogen atoms, repair missing bond information, and repair missing peptides. Finally perform energy minimization on the protein, and optimization of geometry. Molecular docking was performed by Covalent Docking in the Glide module of Schrödinger Maestro software. Protein processing utilizes the Protein Preparation Wizard module. Receptors were preconditioned, optimized and minimized (constrained minimization using OPLS3e force fields). All molecules were prepared with the default settings of the LigPrep module. When screening in the Glide module, import the prepared receptors to specify the appropriate positions in the receptor mesh generation. Finally, the standard docking method (standard precision, SP) was used for molecular docking and screening.
All experiment of this study were statistically analyzed using SPSS 19.0 (IBM Corp., Armonk, NY, USA) and significant differences between variables were determined using one-way analysis of variance (ANOVA). The sample was divided into three replicates, each of which was considered an experimental unit (n=3).
Results and Discussion
PV and AnV value were used to monitor the level of lipid oxidation (Fig. 1). The contents of PV measured in WCM oxidation model with 0%, 5%, 10% and 15% BCPC at 0 d were 49.23±3.25, 46.34±12.58, 45.77±2.21 and 44.65±1.05 μmol LHP/kg muscle, indicating that there were no significant differences (p>0.05) in BCPC addition at the start of storage. During the ice storage, the PV of 0% BCPC initially increased and had achieved their maximum on 4 d, which was significantly higher than the values at 5%, 10%, and 15% BCPC (p<0.05). Similarly, the oxidation systems with 5% and 10% BCPC addition reached the highest oxidation values of 645 μmol LHP/kg and 345 μmol LHP/kg on 6 d and 10 d, which were significantly higher than the values of other additions (p<0.05), respectively. However, no significant lag phase was observed in WCM oxidation model in 15% BCPC group, with a low PV value (≤89.87 μmol LHP/kg) throughout storage. Also, the change of AnV aligned with the PV trend. These results indicated that BCPC exhibits a strong inhibitory effect on Hb-mediated lipid oxidation, with a concentration-dependent antioxidant capacity. BCPC of black chokeberry contains high polyphenol, TPC, flavonoid contents, especilly PCA contents were four-fold higher than those of the blueberry extract (Hwang et al., 2014; Zhao et al., 2022). The phenolic hydroxyl groups in polyphenols can reduce the formation of metHb, thereby decreasing the oxidative activity of Hb (Wu et al., 2022a). Moreover, phenolic compounds could scavenge free radicals and form a chelate with transition metal catalysts to limit lipid oxidation (Zamora and Hidalgo, 2016).
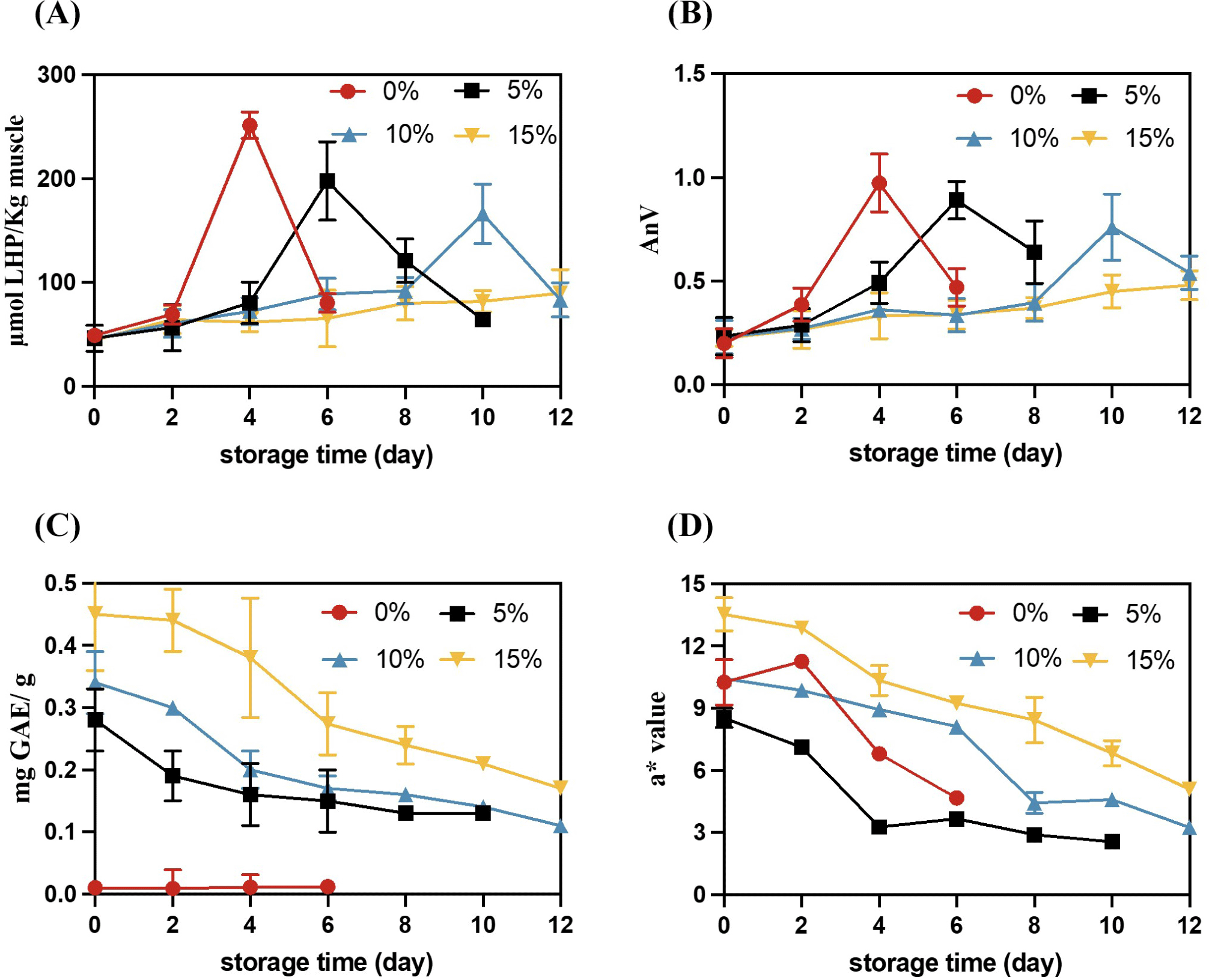
The addition amounts of TPC and BCPC are concentration-dependent, and the initial value of TPC in the WCM oxidation system adding 15% BCPC is 0.45 mg GAE/g DW. With the extension of storage time, TPC gradually decreased and stabilized at around 0.15–0.2 mg GAE/g DW. Until the end of storage, the TPC in the WCM oxidation system of 5% BCPC, 10% BCPC and 15% BCPC decreased 53.57%, 67.65% and 62.22%, respectively (Fig. 1C). TPC was associated with CIE a* value, which also decreased during the storage period (Fig. 1D). Notably, the CIE a* value of 5% BCPC was lower than that of 0% BCPC; this may be because BCPC addition darkened the color of the WCM model. With an increase in BCPC concentration, the CIE a* values of 10% and 15% BCPC exceed those with 0% BCPC. In the present study, the red color of CIE a* value was primarily derived from phenols and Hb. The red loss is partly due to the transition from oxyhemoglobin (oxyHb) to methb, but phenolics slow down the red loss by hindering the process of hydrogen peroxide-mediated oxidation of oxyHb to metHb (Wu et al., 2022a). The other part of the loss of red color is due to the degradation of TPC with prolonged storage, and also the involvement of phenolic hydroxyl groups in anthocyanins in the chain reaction of lipids, which also reduces the anthocyanin content (Ferreres et al., 1996).
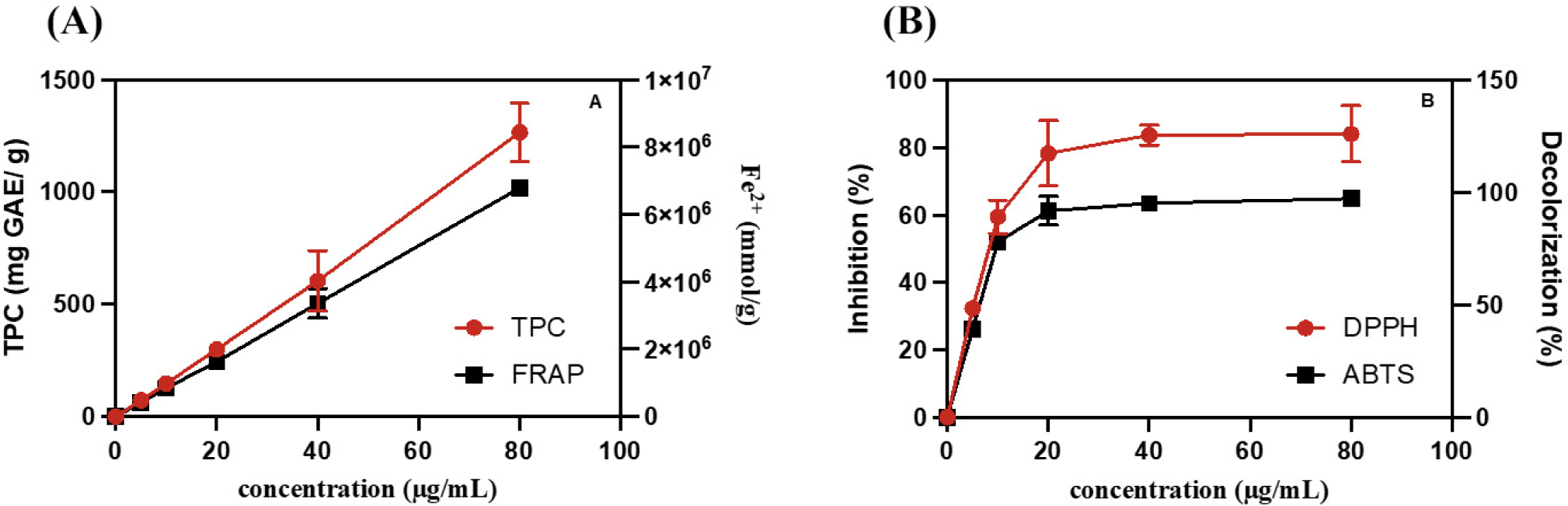
The antioxidant capacity of BCPC in the WCM oxidation model may be involved in free radical scavenging. TPC represents the content of phenol hydroxyl groups in antioxidants. FRAP, DPPH, and ABTS assays were performed to measure the iron-reducing ability, DPPH scavenging ability, and ABTS free radical scavenging ability, respectively (Blainski et al., 2013; Hwang et al., 2014; Valverde Malaver et al., 2015). As Fig. 2A shown, TPC and FRAP proportionally increased with an increase in BCPC concentration. In Fig. 2B, when the BCPC concentration was increased from 5.0 to 20.0 μg/mL, ABTS decolorization rapidly increased and then reached a plateau, with a scavenging rate of approximately 100%. DPPH inhibition also exhibited the same trend, but the maximum plateau value was achieved at approximately 80%. The in vitro antioxidant capacity of FRAP, DPPH, and ABTS is related to their antioxidant activity, which may depend on TPC (Hwang et al., 2014). The above results indicated that BCPC had strong antioxidant and free radical scavenging ability. Therefore, the phenolic monomers in BCPC were further investigated by UV-Vis spectroscopy and UPLC-PDA.
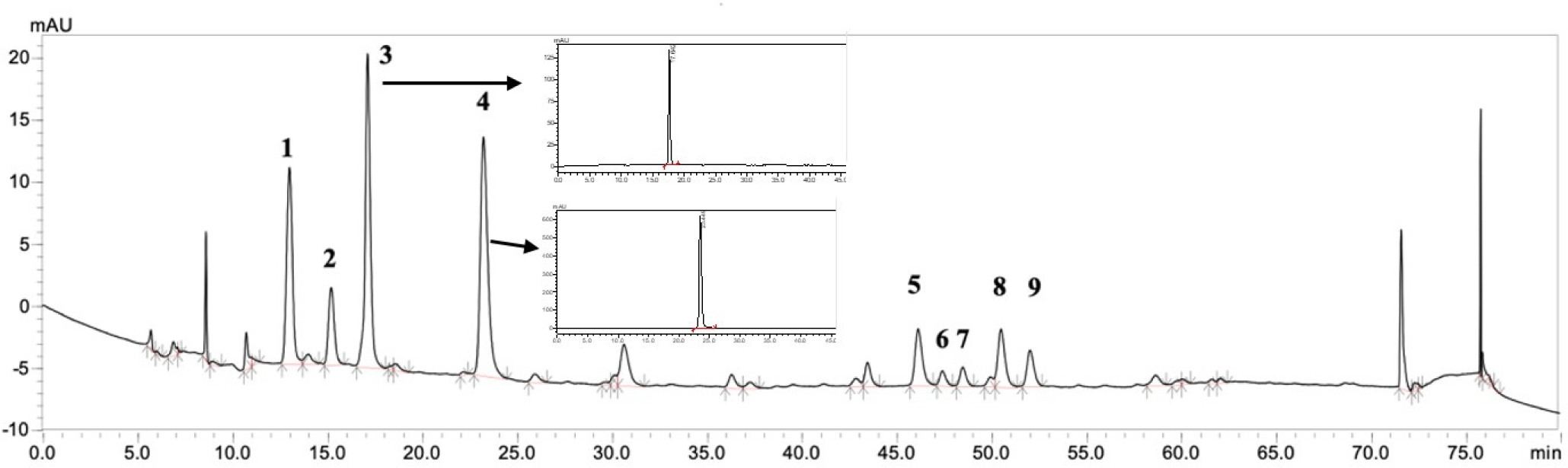
UPLC-MS analysis was performed to determine the types of polyphenol extracts in BCPC. CA (peak 4) and NCA (peak 3) were identified as the two higher content substances (Fig. 3). In addition to these two antioxidants, PCA was identified as the major polyphenol in black chokeberry. In our study, the PCA was accounted for 56.7% of TPC.
Polyphenols can reduce the pro-oxidant activity of Hb by reducing Hb from pro-oxidant metHb to oxyHb (Wu et al., 2022c). Fig. 4 illustrates the UV-visible spectrum of chicken metHb with or without PCA/CA/NCA after 6 d of incubation. The spectrum displayed a significant trough and peak on 0 d. As the storage time prolongs, Hb-PCA and Hb-CA still have obvious peaks and troughs on the 6 d of storage, while the absorption curve of Hb-NCA tends to be flat, indicating that Hb has been denatured. PCA was used as a reducing and chelating agent to maintain Hb in a ferrous state, and that the inhibitory effect of PCA as a reducing agent on Hb-mediated lipid oxidation was higher than that of PCA as a chelating agent (Maestre et al., 2009). Similarly, CA maintained the ferrous state of Hb by chelating with iron ions and effectively reduce the production of oOH triggered by iron ions (Yang et al., 2021). In Fig. 4, the initial absorbance baseline of Hb-PCA was higher than that of Hb-CA and Hb-NCA, which may be because PCA is purple, therefore it increased the baseline value. These results indicate that PCA and CA can delay Hb-mediated lipid oxidation by inhibiting the oxidation of oxyHb to metHb, but NCA cannot effectively inhibit Hb autoxidation.
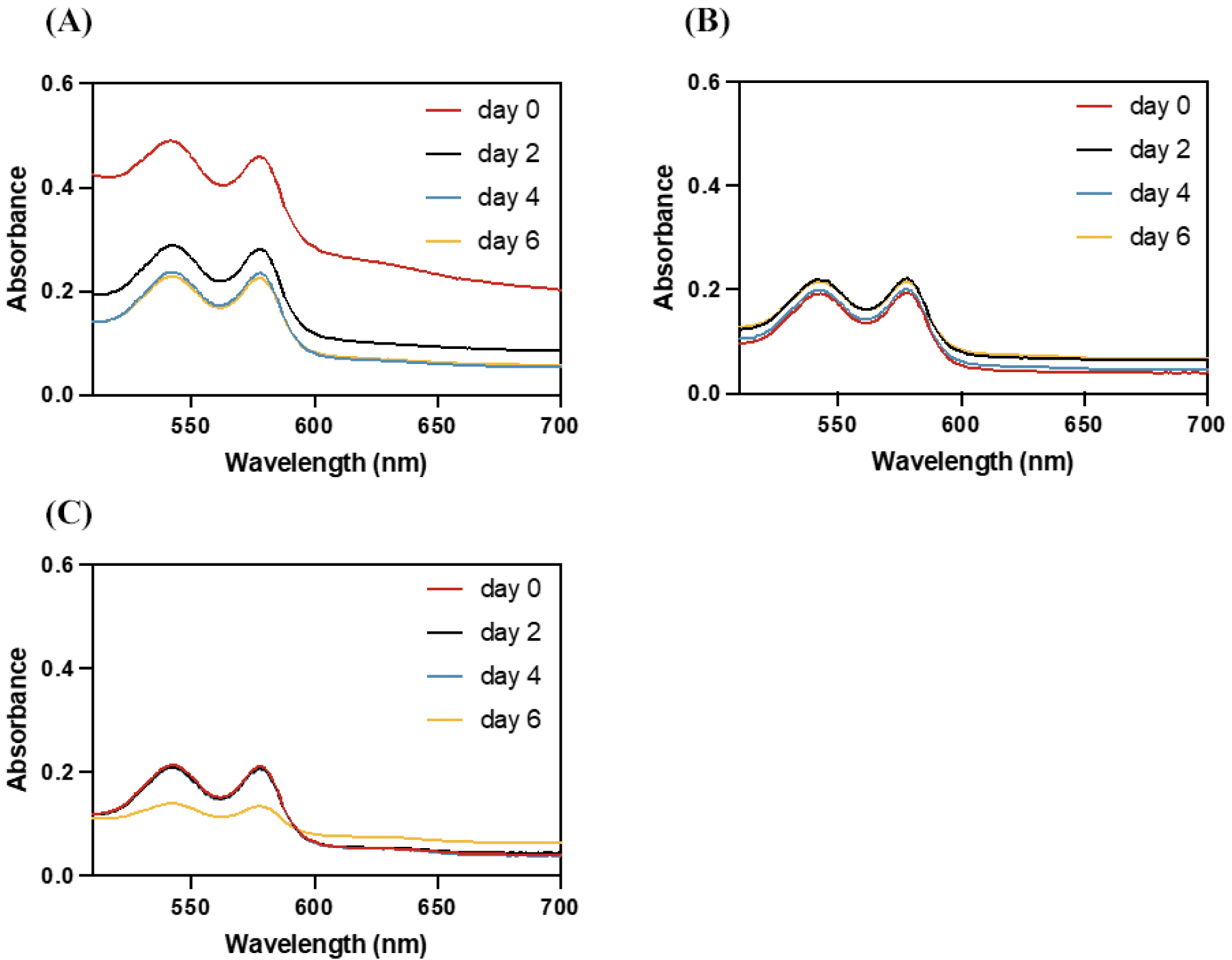
Fig. 5 demonstrates that the interaction and action sites between CA, NCA, PCA-B2 and Hb. CAs are esters formed between caffeic and quinic acids (Xu et al., 2012). As Fig. 5A shown, hydroxyl groups on CA form hydrogen bonds with aspartate (ASP, 126), serine (SER, 130), valine (VAL, 34), tyrosine (TYR, 140), and glutamate (GLU, 138). Compared with PCA-B2 (Mm=578.52), CA’s molecular mass (Mm=354.31) is smaller. These smaller ligands typically bind better to the cavity site (Kamenik et al., 2021), thus CA prone to bind with Hb’s cavity, hindering the solvent entry. Once the solvent enters the cavity, it will reduce the affinity of chicken Hb to oxygen, and further triggers the protonation of distal histidine and weakens the iron binding between the imidazole moiety and Hb, leading to the dissociation of globin and heme (Lei et al., 2022b).
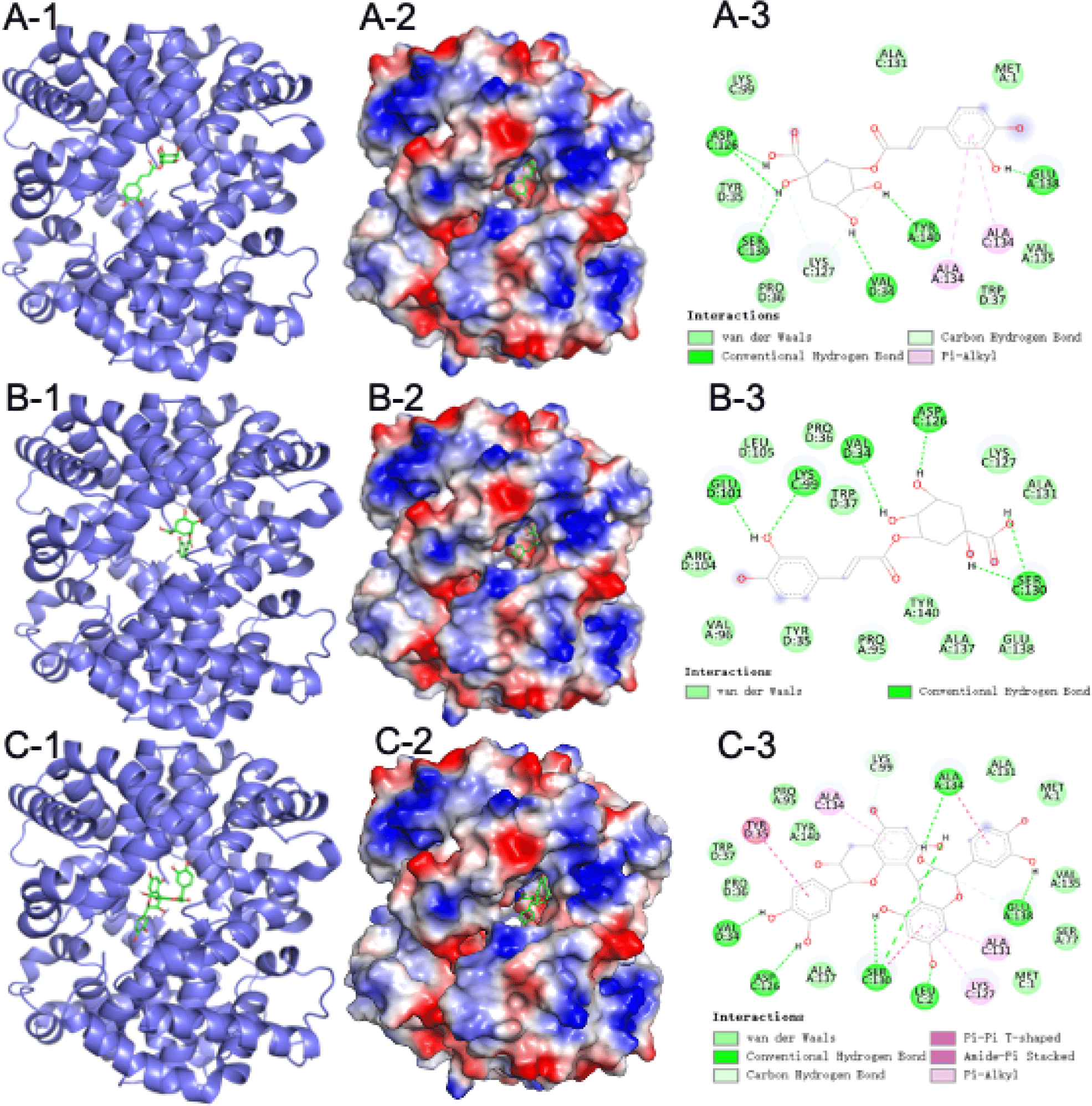
Studies have reported the antioxidant capacity of NCA, comparatively a less-studied isomer of CA (Navarro-Orcajada et al., 2021; Rop et al., 2010). In Fig. 5B, NCA also bound on Hb with six amino acid by hydroxyl groups. Notably, NCA binds to lysine at position 99 (E10) of Hb, this position is a key site in the control of the Hb cavity (Wu et al., 2017; Yin et al., 2017). This binding may lead to detachment of the porphyrin ring from the bead proteins, and the iron ions on the porphyrin ring can trigger lipid oxidation via the Fenton reaction with H2O2, producing the highly oxidizing hydroxyl radical (Maestre et al., 2009).
The inconsistent reducing and chelating abilities of CA, NCA, and PCA to Hb are related to their structures. Molecular docking of CA, NCA, and PCA with Hb was performed to study the impact of their combination with Hb on the structure of Hb. From a chemical point of view, PCAs are an oligomeric polyphenol compound, most of which are oligomers of catechin, epicatechin and their gallic acid esters. PCAs are mainly divided into type A, type B, and type C according to the linking methods of catechin, epicatechin and their gallic acid esters. Among them, B2 is a common type of flower found in Sorbus nigra. cyanins (Esatbeyoglu and Winterhalter, 2010; Sidor et al., 2019; Wu et al., 2004), so PCA-B2 was used for molecular docking experiments in this study.
PCA forms stable compounds with other substances with the help of hydrogen bonds (Nie et al., 2023). As Fig. 5C shown, PCA-B2 bound to Hb via hydrogen bonding to leucine (LEU, 2), valine (VAL, 34), serine (SER, 130), alanine (ALA, 134), glutamate (GLU, 138), respectively. These hydrogen bonds can help PCA-B2 to bind in the Hb cavity, thus PCA-B2 blocks solvent entry into the Hb cavity and delays the oxidation of Hb from the oxyHb to the metHb, further inhibits Hb-mediated lipid oxidation. Remarkably, among the six amino acid sites where PCA-B2 binds to Hb, ALA, VAL, and LEU are all hydrophobic amino acids, which may lead to form a more hydrophilic environment around the Hb cavity, causing the solvent to enter the Hb cavity and accelerating lipid oxidation (Wu et al., 2017). The investigation of Fan et al. (2022) also found that PCA-B2 was able to change the conformation of bovine α-lactalbumin in the hydrophobic cavity by hydrogen bonding to further form a stable structure.
In order to further verify whether PCA, CA, and NCA can inhibit Hb-mediated lipid oxidation, the contents of PCA, CA, and NCA in 15% BCPC were used as the benchmark and were added to the WCM model respectively, and then lipid oxidation analysis was performed. Results of WCM oxidation model with/without the three antioxidants are demonstrated in Fig. 6. As shown in Fig. 6A, 0% BCPC group reached the highest oxidation value of 251.75 μmol LHP/kg muscle on 4 d, while the NCA added group also reached the highest oxidation value on 4 d, but the peak oxidation value was only 111.29 μmol LHP/kg muscle, indicating that although NCA did not delay the arrival of the lipid oxidation peak, it can inhibit the intensity of the lipid oxidation peak. The groups added with PCA and CA reached the highest oxidation peaks on 6 d and 8 d respectively, which were 97.90 μmol LHP/kg muscle and 103.87 μmol LHP/kg muscle. The mixed group of three antioxidants reached the highest oxidation peak on 10 d. The results of AnV were consistent with those of PV (Fig. 6B). All antioxidants could significantly inhibit the formation of PV, but could not significantly reduce the AnV peak (p>0.05).
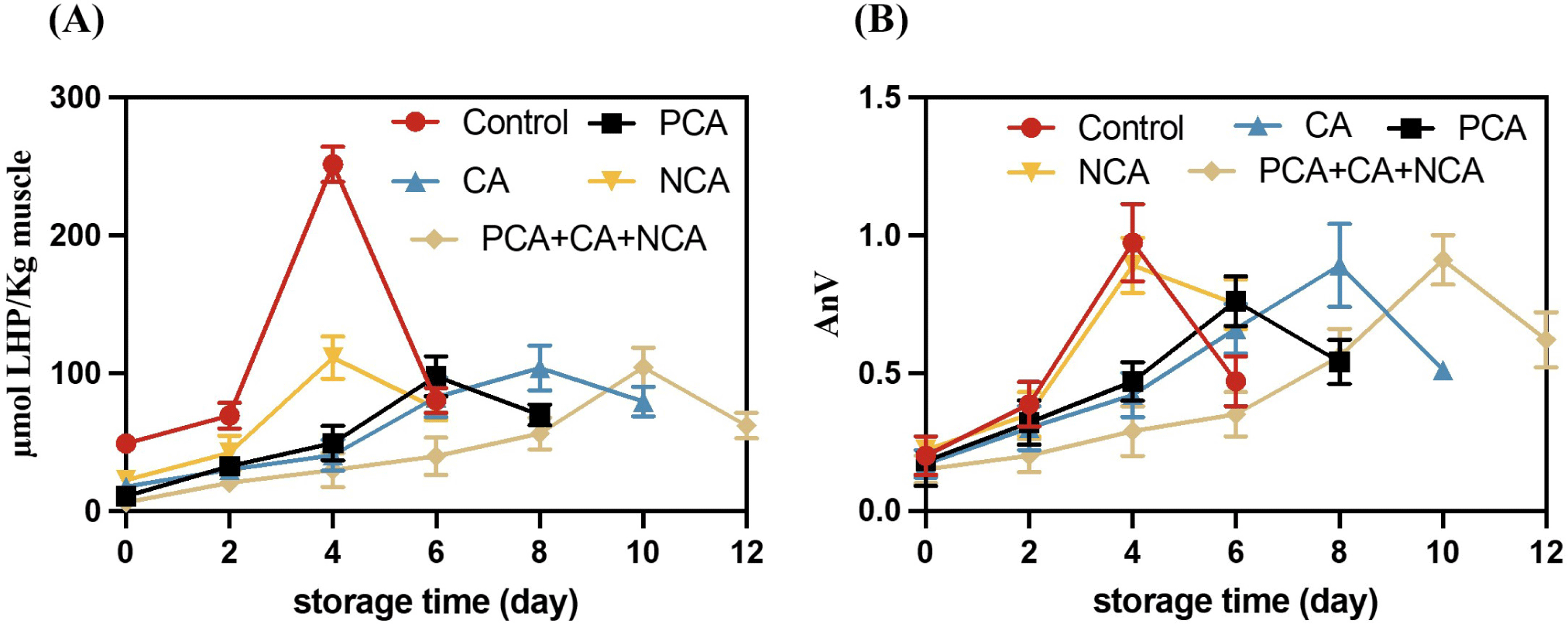
CAs inhibit lipid oxidation by donating hydrogen atoms to reduce free radicals. After donating a hydrogen atom, CA is oxidized to form the respective phenoxy groups, which are rapidly stabilized via resonance stabilization (Liang and Kitts, 2016). Cao et al. (2019) reported that CA acted as a hydrogen donor and inhibited lipid oxidation as well as maintained the color of fish flesh; furthermore, CA exerted an inhibitory effect on lipase and lipoxygenase activities because CA quenched the intrinsic fluorescence of the enzyme via static quenching. NCA as an isomer of CA, also has a similarly strong antioxidant capacity (Thurow, 2012), however, they show a different antioxidant capacity in WCM oxidation model. It might be due to that the differences between CA and NCA are partially because NCA content was less than CA content in the WCM model, which NCA content was 90% of CA content in BCPC. Furthermore, NCA cannot be easily distributed into the lipid phase because of its hydrophilic nature (Navarro-Orcajada et al., 2021). In addition, NCA can be easily degraded under neutral conditions (the pH of the WCM model is 7); this may be one of the reasons why NCA has worse antioxidant capacity than CA (Wang, 2021).
Conclusion
BCPC, as an agricultural by-product, is rich in polyphenols. In this study, BCPC showed significantly inhibition capacity to lipid oxidation in chicken. PCA, CA, and NCA were found to be the most abundant polyphenols in BCPC. Among them, PCA and CA can form strong hydrogen bond interactions with the Hb active site, which is beneficial to stabilizing the heme in the Hb pocket, reducing the transformation of oxyHb to metHb, thereby delaying the release of heme; NCA differs from PCA and CA in that it seizes the binding site between globin and heme on Hb, causing heme to detach from globin. Comparison of the three antioxidant components in the WCM oxidation model of lipid oxidation revealed that the antioxidant capacity of the three mixtures, CA, PCA, and NCA, was in descending order of strength.













