Introduction
The global population has been continuously increasing, and it will increase to 9 billion people by 2050. Therefore, it is estimated that food production will need to be greater than it currently is to sustain this (van Huis et al., 2013). Additionally, as income levels increase, food consumption changes, which increases meat, fish, and poultry intake. The consumption of animal products is expected to increase by 60%–70% (Lalander et al., 2019; Makkar et al., 2014); therefore, a large amount of animal feed is required. Animal feed components include oil, cornmeal, vitamin premixes, minerals, and other ingredients; notably, soybean and fish meal serve as major protein sources (Taufek et al., 2021). However, land availability for soybean cultivation is declining globally, and small pelagic fish, which are used to derive fish meal and oil, are reducing owing to marine overexploitation (Onsongo et al., 2018); therefore, their price is dramatically increasing every year. For these reasons, alternative protein sources are required in the feed industry.
Insects that have been recently introduced as future superfoods in the food industry have a high level of vitamins, amino acids, zinc, iron, and polyunsaturated fatty acids, and are thus suggested as a novel source of alternative high-quality protein that can play an important role in increasing current food production methods (Nowakowski et al., 2021; Nyakeri et al., 2017). In the insect industry, black soldier fly (Hermetia illucens L.) larvae (BSFL) are reported to be rich in lipids, proteins, and minerals (Caligiani et al., 2018), and after partial removal of lipids, may have a 55%–65% protein content (Gold et al., 2018). BSFL are highly useful as feed insects and can be used as a substitute for soybeans, corn, and fish meal feed. Research is actively being conducted to use them as a substitute, such as feeding them to pig feed and feeding them to broiler chicken feed (Crosbie et al., 2021, Dabbou et al., 2018; Kim et al., 2023). Also, Finland has adjusted EU regulations to allow the sale of insect feed as human food, a representative thing of which is BSFL. Additionally, there have been reports of the Kadazan-Dusun people consuming BSFL as food (Chung et al., 2002; Mikkola, 2020). These countries have an interest in BSFL, and the use of BSFL as food is legally supported or regulated in various ways. However, the legal allowance of insects for human consumption differs from country to country, it is important to check the current regulations of a specific country.
Generally, the nutritional value of insects differs among life stages, species, and substrates (dos Santos Aguilar, 2021) and the nutritional composition of BSFL are also influenced by the life cycle rearing substrate. BSFL are polyphagous and grows and feeds on an extensive range of substrates such as by-products and food waste (Lalander et al., 2019). Therefore, they are environmentally friendly because they can grow by ingesting a wide range of waste such as manure, by-products (agriculture and livestock), and carrion (Meneguz et al., 2018; Nyakeri et al., 2017).
On Jeju Island, mandarin is an important industry and a significant source of local income (Kim et al., 2011), and more than 600,000 tons are produced annually (Korean Statistical Information Service, 2021). However, a considerable quantity of mandarin is disposed of because of overproduction, and 30,000–80,000 tons of by-products are produced by juice manufacturing annually (Kim et al., 2017). Moreover, mandarin wastes are difficult to landfill and incinerate because of the regional properties and environmental problems on Jeju Island, and disposal costs are high (Ahn et al., 2019). Therefore, mandarin by-products are major agricultural wastes on Jeju Island and cause local environmental issues. Recycling mandarin by-products into useful resources such as animal feed and functional materials can be beneficial in Jeju Island and can be expected to facilitate an enormous reduction in waste (Choi et al., 2011). Recently, many studies have tried to convert wastes such as by-products (agriculture and livestock), food waste, manure, and faeces into useful resources using BSFL, which are known to eat those wastes (da Silva and Hesselberg, 2020; Kinasih et al., 2018; Shumo et al., 2019; Spranghers et al., 2017). Some studies have pointed out that BSFL have high protein content and growth performance, especially when reared on animal-based substrates (e.g., animal slaughter by-products; Gold et al., 2020; Lopes et al., 2020; Pamintuan et al., 2019).
Overall, the aim of this study was to evaluate the effects of mandarin and poultry slaughter by-products from Jeju Island on the growth performance, physicochemical properties, and antioxidant activities of BSFL.
Materials and Methods
2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), Iron(III) chloride (FeCl3), Folin-Ciocalteu’s phenol reagent, gallic acid, sodium hydroxide, hydrogen peroxide solution, 6-hydroxyl-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) and peroxidase (from horseradish) were from Sigma-Aldrich (St. Louis, MO, USA). 2,2-Diphenyl-1-picrylhydrazyl (DPPH) was from Alfa Aesar (Haverhill, MA, USA). Sodium carbonate was from DC Chemical (Shanghai, China). Acetic acid was from Samchun Chemicals (Pyeongtaek, Korea). Potassium hydroxide, ethyl ether, phenolphthalein, citric acid, hydrochloric acid and ethanol were from Daejung Chemicals and Materials (Siheung, Korea). Sodium acetate was from Kanto Chemical (Tokyo, Japan). Ferrous sulfate (FeSO4) was from Wako Pure Chemical Industries (Osaka, Japan). Phosphate-buffered saline was from Welgene (Gyeongsan, Korea).
Mandarin (M) by-products were compressed to remove moisture, and the remaining residue was used as feed. Poultry (P) by-products were steamed and pressed using a screw press to extract oil and remove moisture, then ground into a powder. Mandarin and poultry by-products were mixed at different ratios (10:0, 9:1, 8:2, 7:3, 6:4, and 5:5, w/w) and used as rearing substrates. The substrates were provided at a rate of 25 g per larva. BSFL used in this study were obtained from Real Nature Farm (Jeju, Korea). The larvae were not fed during the first seven days post-hatching. Beginning on day 8, feeding commenced and continued for a duration of 10 days. After a total rearing period of 17 days, the larvae were killed and used as experimental samples (Table 1). The reared larvae were killed using a freezing method and subsequently evaluated for growth performance. Ten larvae were randomly sampled from each group to measure length (mm), width (mm), and weight (g).
| Rearing information | |||||
|---|---|---|---|---|---|
| Non-feeding period | Feeding period | Total rearing period | Substrates1) | Feeding rate | Killing method |
| 7 days | 10 days | 17 days | Mandarin and poultry by-products | 25 g per larvae | Freezing |
The BSFL were washed under running water for two cycles, then blanched in water (1:4, w/w) at 100°C for 40 s. After that, cooled in the flowing water and hot-air dried at 70°C for 7 h. Dried samples were defatted at 90°C for 1 cycle by using a screw-type oil press machine (Oil love premium, National Engineering, Seoul, Korea), and separated into unrefined BSFL oil and defatted BSFL cake. The unrefined BSFL oil was used for oil refining experiment, and defatted BSFL cake was pulverized using an electric grinder for 1 min and used as defatted BSFL. The defatted BSFL was kept in vacuum-packed for future experiments.
The proximate composition of defatted BSFL samples was analyzed according to AOAC (2005) method. Moisture content (AOAC 950.46) was evaluated by drying a 3 g sample at 105°C for 24 h using a dry oven (HB-502S, Hanbaek Scientific, Bucheon, Korea). Crude fat content was determined by Soxhlet extraction method (AOAC 960.39). Crude protein content (AOAC 928.08) was measured using standard Kjeldahl procedure. Crude ash content (AOAC 920.153) was measured after burning in a furnace (C-FMD2, Changshin Science, Seoul, Korea) at 550°C.
The color of sample was determined in petri-dish on a whiteboard using colorimeter (TCR-200, TIME High Technology, Beijing, China) and CIE L*, CIE a*, and CIE b* values were recorded on CIE scale. Before measurement, the device was calibrated using its own white calibration plate (D65, CIE L*=93.90, CIE a*=3.94, CIE b*=–9.55).
The sample was mixed with distilled water (1:9, w/w) using a homogenizer (T 25 Ultra-Turrax, IKA, Staufen, Germany) for 1 min at 6,000 rpm. Then, the pH value was measured using a pH-meter (FiveEasy Plus F20, Mettler-Toledo, Schwerzenbach, Switzerland).
The total phenolic content (TPC) of sample was evaluated Folin and Denis (1912) method, with a slight modification. 100 mg samples were mixed with 10 mL distilled water and mixture was centrifuged (LaboGene 1248R, GRYOZEN, Daejeon, Korea) at 3,134×g for 20 min. Then, 10 μL Folin-Ciocalteau’s phenol reagent added in the 10 μL of supernatant and stand for 3 min at room temperature. After that, 70 μL of distilled water and 2 M sodium carbonate was added in the mixture. The mixture was incubated in dark at 25°C for 1 h, then absorbance was measured at 725 nm wavelength using spectrophotometer (Epoch, BioTek Instruments, Winooski, VT, USA). The TPC of sample was calculated using standard curves (0, 62.5, 125, 250, 500, 1,000 μg/mL) of gallic acid. Result was expressed as gallic acid equivalent (μg GAE/mg).
The DPPH radical scavenging ability was determined Blois (1958) method, with a slight modification. 100 mg sample was mixed with 10 mL distilled water and mixture was centrifuged at 3,134×g for 20 min. 0.4 mL supernatant and 0.4 mM DPPH solution (in 95% ethanol) was mixed. The mixture was incubated in dark at 25°C for 30 min, after that, centrifuged at 9,220×g for 3 min. Then, absorbance was measured at 517 nm wavelength against a blank (distilled water) by using spectrophotometer (BioTek Instruments). Scavenging rate was expressed as follows:
The ferric ion reducing antioxidant power was determined as described by Di Mattia et al. (2019). The ferric ion reducing antioxidant power (FRAP) reagent was prepared by mixing 300 mM acetate buffer (pH 3.6), 20 mM FeCl3·6H2O, and 10 mM TPTZ (solubilized in 40 mM HCl), ratio of 10:1:1, respectively. 100 mg sample was mixed with 10 mL distilled water and mixture was centrifuged at 3,134×g for 20 min. 40 μL supernatant, 40 μL distilled water, and FRAP reagent was mixed. The mixture was incubated at 37°C for 4 min, then absorbance was measured at 593 nm wavelength using spectrophotometer (BioTek Instruments). The ferric ion reducing antioxidant power was determined as FeSO4 equivalents compared to a calibration curve of FeSO4 at 0–500 μM.
The hydrogen peroxide scavenging activity was determined as described by Müller (1985). 100 mg sample was mixed with 10 mL distilled water and mixture was centrifuged at 3,134×g for 20 min. 20 μL supernatant, 100 μL phosphate-buffered saline (pH 7.4), and 20 μL 1 mM hydrogen peroxide was mixed. The mixture was incubated at 37°C for 5 min. After that, 30 μL 1.25 mM ABTS and 30 μL 1 unit/mL peroxidase was added in the mixture. The mixture was incubated at 37°C for 10 min, then absorbance was measured at 405 nm wavelength using spectrophotometer (BioTek Instruments). The hydrogen peroxide scavenging activity was determined as Trolox equivalents compared to a calibration curve of Trolox at 0–50 mM.
Amino acid composition of sample was determined using an amino acid analyzer (L-8900, Hitachi, Ibaraki, Japan). 5 g sample was mixed with 40 mL of 6 N hydrochloric acid and mixture was hydrolyzed at 110°C for 24 h. Thereafter, excess acid was removed using a vacuum rotary evaporator at 50°C and 50 mL of 0.2 N sodium citrate buffer (pH 2.2) was added. The sample was filtered using a 0.45 μm membrane filter, and amino acid composition was determined by analyzing the 30 μL of filtrate. 30 μL of filtrate was analyzed for determined amino acid composition.
The oil refining procedure was conducted in accordance with the methodology outlined by Jang et al. (2018), with minor modifications. Initially, the unrefined BSFL oil underwent centrifugation at 3,134×g for 20 min to eliminate natural sediment. The refining process was executed in a sequential manner, encompassing degumming, neutralization, and washing stages. To initiate degumming, distilled water (2%, w/w) was incorporated into the unrefined BSFL oil, followed by stirring at 120 rpm and a temperature of 50°C for 1 h in the water bath (C-SKW1, Changshin science, Seoul, Korea). Hydrated phospholipids were subsequently isolated through centrifugation at 2,399×g for 15 min. The non-hydratable phospholipids were then converted into hydratable phospholipids by treating the resultant oil with a 20% citric acid solution (2%, w/w) and stirring at 60°C for 15 min, after which the resulting gums were separated via centrifugation at 1,763×g for 15 min.
For the neutralization phase, a 3 M NaOH solution (1%, w/w) was applied to the degummed oil and stirred at 120 rpm at 50°C for 30 min. Following neutralization, the oil was subjected to centrifugation at 2,399×g for 15 min.
The washing process involved the addition of 15% water relative to the mass of the oil, conducted at 95°C with stirring at 120 rpm for a contact duration of 30 min, while maintaining the oil temperature at 50°C. The oil from this first washing cycle was obtained through centrifugation at 2,399×g for 15 min. A second washing cycle was performed by adding 10% water relative to the mass of the oil from the first washing cycle, at 95°C with stirring 120 rpm, for 20 min, again maintaining the oil temperature at 50°C. The oil obtained from this second washing cycle was centrifuged at 2,399×g for 15 min and was designated as refined BSFL oil.
The acid value of refined and unrefined oil extracted from BSFL was measured by AOAC method. The oil samples (5 g) were dissolved in 100 mL of ethanol-ether (1:2, v/v) mixture and addition of 1% phenolphthalein indicator, then, titrated with 0.1 N potassium hydroxide (KOH) solution until pale red color persists for 30 s. The acid value was calculated using the following Eq. (2):
Where, a=volume of KOH solution of sample titration; b=volume of KOH solution of blank titration; f=titer of 0.1 N KOH solution; s=sample weight (g).
The fatty acid composition of refined BSFL oils was measured as described by Lee et al. (2017). The fatty acid composition was analyzed using an Agilent GC equipped with an SP-2560 (Supelco, Bellefonte, PA, USA) fused silica capillary column (30 m×0.25 mm i.d., film thickness 0.25 μm). Helium served as the carrier gas (0.75 mL/min), with a split ratio of 200:1 and an injector temperature of 225°C. Fatty acid methyl esters were prepared by methylation, using triundecanoin (C11:00) as the internal standard and Supelco 37 Component FAME Mix (Supelco) as the reference. The final isooctane extract was dried over anhydrous MgSO4 and analyzed by GC-MS. Results were expressed as the percentage of the total fatty acid detected based on the total peak area.
All experiments were performed in triplicate. Result was presented means±SD. The statistical analysis of treatments was performed with the analysis of variance (ANOVA) in Minitab 18 (Minitab, State College, PA, USA) software. Tukey’s test (p<0.05) was used to detect significant among mean values of samples in all test intervals. Pearson’s correlation heatmap diagram was performed with software package of heatmap.2 in R software 4.2.1 (https://www.r-project.org/).
Results and Discussion
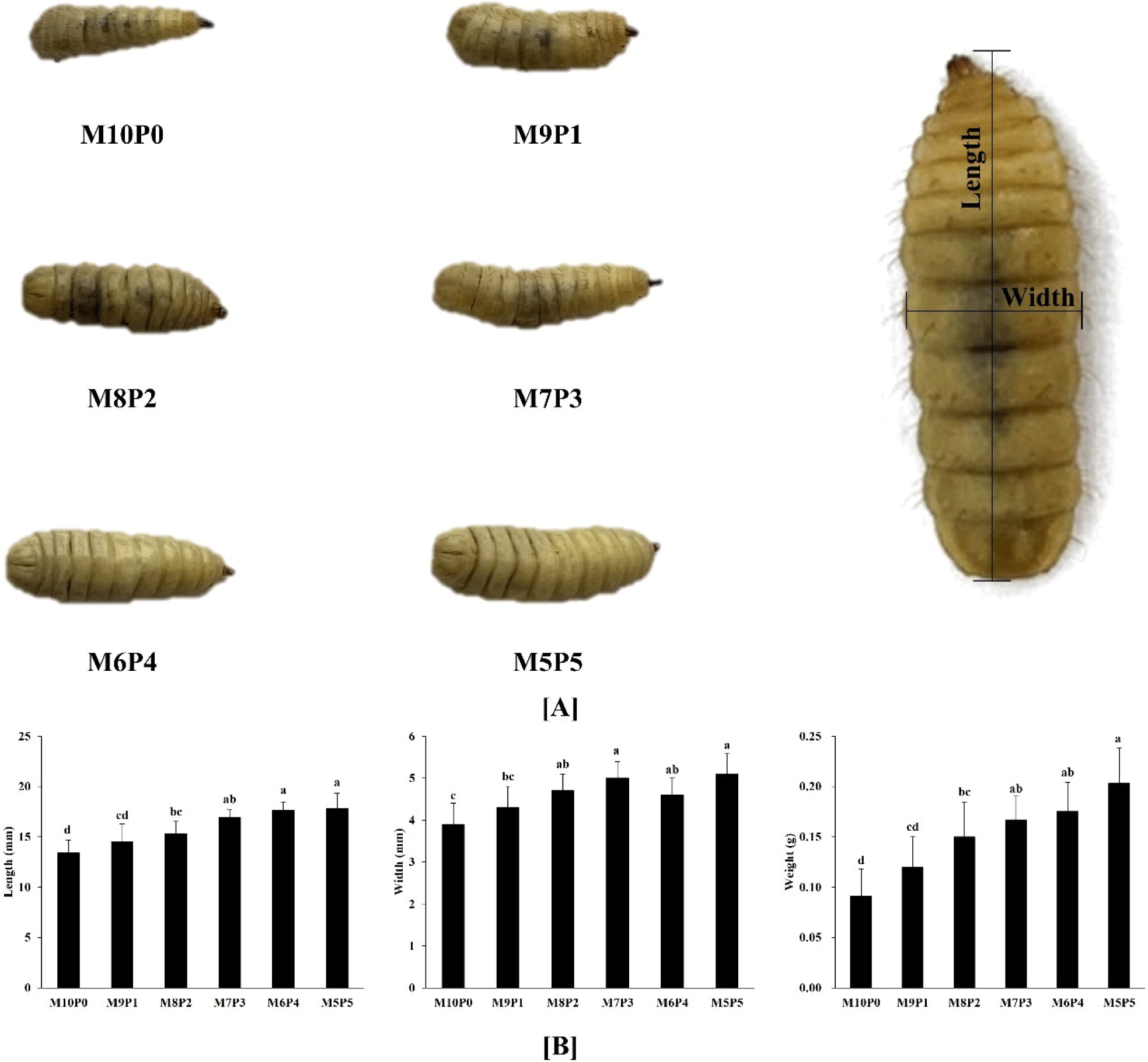
The effects of the mandarin and poultry waste ratio on the length, width, weight, and appearance of the BSFL are shown in Fig. 1. The length, width, and weight of the M10P0 fed group were significantly (p<0.05) lower than those of the other groups. In particular, the body weights of M10P0 and M5P5 were 0.091±0.027 g and 0.203±0.035 g, respectively, which is approximately a twofold difference. In other words, growth performance was influenced by the poultry by-product ratio. Barragan-Fonseca et al. (2017) explained the same results in their study in which the weights of BSFL fed on vegetable and meat waste were 0.13 g and 0.158 g, respectively. Generally, rearing substrates are known to affect the growth performance of larvae, including body weight, body size, and nutrient composition (El-Dakar et al., 2021). In particular, Amrul et al. (2022) reported that BSFL reared on organic wastes with higher protein content exhibited superior growth performance, which aligns with our findings. Given that poultry by-products have a higher protein content than mandarin by-products, our results confirm that the M5P5 group, which consumed the highest proportion of poultry by-products, achieved the greatest body weight.
The proximate composition of the defatted BSFL fed with various mixing ratios of mandarin and poultry by-products is shown in Table 2. M10P0 was found to have significantly (p<0.05) higher crude fat and crude ash contents and a lower crude protein content than the other groups (18.31±0.17%, 11.58±0.06%, and 50.29±0.13%, respectively). As the poultry by-product ratio in the substrate increased, crude fat and ash decreased, and the content of protein tended to increase. Furthermore, M5P5 had significantly (p<0.05) lower crude fat and higher protein content than the other groups (8.09±0.22% and 64.30±0.04%, respectively). The crude protein content of mandarin by-products is 5%–8%, the crude fiber content is 13%–20% (Ministry of Agriculture, Food and Rural Affairs [MAFRA], 2022).
M10P0, reared on substrates containing 100% mandarin by-product; M9P1, reared on substrates containing 90% mandarin by-product and 10% poultry by-product; M8P2, reared on substrates containing 80% mandarin by-product and 20% poultry by-product; M7P3, reared on substrates containing 70% mandarin by-product and 30% poultry by-product; M6P4, reared on substrates containing 60% mandarin by-product and 40% poultry by-product; M5P5, reared on substrates containing 50% mandarin by-product and 50% poultry by-product.
Song (2015) reported that the crude protein, crude fat, and crude ash contents of dried mandarin by-products were 8.2%, 3.2%, and 1.5%, respectively. Also, Alnaimy et al. (2017) reported that crude protein, crude fat, crude fiber, and crude ash contents were 8.25%, 3.78%, 10.82%, and 3.17% in fresh citrus pulp, respectively, and 9.66%, 4.43%, 12.68%, and 3.71% in dried form. The protein and fat content of poultry by-products are 13%–26% and 1%–34%, respectively (Henry et al., 2019). Lee (1997a) reported that the nutritional composition of poultry by-products included crude protein contents of 49.51% in the head, 58.76% in the feet, 64.67% in the viscera, 82.99% in the blood, and 86.71% in the feathers, while crude fat contents were 26.19%, 13.73%, 23.96%, 6.96%, and 2.96%, respectively, and crude ash contents were 20.38%, 21.69%, 8.62%, 3.56%, and 0.96%, respectively. Additionally, the author reported that the crude protein, crude fat, and crude ash contents of their mixtures were 71.32%, 14.09%, and 9.99%, respectively (Lee, 1997b).
Our results indicate that various substrates affect proximate composition, and similar findings were also obtained by Ewald et al. (2020) and Lopes et al. (2020) in their study on BSFL fed with bread and mussels (Mytilus edulis) and bread and rainbow trout (Oncorhynchus mykiss) by-products, respectively. Ewald et al. (2020) reported that when larvae were fed bread and mussel mixtures, the higher the mussel content, the lower the fat and higher the protein content of the larvae. Furthermore, Lopes et al. (2020) reported that a higher protein content (aquaculture by-product) and lower non-fiber carbohydrate (bread) content in substrates resulted in the larvae having a higher protein content, weight, and growth rate. However, those studies used bread with aquaculture by-products as BSFL-rearing substrates, whereas this study used fruit and poultry by-products. Many researchers have reported that the rearing substrate affects the nutrient composition of insects (Barragan-Fonseca et al., 2018; Dreassi et al., 2017; Mancini et al., 2019). Additionally, plant by-products of fruits, vegetables, and grain products are known to have high carbohydrate content, and animal-based by-products of poultry and aquaculture are known to have high protein and lipid content (Gold et al., 2020; Jucker et al., 2017; Nguyen et al., 2015). This study demonstrated that animal-based by-product substrates (poultry by-product) were effective in increasing the growth performance and protein content of BSFL. Although the various feeds were not evaluate proximate composition the results of this study also supported that the type of feed affects nutrient composition and influences the growth performance of various living things.
Table 3 shows the effect of rearing substrates on the color and pH value of defatted BSFL. The CIE L*, CIE a*, and CIE b* values of defatted BSFL according to the ratio of mandarin to poultry by-products exhibited a similar tendency. The group fed solely on mandarin waste had the lowest CIE L*, CIE a*, and CIE b* values (p<0.05) compared to the other groups, with values of 47.70±0.22, 0.38±0.07, and 4.85±0.34, respectively. The CIE L*, CIE a*, and CIE b* values increased when mandarin and poultry by-products were fed at a ratio of 9:1; however, they decreased as the ratio of poultry by-products increased. The color value increased at a ratio of 6:4 (M:P), and the highest CIE L*, CIE a*, and CIE b* values were observed in the M5P5 group (52.91±0.10, 1.48±0.12, and 6.42±0.16, respectively). The color of BSFL is an important factor as it can influence its application in various industries, including animal feed and food processing. Larouche et al. (2019) reported that BSFL tend to darken during processing, and color can vary significantly depending on the feeding substrate and processing methods. In particular, lighter-colored BSFL are often preferred in certain applications, such as protein extraction for animal feed or human food, as darker coloration may be perceived as less desirable. Therefore, maintaining a consistent color through controlled feeding conditions is essential to ensure the quality and acceptability of BSFL-based products.
M10P0, reared on substrates containing 100% mandarin by-product; M9P1, reared on substrates containing 90% mandarin by-product and 10% poultry by-product; M8P2, reared on substrates containing 80% mandarin by-product and 20% poultry by-product; M7P3, reared on substrates containing 70% mandarin by-product and 30% poultry by-product; M6P4, reared on substrates containing 60% mandarin by-product and 40% poultry by-product; M5P5, reared on substrates containing 50% mandarin by-product and 50% poultry by-product.
The pH impacts microbial spoilage, proliferation, and metabolism and is an important parameter to consider when estimating product shelf life (Larouche et al., 2019; Nam and Chun, 2021). The pH value of M10P0 (8.52±0.03) was significantly higher than that of the other samples (p<0.05). The pH value significantly decreased as the ratio of poultry by-products increased; moreover, M5P5 (8.07±0.01) had the lowest pH value in defatted BSFL fed on different substrates (p<0.05). According to Larouche et al. (2019), the pH of BSFL ranged from 6.1 to 8.7 when killed by different methods (mechanical disruption, heating, freezing, and asphyxiation). Saucier et al. (2022) reported that the pH value of BSFL after scalding and hot air drying ranged from 7.4 to 7.7.
The phenolic hydroxyl group of phenolic compounds tends to combine with proteins and has potential anticancer, antimicrobial, and antioxidant activities (Lee et al., 2012). The TPC and antioxidant capacity of defatted BSFL are shown in Fig. 2. The TPC of M10P0 was 3.74±0.21 μg GAE/mg, which was significantly (p<0.05) lower than that of the other groups. The TPC of M9P1, M8P2, and M7P3 were significantly (p<0.05) higher than that of M10P0; however, there was no significant (p>0.05) difference between these groups. The M6P4 and M5P5 groups were significantly (p<0.05) higher than the other groups, at 5.10±0.13 μg GAE/mg and 5.12±0.13 μg GAE/mg, respectively.
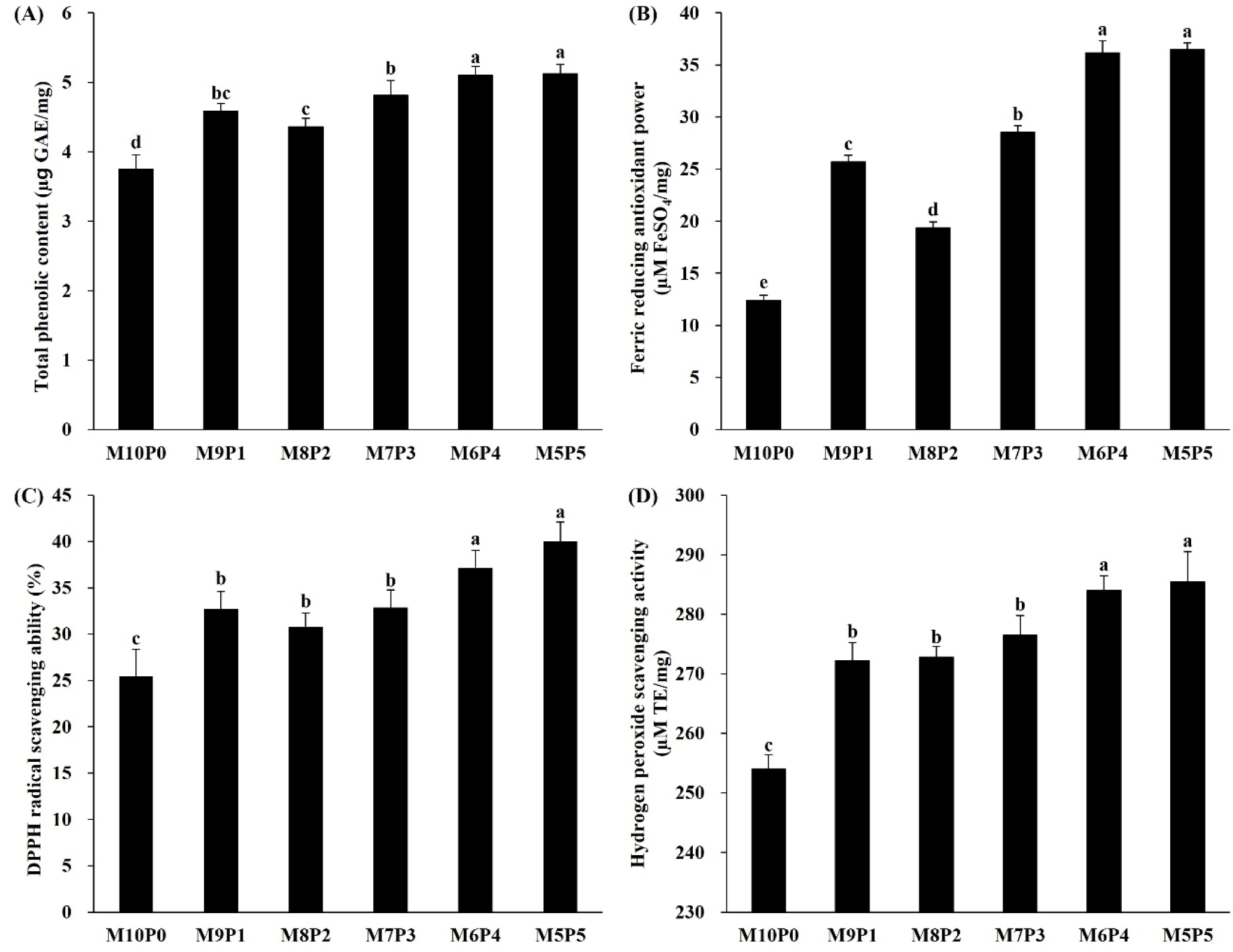
The FRAP assay is a convenient and reproducible way of evaluating antioxidant capacity, and the ability of a compound to transform from Fe3+/ferricyanide complex to Fe2+/ferrous serves as an indicator of antioxidant capacity (Aryal et al., 2019). The lowest (p<0.05) value of FRAP was obtained from M10P0 (12.40±0.52 μM FeSO4/mg). The FRAP values of BSFL were enhanced by increasing the poultry by-product in the substrate mixture, and M6P4 and M5P5 were significantly (p<0.05) higher than the other groups, at 36.15±1.16 and 36.49±0.61 μM FeSO4/mg, respectively.
DPPH assay is a facile and fast method of antioxidant measurement. DPPH is a stable free radical that produces violet solution in ethanol; moreover, it is reduced by the extinction of an antioxidant material to produce a colorless ethanol solution (Mensor et al., 2001). The DPPH radical scavenging ability of M10P0 was 25.45±2.91%, which was significantly (p<0.05) lower than that of the other groups. Meanwhile, M9P1, M8P2, and M7P3 had DPPH radical scavenging abilities of 30.80%–32.87%. However, no DPPH radical scavenging ability difference (p>0.05) was observed between these group. The DPPH radical scavenging abilities of M6P4 and M5P5 ranged from 37.18% to 40.05%, which were significantly (p<0.05) higher than those of the other groups.
Hydrogen peroxide is a reactive oxygen species that is produced endogenously as a consequence of normal cell function or derived from external sources, and causes protein, DNA, and lipid damage (Martindale and Holbrook, 2002). The H2O2 scavenging activity exhibited a similar tendency to the DPPH radical scavenging ability. The H2O2 scavenging activity of M10P0 (254.07±2.38 μM TE/mg) was significantly (p<0.05) lower than that of other groups. The H2O2 scavenging activity was significantly increased when the mandarin and poultry by-products were fed at a ratio of 9:1. Furthermore, M6P4 and M5P5 (284.05±2.41 and 285.40±5.09 μM TE/mg) had significantly higher H2O2 scavenging activities than M9P1, M8P2, and M7P3 (272.21–276.46 μM TE/mg).
Studies analyzing the antioxidant activities of BSFL fed on different substrates have rarely been reported. In this study, higher animal-based substrate (poultry by-product) ratios in the substrate mixture were found to enhance the antioxidant activity of BSFL. The overall antioxidant activities (DPPH radical scavenging ability, FRAP value, and H2O2 scavenging activity) and TPC were lowest for M10P0 and enhanced by increasing the poultry by-product ratio in the substrate mixture. Furthermore, M6P4 and M5P5 exhibited the highest antioxidant activities. Zhou et al. (2019) when comparing the basal feed and feeds containing 100 mg/kg and 200 mg/kg of baicalein, a flavonoid compound with antioxidant activity, the group fed 200 mg/kg of baicalein showed the best growth overall. Therefore, in this study, it is thought that the group fed 6:4 and 5:5, which have high antioxidant activity, will show good effects on weight gain and average body weight.
Amino acids are necessary for the growth and development of livestock; in particular, essential amino acids cannot be synthesized by livestock and must be supplied through the diet (Choi et al., 2021; Craig et al., 2017). Table 4 shows the effect of the rearing substrates on the amino acid composition of defatted BSFL. Aspartate, glutamate, valine, leucine, and lysine levels were higher than those of other amino acids. Liland et al. (2017) reported that aspartate and glutamate were the predominant amino acids in BSFL. According to Hopkins et al. (2021), leucine and glutamate are the most abundant essential amino acids and non-essential amino acids in BSFL. Among the amino acids, glutamate had the highest content, ranging from 56.9 to 69.2 g/kg, followed by aspartate, valine, leucine, and lysine, which were 47.1–63.8 g/kg, 29.3–37.7 g/kg, 32.9–43.1 g/kg, and 32.0–40.7 g/kg, respectively. Furthermore, the M6P4 and M5P5 groups had significantly (p<0.05) higher contents of all amino acids, and the total amino acid contents were 566.0 g/kg and 576.4 g/kg, respectively. However, these high levels of amino acids were expected because of the higher crude protein content in M6P4 and M5P5 than in the other samples. According to Lalander et al. (2019), rearing substrates influence the amino acid composition of BSFL; however, they reported that this influence does not appear to be significant.
M10P0, reared on substrates containing 100% mandarin by-product; M9P1, reared on substrates containing 90% mandarin by-product and 10% poultry by-product; M8P2, reared on substrates containing 80% mandarin by-product and 20% poultry by-product; M7P3, reared on substrates containing 70% mandarin by-product and 30% poultry by-product; M6P4, reared on substrates containing 60% mandarin by-product and 40% poultry by-product; M5P5, reared on substrates containing 50% mandarin by-product and 50% poultry by-product.
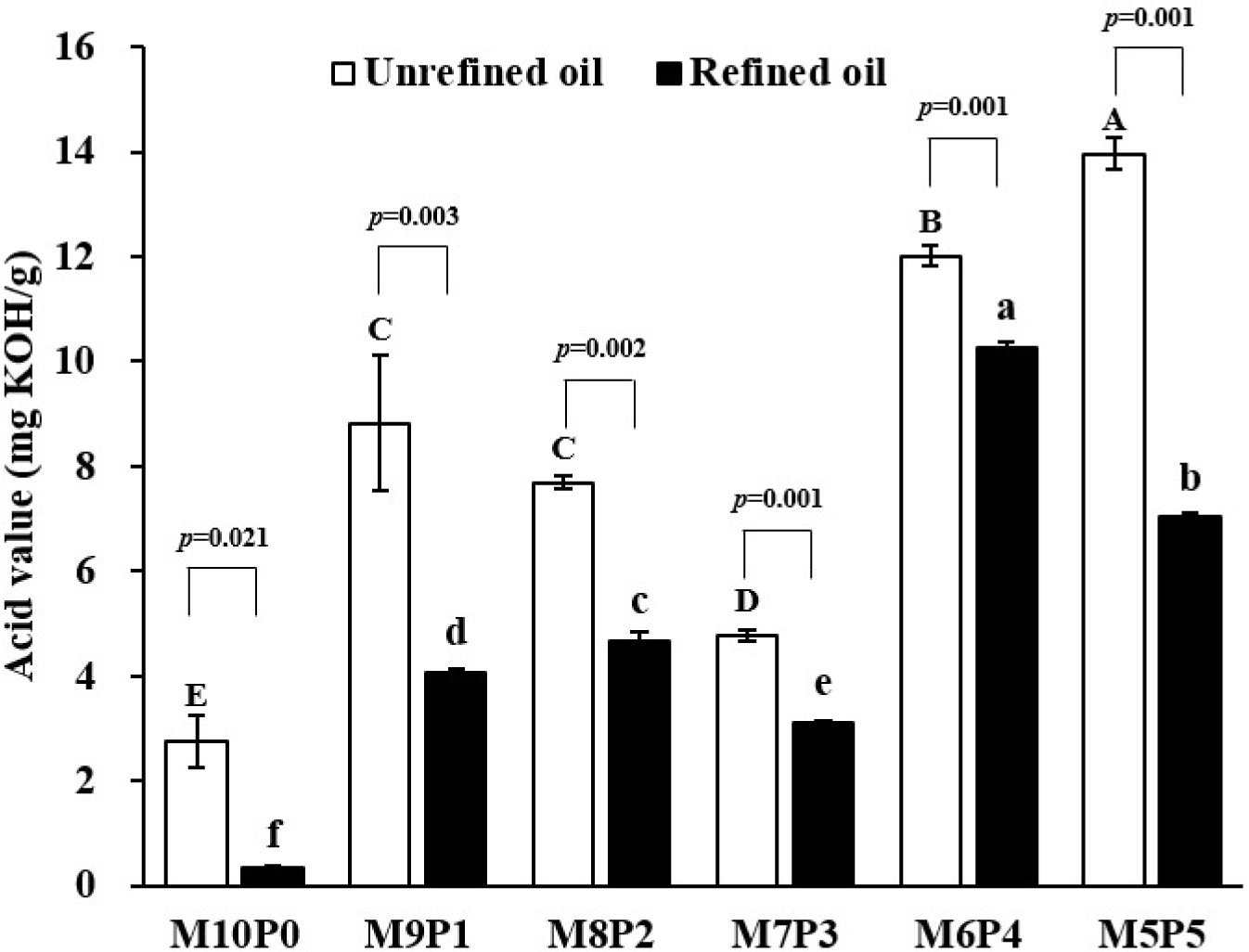
The acid values of the unrefined and refined BSFL oils are shown in Fig. 3. The acid value is used as a quality standard to measure the degree of acidification by measuring the free fatty acids contained in oil. The acid value of unrefined BSFL oils ranged from 2.76 to 13.96 KOH mg/g and refined BSFL oils ranged from 0.35 to 10.27 KOH mg/g. The acid value of BSFL oils was lowest in M10P0, highest in M6P4 and M5P5, and the acid values were decreased significantly (p<0.05) after the oil refining process. In Korea, the MAFRA (2022) stipulated the acid value of animal oils to be 30 mg KOH/g or less according to the standards and specifications for each item of raw materials of feedstuff; both oils before and after refining were within the acceptable range. A similar result was obtained by Mai et al. (2019), who reported that the acid value of crude BSFL oil was 11.876 mg KOH/g oil, and it decreased to 0.9 mg KOH/g oil after refining. According to Park et al. (2020), during the refining process of Berryteuthis magister viscera oil, the acid value decreased with the amount of NaOH solution used in the neutralization process. Based on these results, to reduce the acid value of BSFL oil, the amount of NaOH solution used in the neutralization process should be increased.
The fatty acid composition of the refined BSFL oils is shown in Table 5. The saturated fatty acid content of the BSFL oils is higher than that of unsaturated fatty acids, and this composition is similar to that of beef tallow (Park et al., 2019). The refined BSFL oils had 53.69%–58.97% saturated fatty acids and 41.04%–46.35% unsaturated fatty acids. The predominant saturated fatty acid in the refined BSFL oils was lauric acid (28.34%–29.31%), followed by palmitic acid (17.21%–19.65%). In BSFL oils, lauric acid has the highest content among the saturated fatty acids (St-Hilaire et al., 2007). Lauric acid is a medium-chain fatty acid and is abundant in coconut oil. Medium-chain fatty acids have antibacterial properties that kill bacteria and can be used as natural antibiotics (Nakatsuji et al., 2009). Lauric acid reduces total serum cholesterol and improves the synthesis of high-density lipoprotein cholesterol (Sheela et al., 2016). The predominant unsaturated fatty acid in the refined BSFL oils was oleic acid (21.17%–26.42%), followed by linoleic acid (9.53%–12.16%). Oleic acid lowers systolic blood pressure in the cardiovascular system and inhibits platelet aggregation (Karacor and Cam, 2015). Linoleic acid and linolenic acid are essential fatty acids that cannot be made and should be consumed in the diet of all mammals (Simopoulos, 2008)
M10P0, reared on substrates containing 100% mandarin by-product; M9P1, reared on substrates containing 90% mandarin by-product and 10% poultry by-product; M8P2, reared on substrates containing 80% mandarin by-product and 20% poultry by-product; M7P3, reared on substrates containing 70% mandarin by-product and 30% poultry by-product; M6P4, reared on substrates containing 60% mandarin by-product and 40% poultry by-product; M5P5, reared on substrates containing 50% mandarin by-product and 50% poultry by-product.
To better understand the effects of rearing substrates (mandarin and poultry by-products) on growth performance, physicochemical properties, and antioxidant activities of BSFL, a correlation matrix was generated using Pearson’s correlation coefficient (Fig. 4). While a strong positive correlation was observed between the poultry by-product ratio and various traits, it is more critical to evaluate the independent effects of each factor (mandarin and poultry by-products) and their interaction rather than focusing solely on overall correlations.
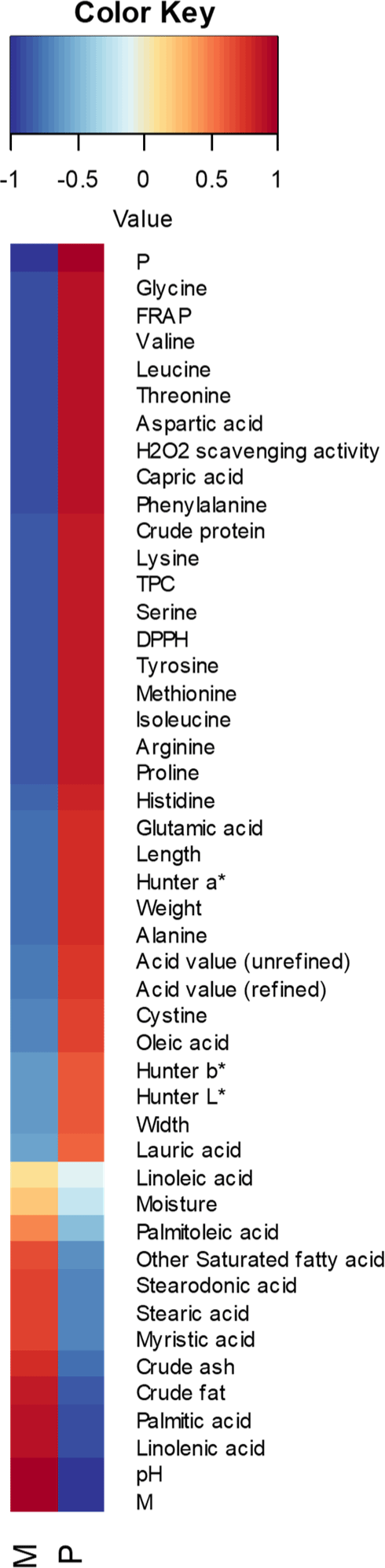
A higher proportion of poultry by-products significantly enhanced insect growth performance, as indicated by positive correlations with length (r=0.784) and weight (r=0.778). Similarly, antioxidant activities (TPC, FRAP, DPPH radical scavenging ability, H2O2 scavenging activity) were strongly correlated with poultry by-product content (r=0.863–0.907), and crude protein (r=0.875) and amino acids (r=0.688–0.910) also exhibited positive relationships. However, these trends may vary depending on the specific interactions between different substrate components.
Moving forward, future research should focus on evaluating the distinct contributions of mandarin and poultry by-products, as well as their synergistic or antagonistic effects. Understanding these interactions will provide deeper insights into how substrate composition influences BSFL metabolism and physiology, ultimately optimizing production efficiency and product quality.
Conclusion
This study highlights the potential of BSFL as a sustainable bioconversion tool for upcycling poultry by-products into valuable protein and bioactive compounds. By utilizing food waste, particularly protein-rich animal by-products, BSFL can contribute to reducing environmental burdens while enhancing the efficiency of alternative protein production. The findings suggest that rearing conditions can improve protein content, antioxidant properties, and overall insect biomass yield. Moving forward, future studies should delve deeper into the metabolic mechanisms underlying BSFL’s ability to convert waste into high-value nutrients. Additionally, investigating the scalability and industrial feasibility of using BSFL for waste valorization will be essential for bridging the gap between laboratory research and real-world applications. Ultimately, this study contributes to the growing body of research supporting insect-based bioconversion as a circular economy approach, paving the way for more sustainable food systems and waste management solutions. Furthermore, BSFL are currently not recognized as edible insects in Korea. However, if their nutritional value and safety are established and recognized as food ingredients, they could become an environmentally friendly future protein source that contributes to carbon neutrality.













